Synergistic Effect of Dual Particle-Size AuNPs on TiO2 for Efficient Photocatalytic Hydrogen Evolution
Abstract
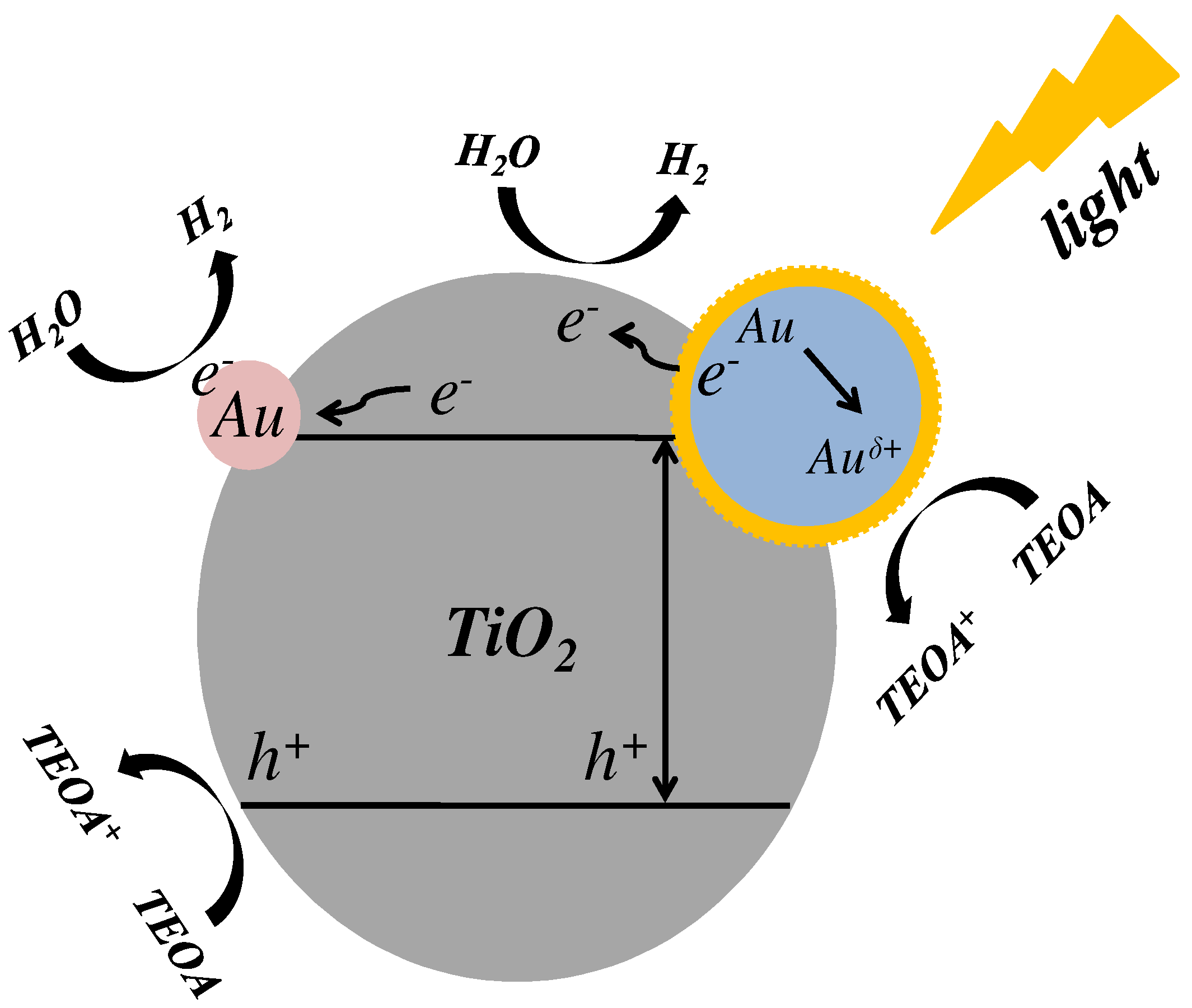
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of TiO2 Nanosheets
2.3. Fabrication of Large (45.0 ± 9.8 nm) AuNPs/TiO2 Photocatalysts
2.4. Fabrication of Small (16.9 ± 5.5 nm) AuNPs/TiO2 Photocatalysts
2.5. Preparation of Dual Particle-Size AuNPs/TiO2 Photocatalysts
2.6. Preparation of GDC-Directed Dual Particle-Size AuNPs/TiO2 Photocatalysts
2.7. Characterization Methods
2.8. Photocatalytic Activity Evaluation
2.9. Electrochemical Characterization
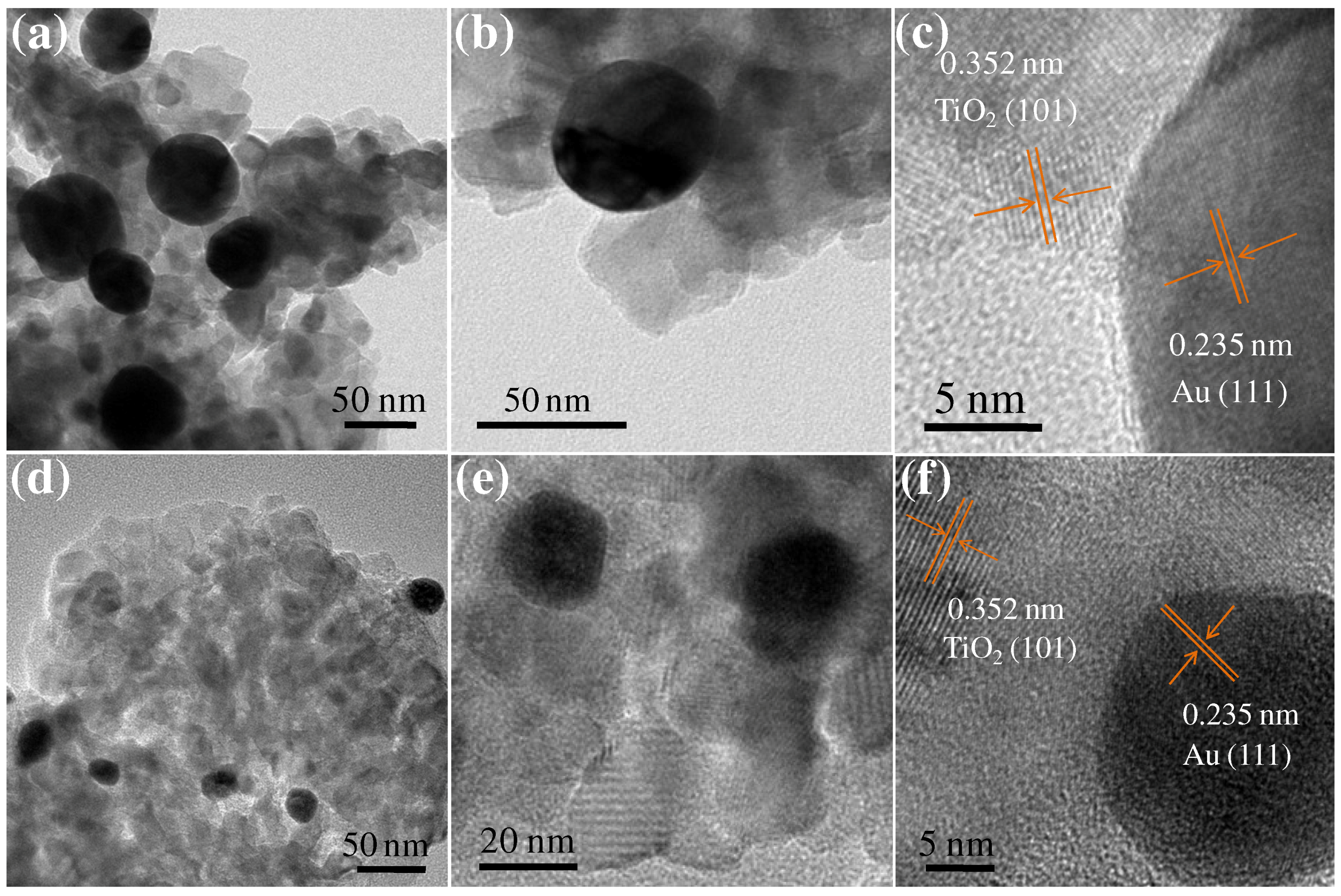
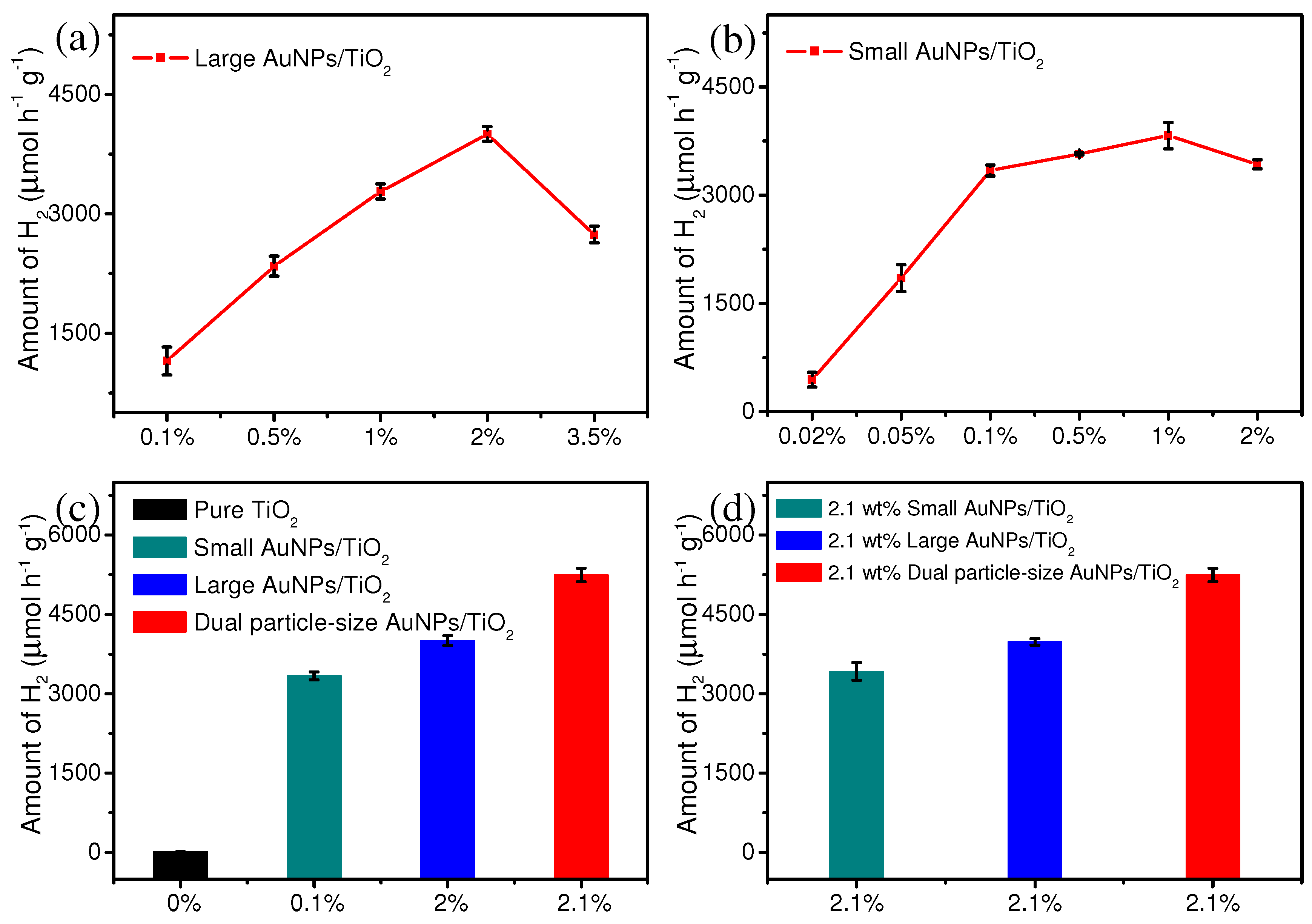
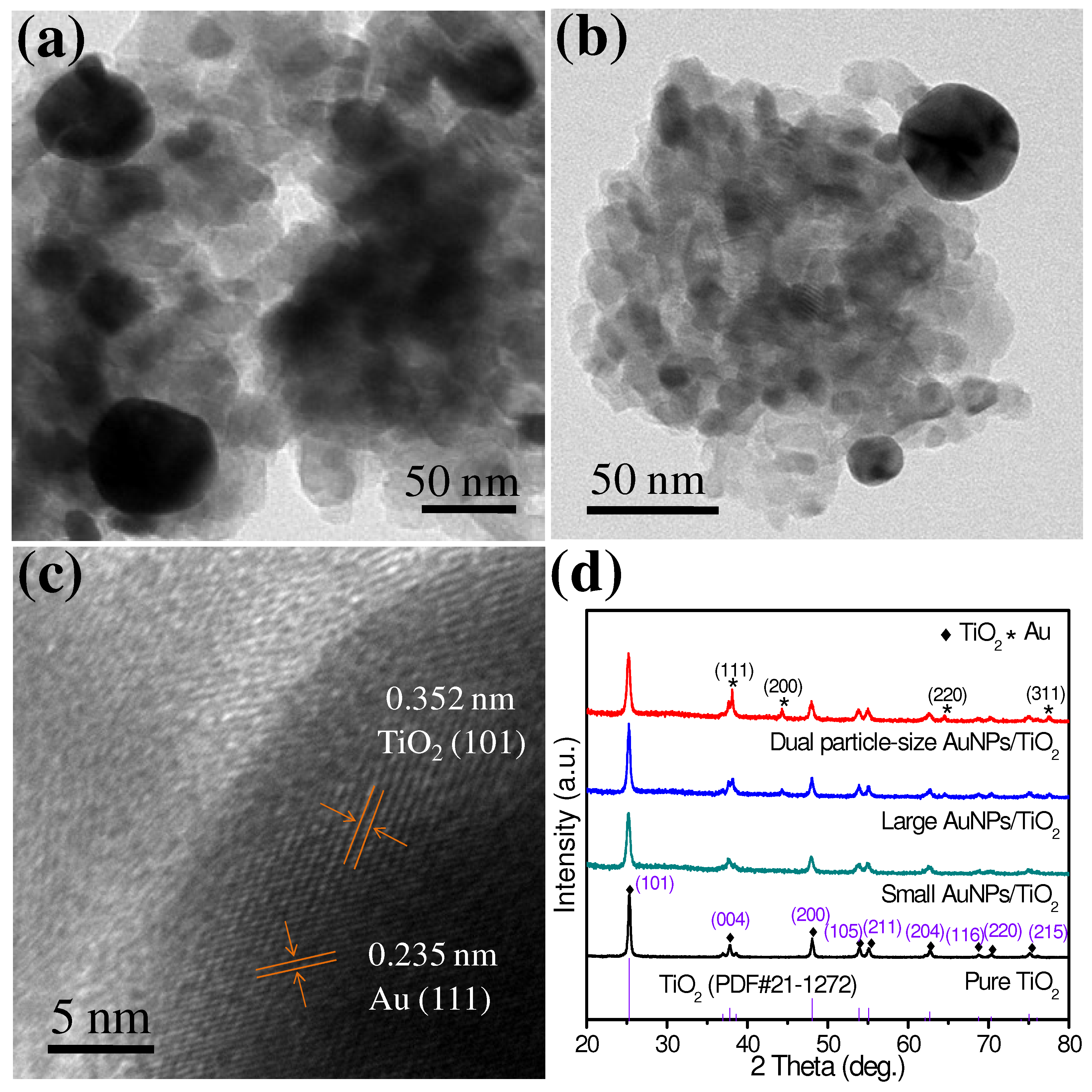
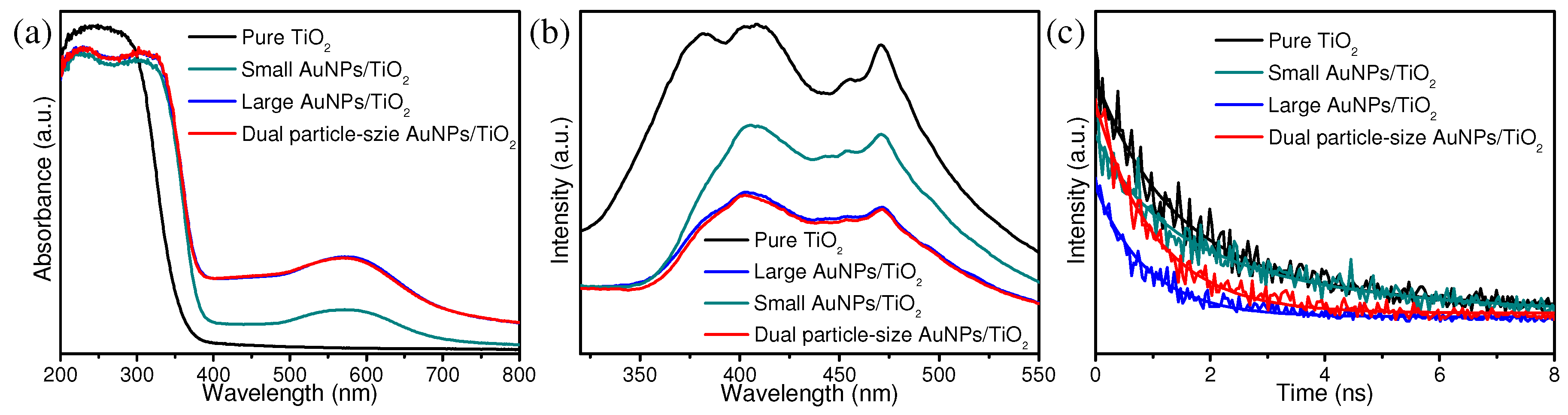
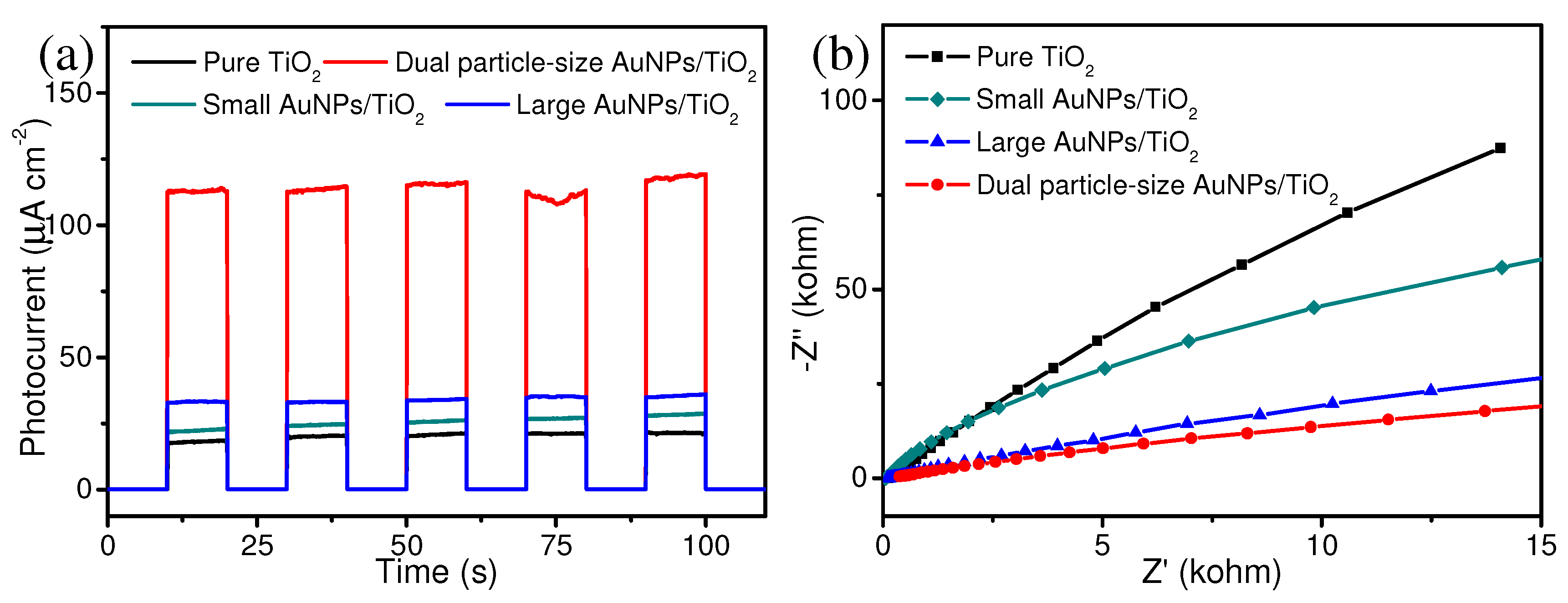
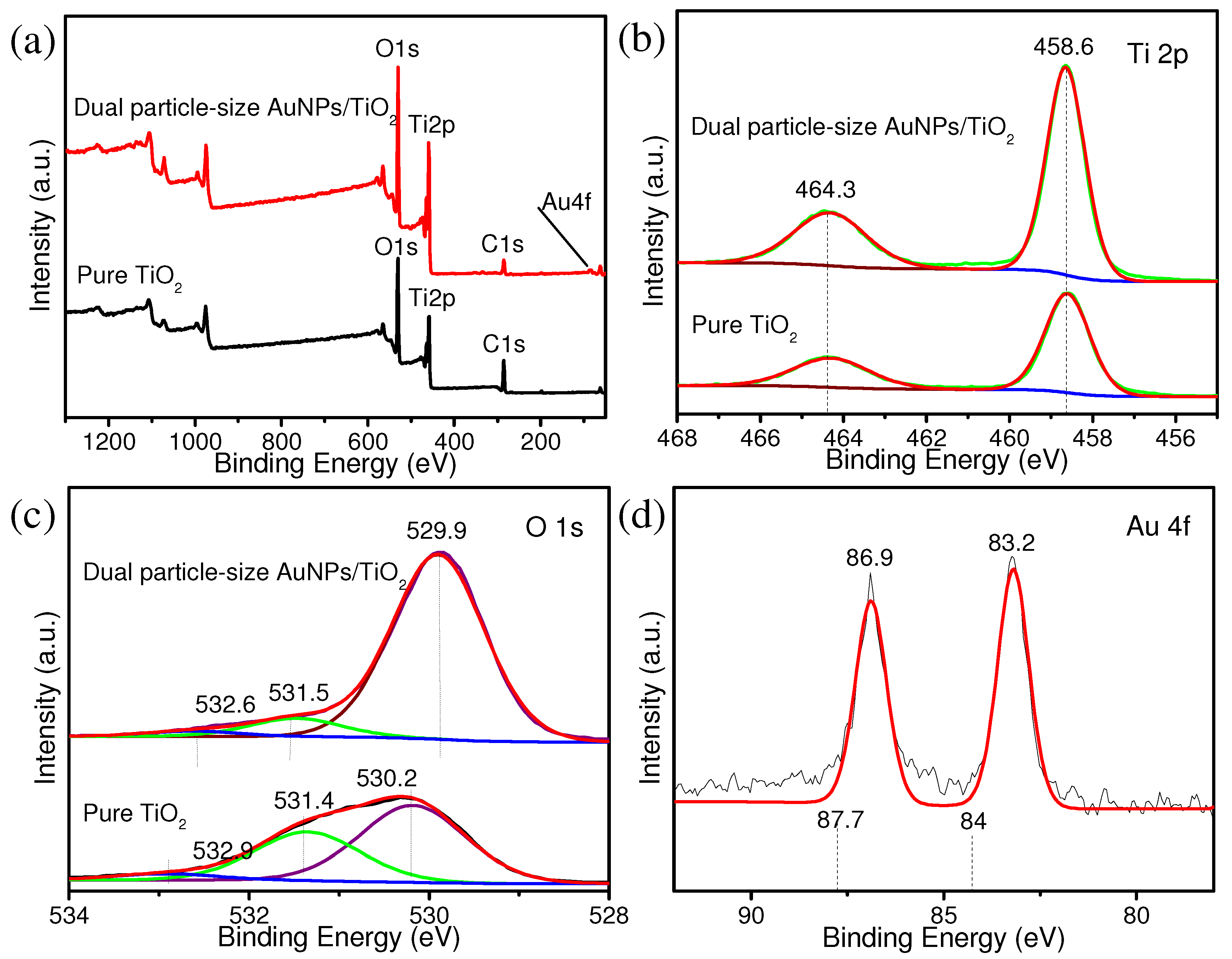
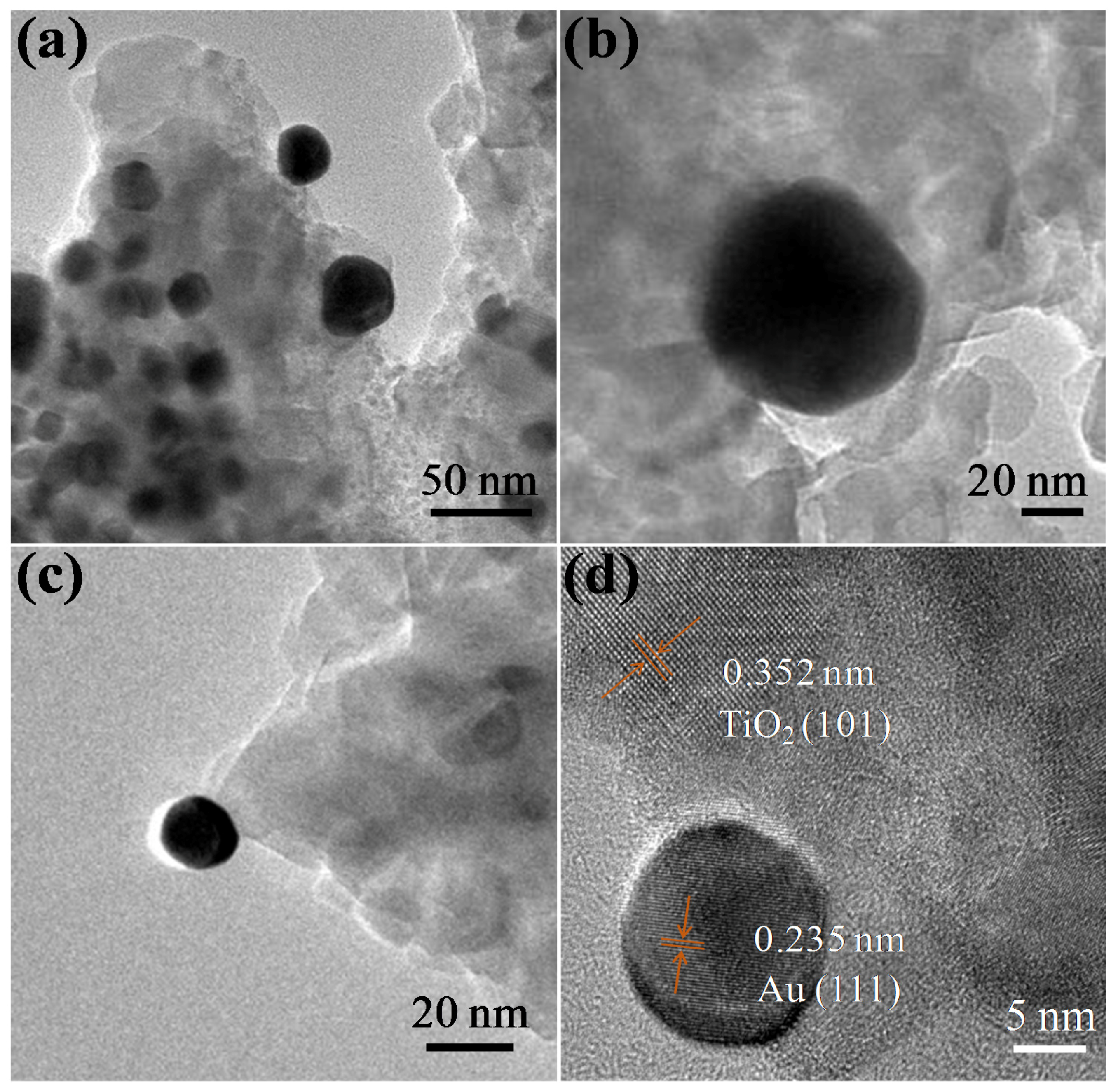
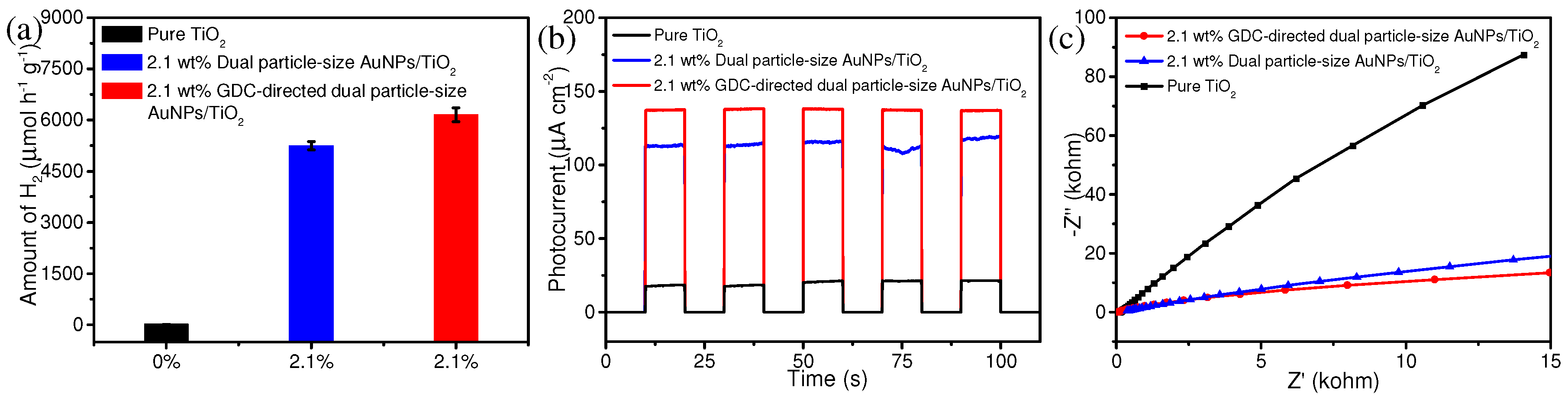
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions. Adv. Mater. 2015, 28, 1917–1933. [Google Scholar] [CrossRef]
- Cui, G.; Wang, W.; Ma, M.; Xie, J.; Shi, X.; Deng, N.; Xin, J.; Tang, B. IR-driven photocatalytic water splitting with WO2-NaxWO3 hybrid conductor material. Nano Lett. 2015, 15, 7199–7203. [Google Scholar] [CrossRef]
- Yu, J.; Ma, T.; Liu, S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef]
