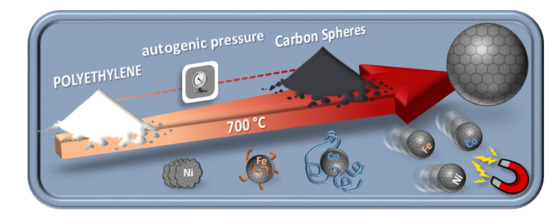
From Polyethylene to Highly Graphitic and Magnetic Carbon Spheres Nanocomposites: Carbonization under Pressure
Abstract
1. Introduction
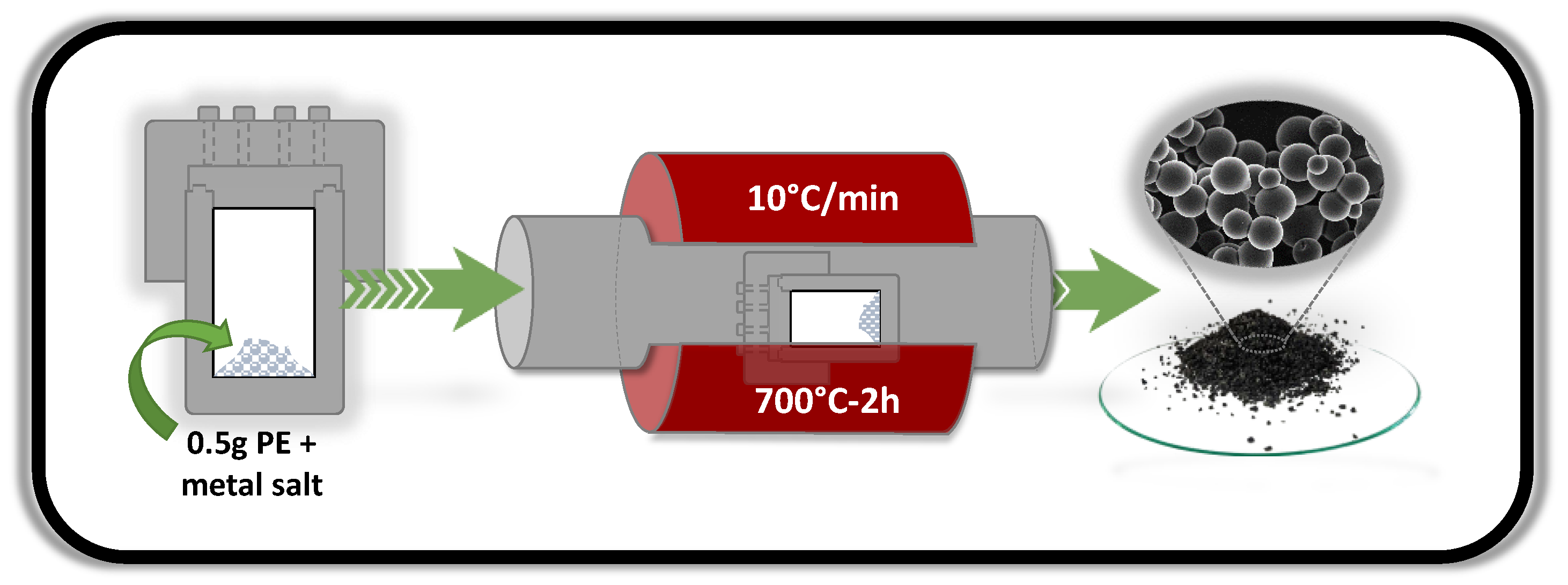
2. Materials and Methods
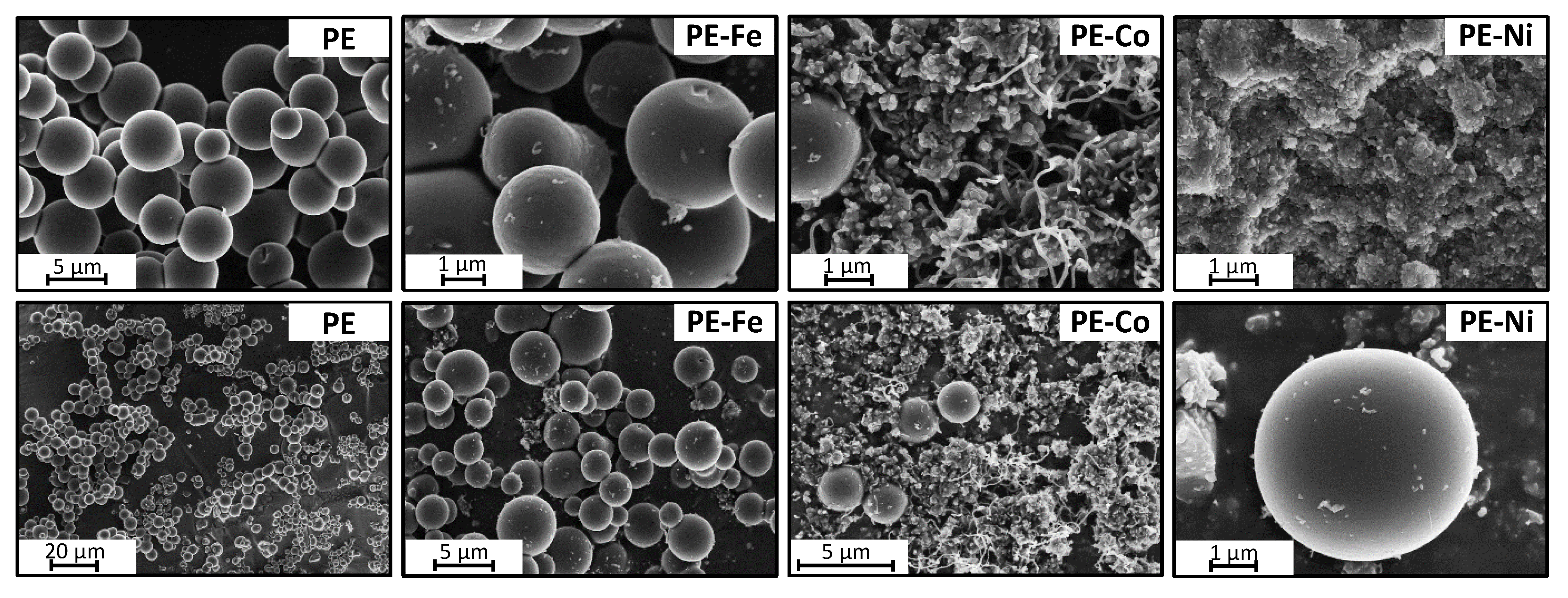
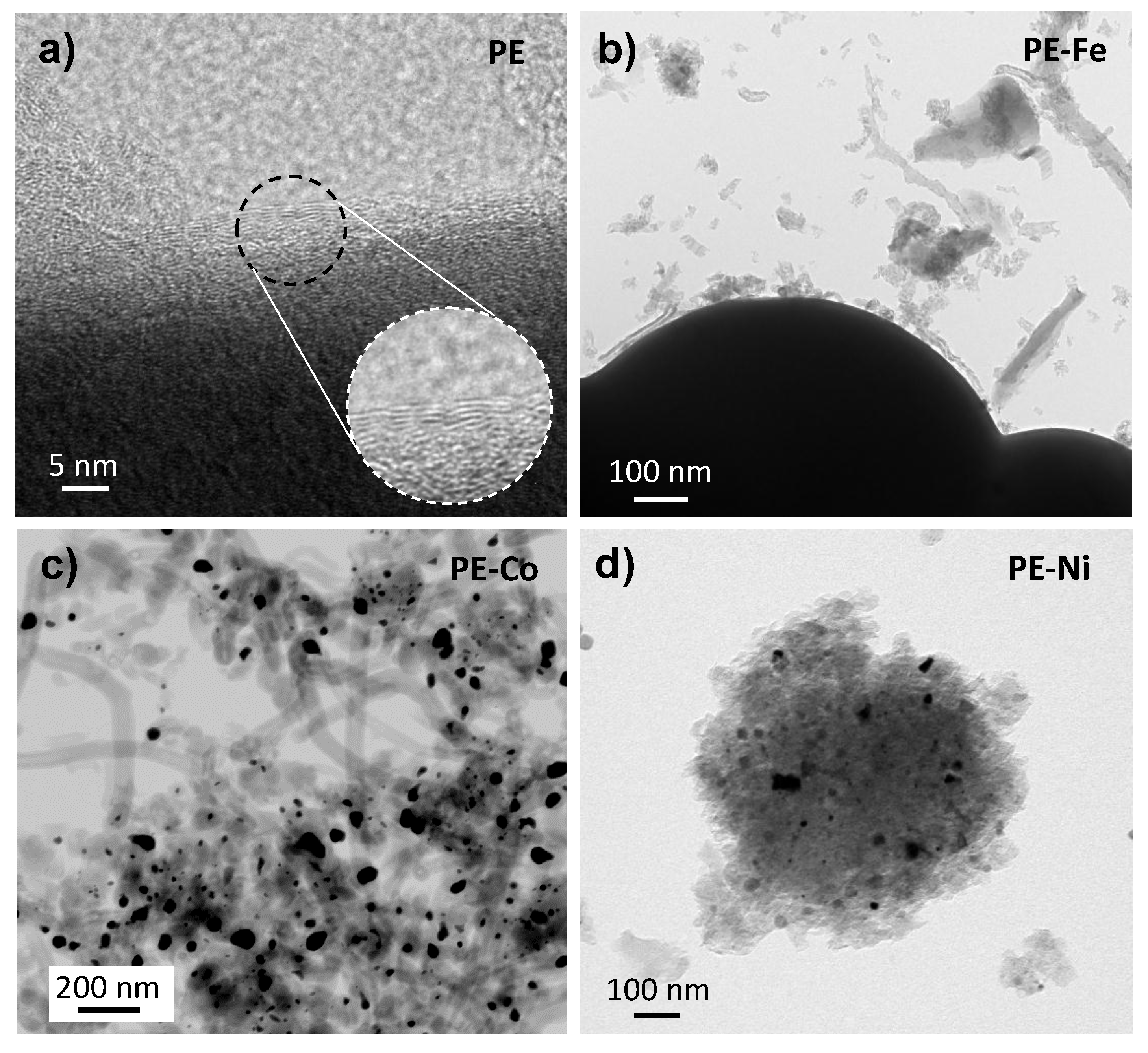
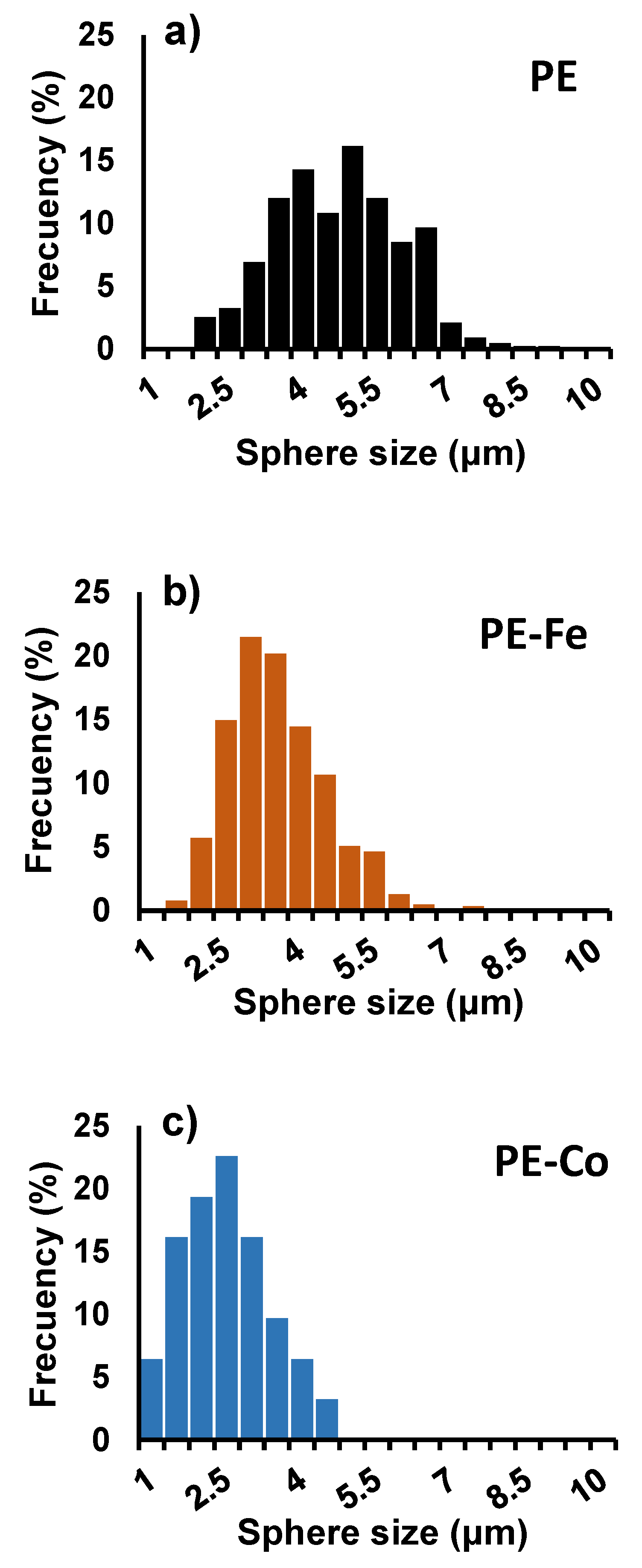
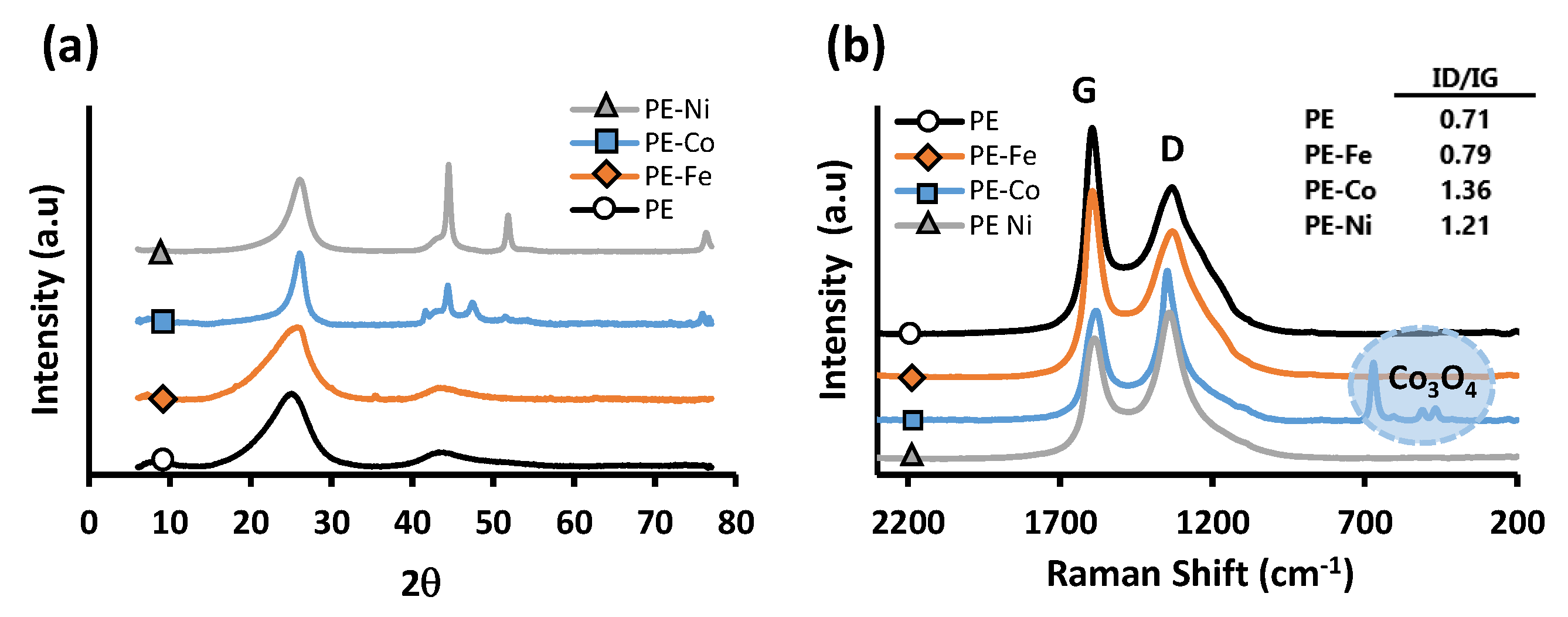
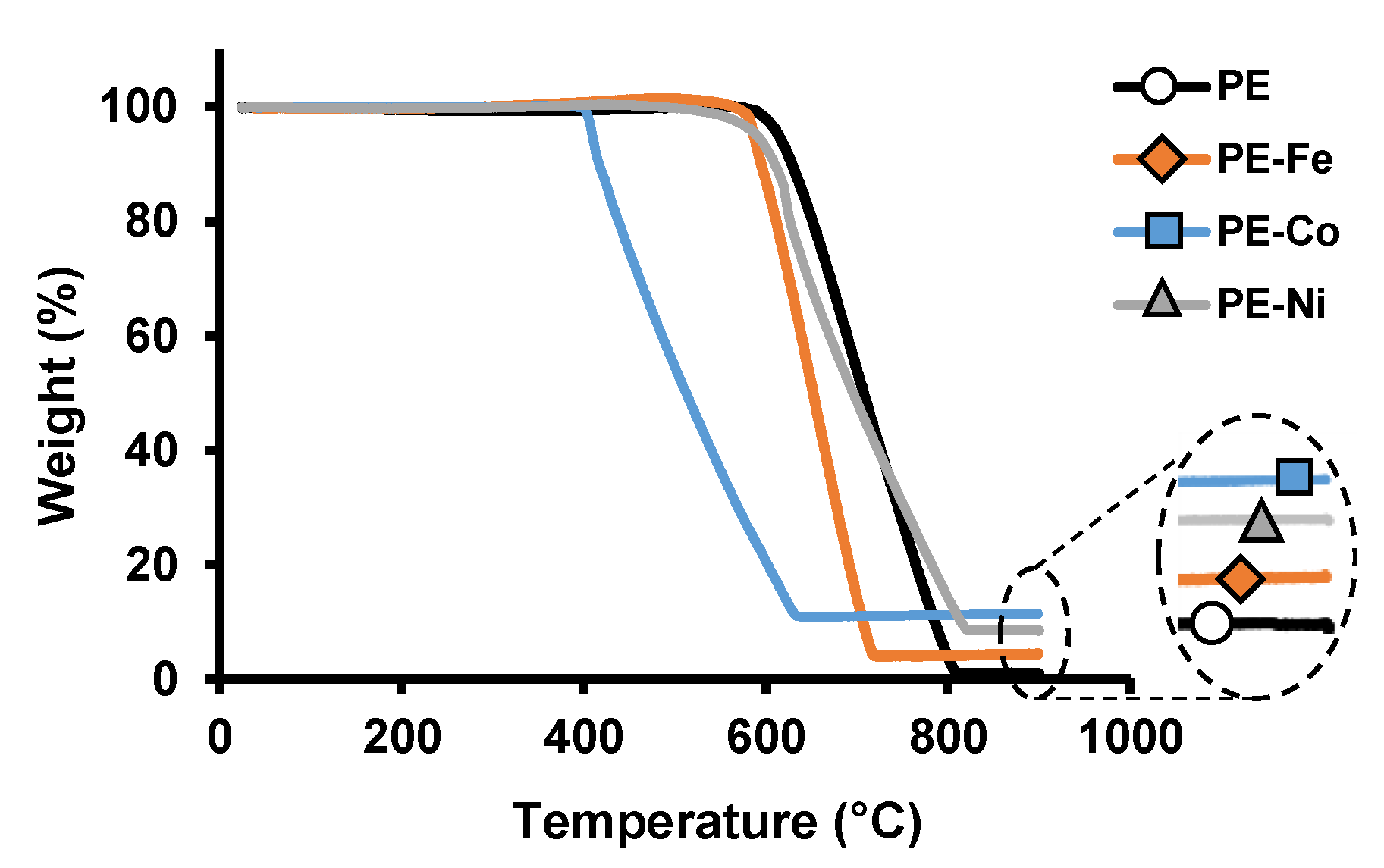
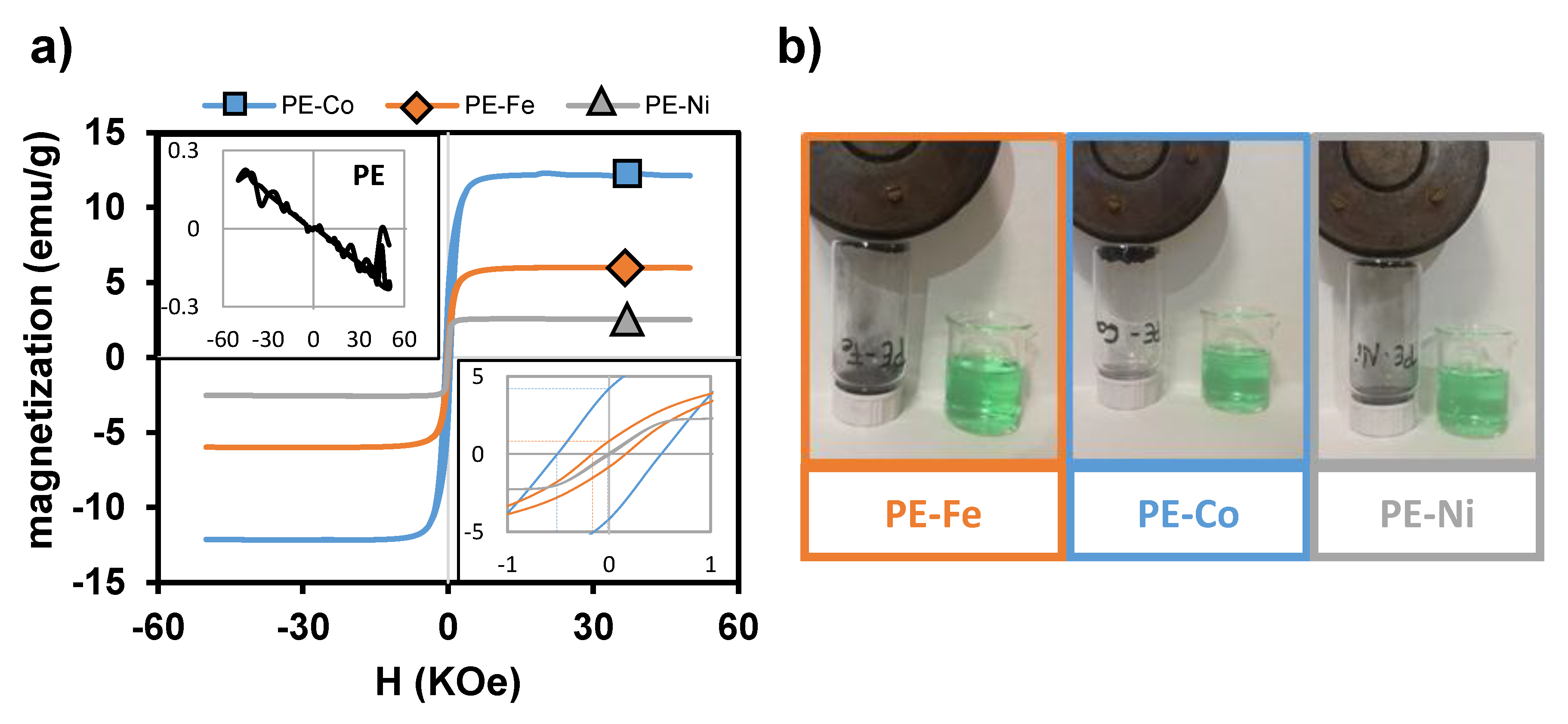
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moreno-Castilla, C.; Maldonado-Hódar, F.J. