Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Functionalization of CeO2 Nanoparticles with NiO, Fe2O3, Co3O4, and PdO
2.2.2. Characterization of the Nanoparticles
2.2.3. Equilibrium Adsorption Isotherms
2.2.4. Thermogravimetric Analysis of Asphaltenes
3. Modeling
3.1. Simplex-Centroid Mixture Design
3.2. Solid-Liquid Equilibrium (SLE) Model
3.3. Estimation of Activation Energy and Reaction Kinetic Rate
4. Results and Discussion
4.1. Nanoparticle Characterization
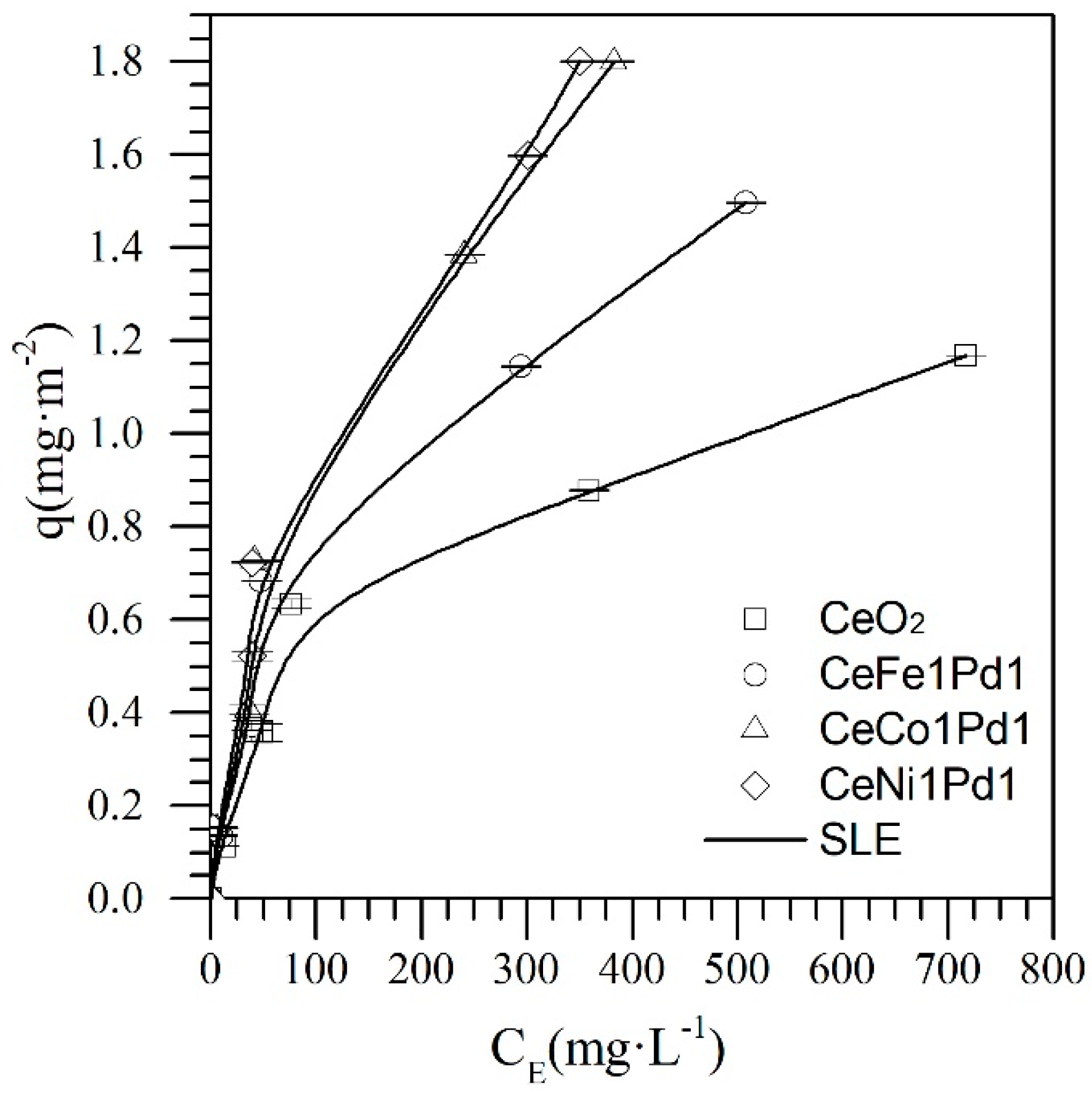
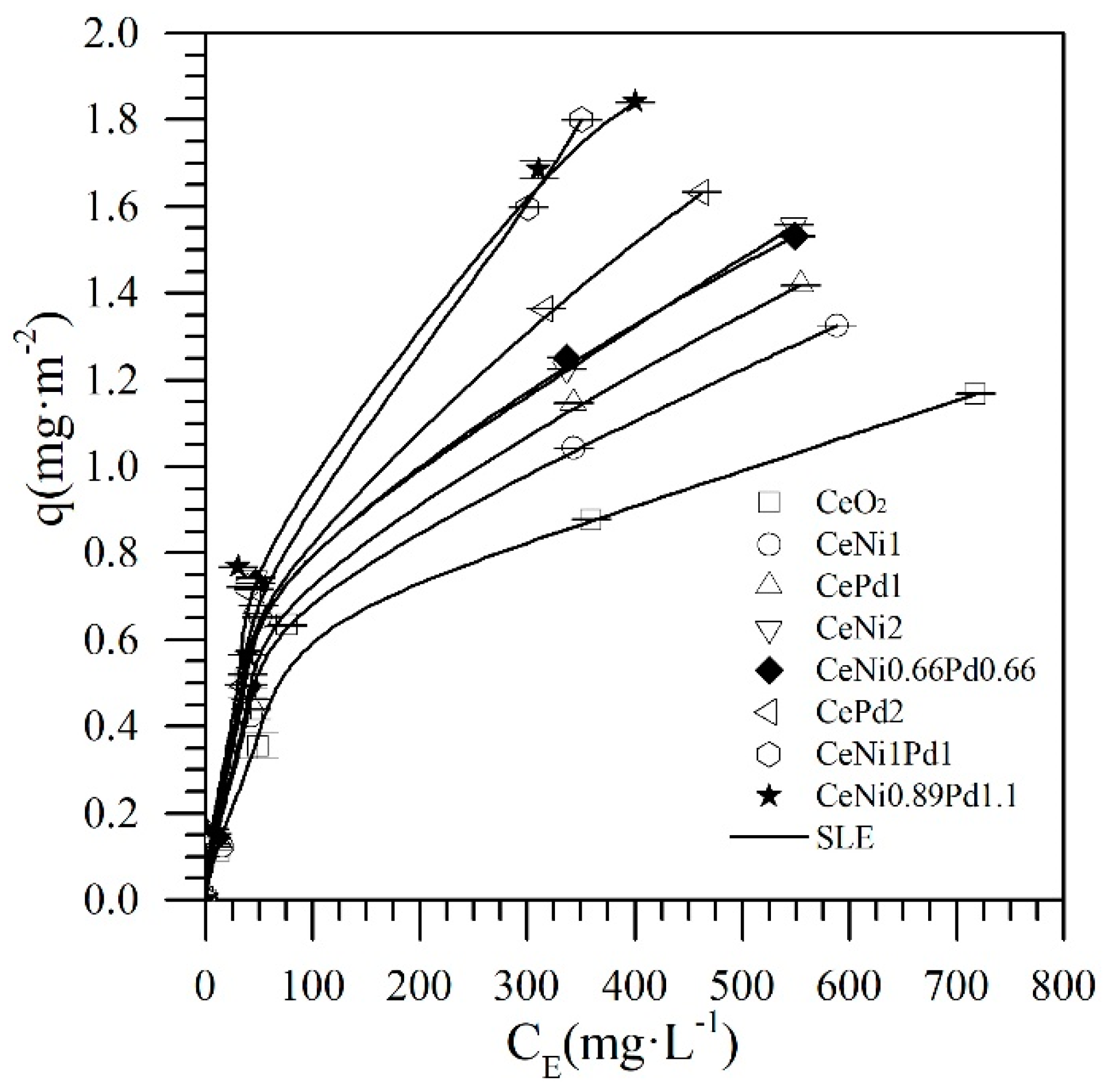
4.2. Asphaltene Adsorption onto Nanoparticles
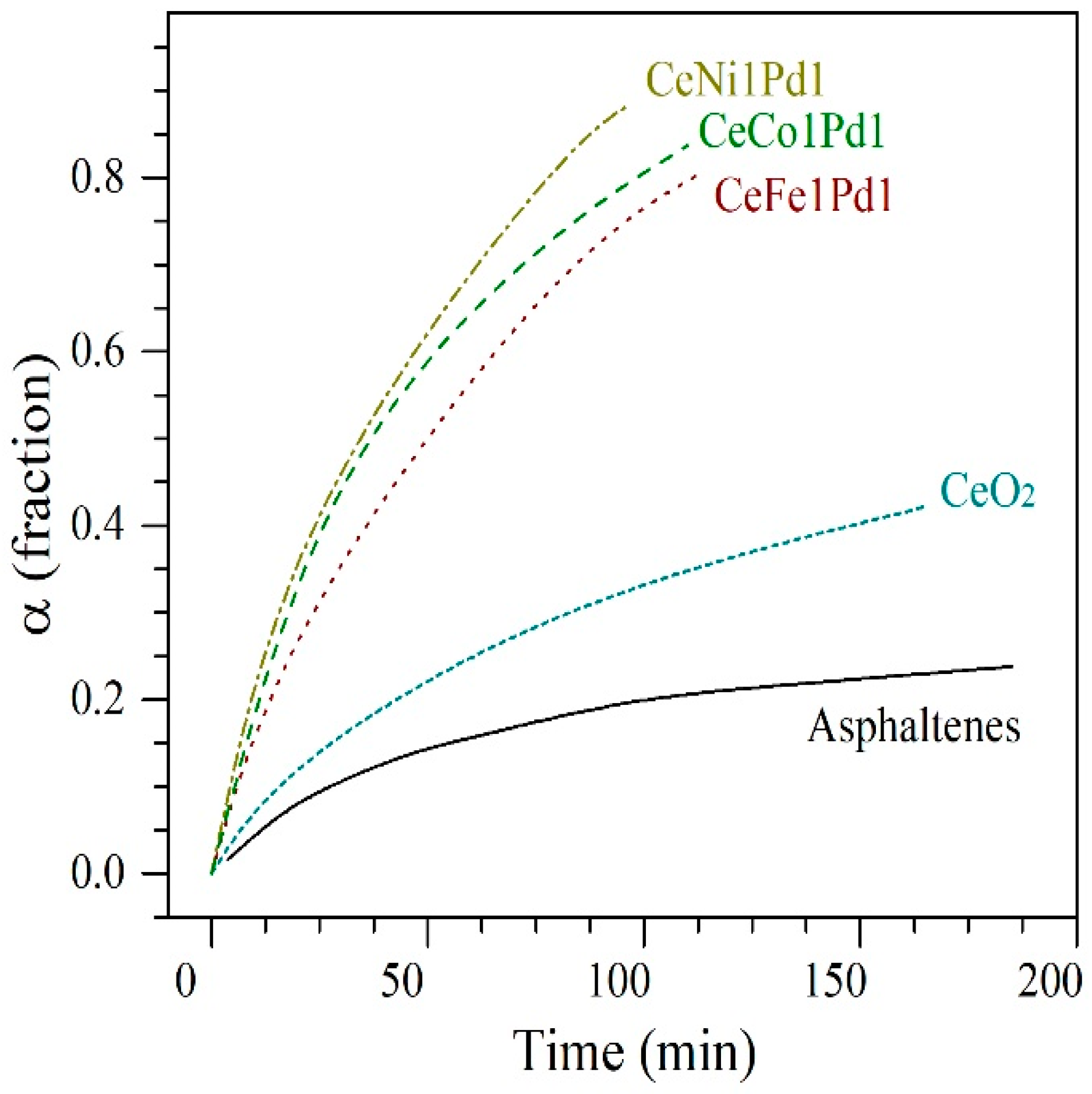