- Irfan, R.M.; Jiang, D.; Sun, Z.; Zhang, L.; Cui, S.; Du, P. Incorporating a molecular co-catalyst with a heterogeneous semiconductor heterojunction photocatalyst: Novel mechanism with two electron-transfer pathways for enhanced solar hydrogen production. J. Catal. 2017, 353, 274–285. [Google Scholar] [CrossRef]
- Cerro-Prada, E.; García-Salgado, S.; Quijano, M.Á.; Varela, F. Controlled synthesis and microstructural properties of sol-gel TiO2 nanoparticles for photocatalytic cement composites. Nanomaterials 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Whangboc, M.H. Facile in situ synthesis of visible-light plasmonic photocatalysts M@TiO2 (M = Au, Pt, Ag) and evaluation of their photocatalytic oxidation of benzene to phenol. J. Mater. Chem. 2011, 21, 9079–9087. [Google Scholar] [CrossRef]
- Kamat, P.V. Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Iwase, A.; Kato, H.; Kudo, A. The effect of Au cocatalyst loaded on La-doped NaTaO3 on photocatalytic water splitting and O2 photoreduction. Appl. Catal. B Environ. 2013, 136–137, 89–93. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Radzig, M.; Koksharova, O.; Khmel, I.; Ivanov, V.; Yorov, K.; Kiwi, J.; Rtimi, S.; Tastekova, E.; Aybush, A.; Nadtochenko, V. Femtosecond spectroscopy of Au hot-electron injection into TiO2: Evidence for Au/TiO2 plasmon photocatalysis by bactericidal Au ions and related phenomena. Nanomaterials 