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Grigoras, M.; Catargiu, A.M.; Tudorache, F.; Dobromir, M. Chemical synthesis and characterization of self-doped N-propanesulfonic acid polyaniline derivatives. Iran. Polym. J. 2012, 21, 131–141. [Google Scholar] [CrossRef]
- Grigoras, M.; Catargiu, A.M.; Tudorache, F. Molecular composites obtained by polyaniline synthesis in the presence of p-octasulfonated calixarene macrocycle. J. Appl. Polym. Sci. 2013, 127, 2796–2802. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Meng, X. Controllable incorporation of Ag and Ag-Au nanoparticles in carbon spheres for tunable optical and catalytic properties. J. Mater. Chem. 2010, 20, 5436–5445. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Frackowiak, E.; Abbas, Q.; Béguin, F. Carbon carbon supercapacitors. J. Energy Chem. 2013, 22, 226–240. [Google Scholar] [CrossRef]
- Himeno, S.; Komatsu, T.; Fujita, S. High-Pressure Adsorption Equilibria of Methane and Carbon Dioxide on Several Activated Carbons. J. Chem. Eng. Data 2005, 50, 369–376. [Google Scholar] [CrossRef]
- Schaefer, S.; Fierro, V.; Izquierdo, M.T.; Celzard, A. Assessment of hydrogen storage in activated carbons produced from hydrothermally treated organic materials. Int. J. Hydrog. Energy 2016, 41, 12146–12156. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Chen, M.; Jiao, Y.; Wang, J. Electrochemical Performances of Nanoparticle Fe3O4/Activated Carbon Supercapacitor Using KOH Electrolyte Solution. J. Phys. Chem. C 2009, 113, 2643–2646. [Google Scholar] [CrossRef]
- Qiang, L.; Hu, Z.; Li, Z.; Yang, Y.; Wang, X.; Zhou, Y.; Zhang, X.; Wang, W.; Wang, Q. Hierarchical porous biomass carbon derived from cypress coats for high energy supercapacitors. J. Mater. Sci. Mater. Electron. 2019. [Google Scholar] [CrossRef]
- Liu, J.; Wickramaratne, N.P.; Qiao, S.Z.; Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 2015, 14, 763–774. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Li, M.; Lu, L.; Xue, J. Fe3O4 Nanoparticles embedded in uniform mesoporous carbon spheres for superior High-rate battery applications. Adv. Funct. Mater. 2014, 24, 319–326. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Fernández-Sáez, N.; Carrasco-Marín, F. Development of Vanadium-Coated Carbon Microspheres: Electrochemical Behavior as Electrodes for Supercapacitors. Adv. Funct. Mater. 2018, 28, 1802337. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated Carbon Spheres for CO2 Adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Li, Z.-S.; Huang, Y.-G.; Li, Q.-Y.; Wang, X.-Y. A novel hybrid supercapacitor based on spherical activated carbon and spherical MnO2 in a non-aqueous electrolyte. J. Mater. Chem. 2010, 20, 3883–3889. [Google Scholar] [CrossRef]
- Ariyadejwanich, P.; Tanthapanichakoon, W.; Nakagawa, K.; Mukai, S.; Tamon, H. Preparation and characterization of mesoporous activated carbon from waste tires. Carbon 2003, 41, 157–164. [Google Scholar] [CrossRef]
- Castelo-Quibén, J.; Elmouwahidi, A.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Metal-carbon-CNF composites obtained by catalytic pyrolysis of urban plastic residues as electro-catalysts for the reduction of CO2. Catalysts 2018, 8, 198. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Castelo-Quibén, J.; Vivo-Vilches, J.F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from agricultural waste solvothermally doped with sulphur as electrodes for supercapacitors. Chem. Eng. J. 2018, 334. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastic Europe—The Facts. 2017. Available online: https://www.plasticseurope.org/de (accessed on 17 March 2019).