4.3. Catalytic Steam Gasification of n-C7 Asphaltenes
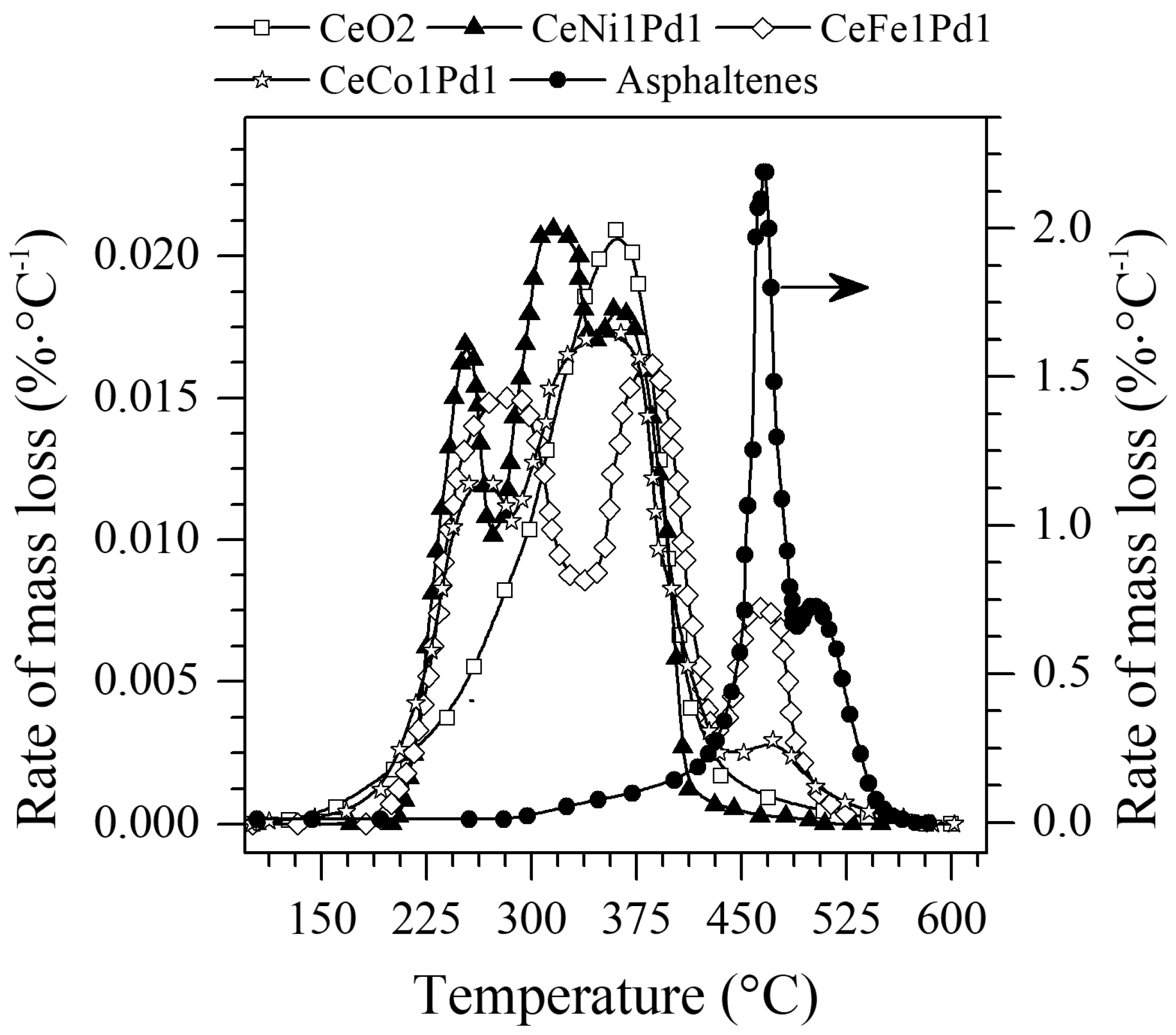
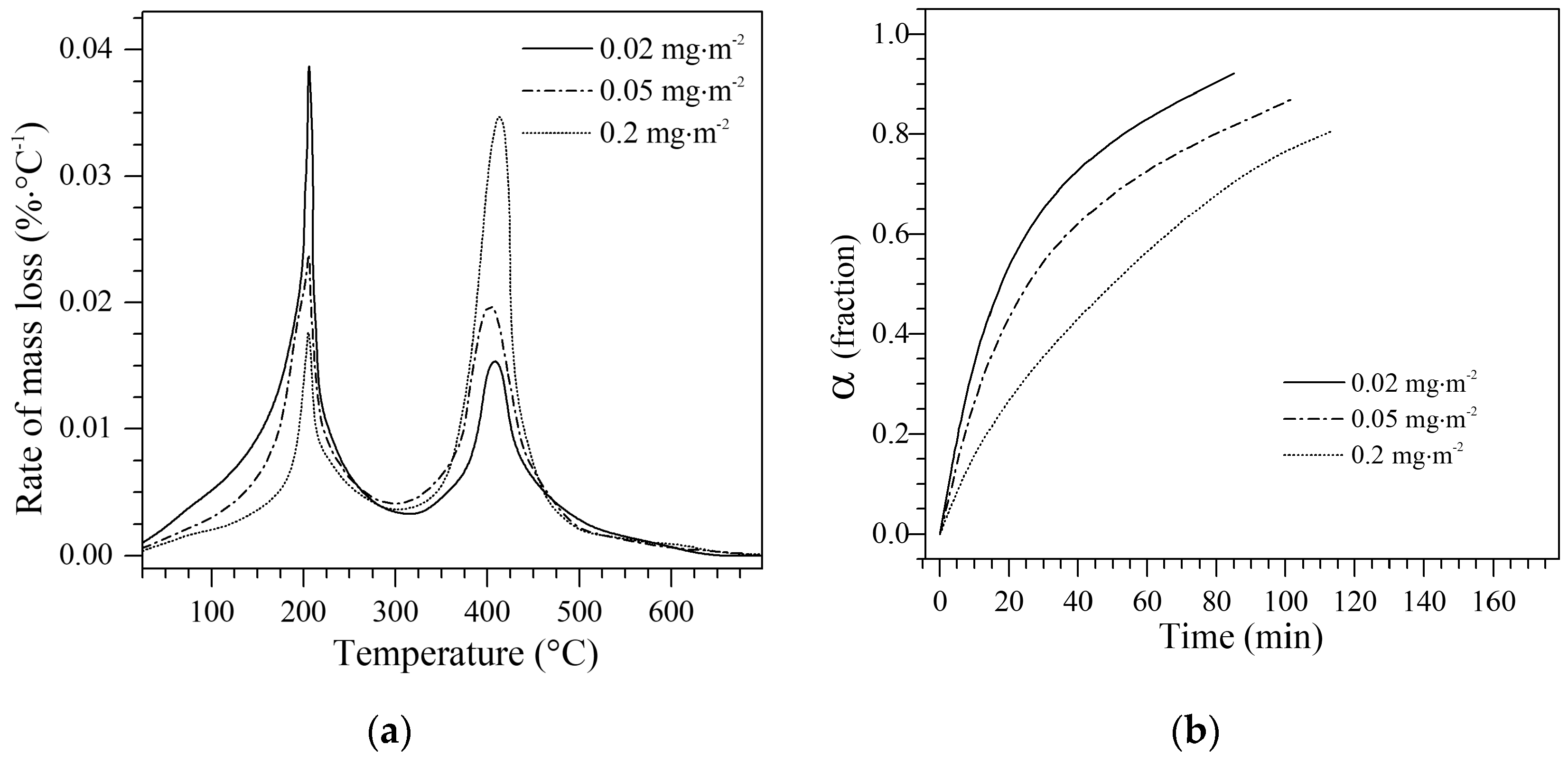
4.3.1. Mass Loss Analysis