2019, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Liu, L.; Ouyang, S.; Ye, J. Gold-nanorod-photosensitized titanium dioxide with wide-range visible-light harvesting based on localized surface plasmon resonance. Angew. Chem. Int. Ed. 2013, 125, 6821–6825. [Google Scholar] [CrossRef]
- Costi, R.; Saunders, A.E.; Elmalem, E.; Salant, A.; Banin, U. Visible light-induced charge retention and photocatalysis with hybrid CdSe-Au nanodumbbells. Nano Lett. 2008, 8, 637–641. [Google Scholar] [CrossRef]
- Murdoch, M.; Waterhouse, G.I.N.; Nadeem, M.A.; Metson, J.B.; Keane, M.A.; Howe, R.F.; Llorca, J.; Idriss, H. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat. Chem. 2011, 3, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, S.; Yin, S.; Xue, H. Over two-orders of magnitude enhancement of the photocatalytic hydrogen evolution activity of carbon nitride via mediator-free decoration with gold-organic microspheres. Chem. Commun. 2017, 53, 11814–11817. [Google Scholar] [CrossRef]
- Marchal, C.; Cottineau, T.; Méndez-Medrano, M.G.; Colbeau-Justin, C.; Caps, V.; Keller, V. Au/TiO2-gC3N4 nanocomposites for enhanced photocatalytic H2 production from water under visible light irradiation with very low quantities of sacrificial agents. Adv. Energy Mater. 2018, 8, 1702142. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Silva, C.G.; Juárez, R.; Marino, T.; Molinari, R.; García, H. Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J. Am. Chem. Soc. 2010, 133, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lv, P.; Li, H.; Wang, F.; Su, W.; Zhang, L. One-step synthesis of Au-Ag alloy nanoparticles using soluble starch and their photocatalytic performance for 4-nitrophenol degradation. J. Mater. Sci. 2018, 53, 15895–15906. [Google Scholar] [CrossRef]
- Wang, H.; You, T.; Shi, W.; Li, J.; Guo, L. Au/TiO2/Au as a plasmonic coupling photocatalyst. J. Phys. Chem. C 2012, 116, 6490–6494. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, C.; Liu, J.; Yu, M.; Zheng, J. One-Step interfacial synthesis and assembly of ultrathin luminescent AuNPs/Silica membranes. Adv. Mater. 2012, 24, 3218–3222. [Google Scholar] [CrossRef]
- Sun, X.; Dong, S.; Wang, E. Large-scale synthesis of micrometer-scale single-crystalline Au plates of nanometer thickness by a wet-chemical route. Angew. Chem. Int. Ed. 2004, 116, 6520–6523. [Google Scholar] [CrossRef]
- Zhuang, Z.; Sheng, W.; Yan, Y. Synthesis of monodispere Au@Co3O4 core-shell nanocrystals and their enhanced catalytic activity for oxygen evolution reaction. Adv. Mater. 2014, 26, 3950–3955. [Google Scholar] [CrossRef]
- Manga, K.K.; Zhou, Y.; Yan, Y.; Loh, K.P. Multilayer hybrid films consisting of alternating graphene and titania nanosheets with ultrafast electron transfer and photoconversion properties. Adv. Funct. Mater. 2009, 19, 3638–3643. [Google Scholar] [CrossRef]