- Inagaki, M.; Park, K.C.; Endo, M. Carbonization under pressure. New Carbon Mater. 2010, 25, 409–420. [Google Scholar] [CrossRef]
- Pol, S.V.; Pol, V.G.; Sherman, D.; Gedanken, A. A solvent free process for the generation of strong, conducting carbon spheres by the thermal degradation of waste polyethylene terephthalate. Green Chem. 2009, 11, 448–451. [Google Scholar] [CrossRef]
- Kotolevich, Y.; Martynyuk, O.; Martínez-González, S.; Tiznado, H.; Pestryakov, A.; Avalos Borja, M.; Cortés Corberán, V.; Bogdanchikova, N. Novel route of synthesis of ultra-small Au nanoparticles on SiO2 supports. Fuel 2019, 236, 589–597. [Google Scholar] [CrossRef]
- Thomsen, C.; Reich, S. Double resonant raman scattering in graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman Spectroscopy of Carbon Materials: Structural Basis of Observed Spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Markovsky, B.; Exnar, I.; Zinigrad, E.; Grinblat, J.; Haik, O.; Martha, S.K.; Gofer, Y.; Deghenghi, G.; Aurbach, D.; Wang, D.; et al. LiMnPO4 as an Advanced Cathode Material for Rechargeable Lithium Batteries. J. Electrochem. Soc. 2009, 156, A541. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Aravindan, V.; Chuiling, W.; Reddy, M.V.; Madhavi, S.; Chowdari, B.V.R. Carbon coated nano-LiTi2(PO4)3 electrodes for non-aqueous hybrid supercapacitors. Phys. Chem. Chem. Phys. 2012, 14, 5808. [Google Scholar] [CrossRef]
- Pang, L.S.K.; Saxby, J.D.; Chatfield, S.P. Thermogravimetric analysis of carbon nanotubes and nanoparticles. J. Phys. Chem. 1993, 97, 6941–6942. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Rivera-Utrilla, J.; López-Peinado, A.; Fernández-Morales, I.; López-Garzón, F.J. Gasification reaction of a lignite char catalysed by Cr, Mn, Fe, Co, Ni, Cu and Zn in dry and wet air. Fuel 1985, 64, 1220–1223. [Google Scholar] [CrossRef]
- Frusteri, L.; Cannilla, C.; Barbera, K.; Perathoner, S.; Centi, G.; Frusteri, F. Carbon growth evidences as a result of benzene pyrolysis. Carbon 2013, 59, 296–307. [Google Scholar] [CrossRef]
- Fujiwara, M.; Oki, E.; Hamada, M.; Tanimoto, Y.; Mukouda, I.; Shimomura, Y. Magnetic Orientation and Magnetic Properties of a Single Carbon Nanotube. J. Phys. Chem. A 2001, 105, 4383–4386. [Google Scholar] [CrossRef]
- Heremans, J.; Olk, C.H.; Morelli, D.T. Magnetic susceptibility of carbon structures. Phys. Rev. B 1994, 49, 15122–15125. [Google Scholar] [CrossRef]
- El-Gendy, A.A.; Ibrahim, E.M.M.; Khavrus, V.O.; Krupskaya, Y.; Hampel, S.; Leonhardt, A.; Büchner, B.; Klingeler, R. The synthesis of carbon coated Fe, Co and Ni nanoparticles and an examination of their magnetic properties. Carbon 2009, 47, 2821–2828. [Google Scholar] [CrossRef]
- Sun, X.; Gutierrez, A.; Yacaman, M.J.; Dong, X.; Jin, S. Investigations on magnetic properties and structure for carbon encapsulated nanoparticles of Fe, Co, Ni. Mater. Sci. Eng. A 2000, 286, 157–160. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelo-Quibén, J.; Pastrana-Martínez, L.M.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. From Polyethylene to Highly Graphitic and Magnetic Carbon Spheres Nanocomposites: Carbonization under Pressure. Nanomaterials 2019, 9, 606. https://doi.org/10.3390/nano9040606
Castelo-Quibén J, Pastrana-Martínez LM, Carrasco-Marín F, Pérez-Cadenas AF. From Polyethylene to Highly Graphitic and Magnetic Carbon Spheres Nanocomposites: Carbonization under Pressure. Nanomaterials. 2019; 9(4):606. https://doi.org/10.3390/nano9040606
Chicago/Turabian StyleCastelo-Quibén, Jesica, Luisa M. Pastrana-Martínez, Francisco Carrasco-Marín, and Agustín F. Pérez-Cadenas. 2019. "From Polyethylene to Highly Graphitic and Magnetic Carbon Spheres Nanocomposites: Carbonization under Pressure" Nanomaterials 9, no. 4: 606. https://doi.org/10.3390/nano9040606
APA StyleCastelo-Quibén, J., Pastrana-Martínez, L. M., Carrasco-Marín, F., & Pérez-Cadenas, A. F. (2019). From Polyethylene to Highly Graphitic and Magnetic Carbon Spheres Nanocomposites: Carbonization under Pressure. Nanomaterials, 9(4), 606. https://doi.org/10.3390/nano9040606