4.3.2. Analysis of the Gaseous Products Evolved during the Steam Gasification Process
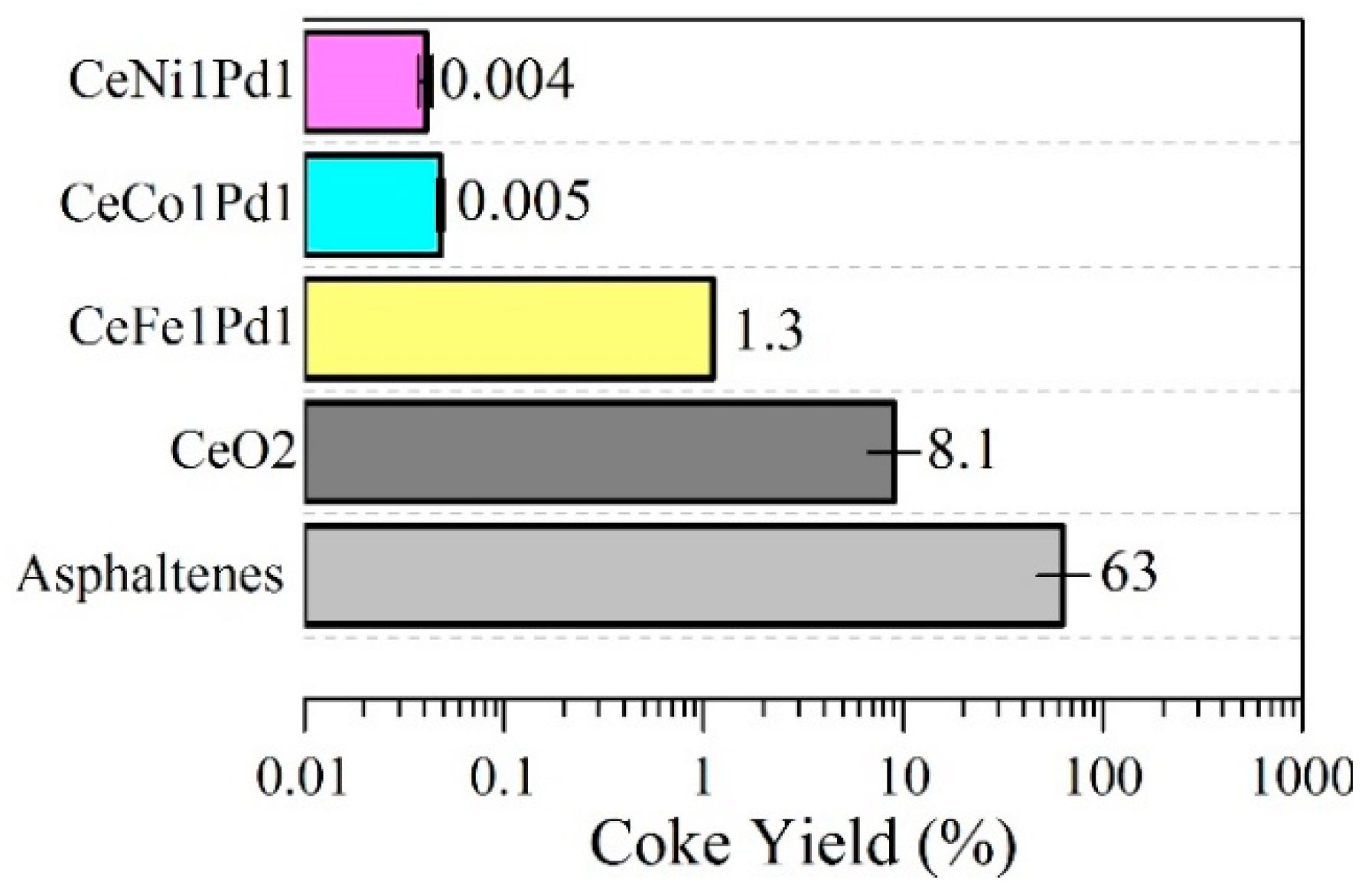
4.3.3. Coke Yield