- Oros-Ruiz, S.; Zanella, R.; López, R.; Hernández-Gordillo, A.; Gómez, R. Photocatalytic hydrogen production by water/methanol decomposition using Au/TiO2 prepared by deposition-precipitation with urea. J. Hazard. Mater. 2013, 263, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Bumajdad, A.; Madkour, M.; Abdel-Moneam, Y.; El-Kemary, M. Nanostructured mesoporous Au/TiO2 for photocatalytic degradation of a textile dye: The effect of size similarity of the deposited Au with that of TiO2 pores. J. Mater. Sci. 2014, 49, 1743–1754. [Google Scholar] [CrossRef]
- Chen, R.; Lu, J.; Liu, S.; Zheng, M.; Wang, Z. The preparation of Cu2O@Au yolk/shell structures for efficient photocatalytic activity with a self-generated acid etching method. J. Mater. Sci. 2018, 53, 1781–1790. [Google Scholar] [CrossRef]
- Torimoto, T.; Horibe, H.; Kameyama, T.; Okazaki, K.; Ikeda, S.; Matsumura, M.; Ishikawa, A.; Ishihara, H. Plasmon-enhanced photocatalytic activity of cadmium sulfide nanoparticle immobilized on silica-coated gold particles. J. Phys. Chem. Lett. 2011, 2, 2057–2062. [Google Scholar] [CrossRef]
- Chen, W.; Chang, H.; Lu, J.; Huang, Y.; Harroun, S.G.; Tseng, Y.; Li, Y.; Huang, C.; Chang, H. Self-assembly of antimicrobial peptides on gold nanodots: Against multidrug-resistant bacteria and wound-healing application. Adv. Funct. Mater. 2015, 25, 7189–7199. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.; Kang, X.; Liu, G.; Cheng, H. An amorphous carbon nitride photocatalyst with greatly extended visible-light-responsive range for photocatalytic hydrogen generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Jovic, V.; Chen, W.; Sun-Waterhouse, D.; Blackford, M.G.; Idriss, H.; Waterhouse, G.I.N. Effect of gold loading and TiO2 support composition on the activity of Au/TiO2 photocatalysts for H2 production from ethanol-water mixtures. J. Catal. 2013, 305, 307–317. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, J.; Wang, H.; Lia, X.; Xu, J.; Wu, J.; Xiao, G.; Xiao, F.; Liu, X. Ligand-triggered electrostatic self-assembly of CdS nanosheet/Au nanocrystal nanocomposites for versatile photocatalytic redox applications. Nanoscale 2016, 8, 19161–19173. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Miao, J.; Liu, B. Layer-by-layer self-assembly of CdS quantum dots/graphene nanosheets hybrid films for photoelectrochemical and photocatalytic applications. J. Am. Chem. Soc. 2014, 136, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Wang, N.; Xu, H.; Wang, W.; Liu, Y.; Kuo, L.; Yadav, T.P.; Wu, J.; Joyner, J.; Song, Y.; et al. Gold nanoparticles and g-C3N4-intercalated graphene oxide membrane for recyclable surface enhanced raman scattering. Adv. Funct. Mater. 2017, 27, 1701714. [Google Scholar] [CrossRef]
- Rather, R.A.; Singh, S.; Pal, B. Visible and direct sunlight induced H2 production from water by plasmonic Ag-TiO2 nanorods hybrid interface. Sol. Energy Mater. Sol. C 2017, 160, 463–469. [Google Scholar] [CrossRef]
- Tamiolakis, I.; Fountoulaki, S.; Vordos, N.; Lykakisb, I.N.; Armatas, G.S. Mesoporous Au-TiO2 nanoparticle assemblies as efficient catalysts for the chemoselective reduction of nitro compounds. J. Mater. Chem. A 2013, 1, 14311–14319. [Google Scholar] [CrossRef]
- Kruse, N.; Chenakin, S. XPS characterization of Au/TiO2 catalysts: Binding energy assessment and irradiation effects. Appl. Catal. A Gen. 2011, 391, 367–376. [Google Scholar] [CrossRef]
- Rogers, C.; Perkins, W.S.; Veber, G.; Williams, T.E.; Cloke, R.R.; Fischer, F.R. Synergistic enhancement of electrocatalytic CO2 reduction with gold nanoparticles embedded in functional graphene nanoribbon composite electrodes. J. Am. Chem. Soc. 2017, 139, 4052–4061. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, X.; Cao, J.; Wu, S.; Yan, W.; Liu, G. Plasmonic Au nanoparticles embedding enhances the activity and stability of CdS for photocatalytic hydrogen evolution. Chem. Commun. 2016, 52, 2394–2397. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Pal, A.; Chatterjee, K.; Rana, T.; Bhattacharya, G.; Roy, S.; Chowdhury, P.; Sharma, G.; Biswas, S. Fabrication of efficient dye-sensitized solar cells with photoanode containing TiO2-Au and TiO2-Ag plasmonic nanocomposites. J. Mater. Sci. 2018, 29, 18209–18220. [Google Scholar] [CrossRef]
- Kumar, D.P.; Reddy, N.L.; Karthik, M.; Neppolian, B.; Madhavan, J.; Shankar, M.V. Solar light sensitized p-Ag2O/n-TiO2 nanotubes heterojunction photocatalysts for enhanced hydrogen production in aqueous-glycerol solution. Sol. Energy Mater. Sol. C 2016, 154, 78–87. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Zhang, Q.; Du, C.; Sun, S.; Steinkruger, J.D.; Zhou, C.; Yang, S. Synergistic Effect of Dual Particle-Size AuNPs on TiO2 for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials 2019, 9, 499. https://doi.org/10.3390/nano9040499
Zhao Q, Zhang Q, Du C, Sun S, Steinkruger JD, Zhou C, Yang S. Synergistic Effect of Dual Particle-Size AuNPs on TiO2 for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials. 2019; 9(4):499. https://doi.org/10.3390/nano9040499
Chicago/Turabian StyleZhao, Qian, Qiaoli Zhang, Cui Du, Shasha Sun, Jay D. Steinkruger, Chen Zhou, and Shengyang Yang. 2019. "Synergistic Effect of Dual Particle-Size AuNPs on TiO2 for Efficient Photocatalytic Hydrogen Evolution" Nanomaterials 9, no. 4: 499. https://doi.org/10.3390/nano9040499
APA StyleZhao, Q., Zhang, Q., Du, C., Sun, S., Steinkruger, J. D., Zhou, C., & Yang, S. (2019). Synergistic Effect of Dual Particle-Size AuNPs on TiO2 for Efficient Photocatalytic Hydrogen Evolution. Nanomaterials, 9(4), 499. https://doi.org/10.3390/nano9040499