4.4. Effect of n-C7 Asphaltene Amount Adsorbed on the Decomposition Temperature
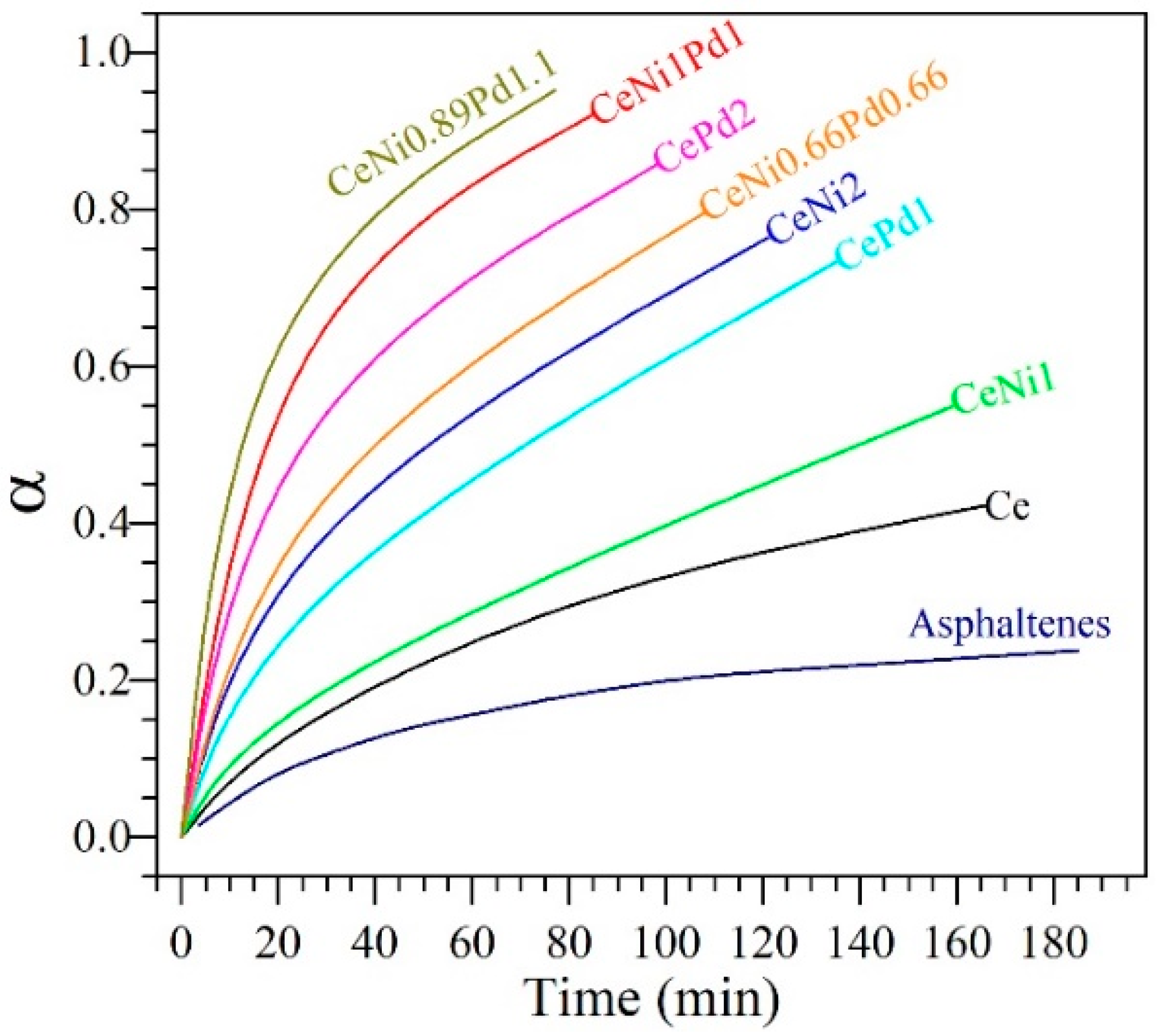
4.5. Maximization of Conversion of Asphaltenes during Steam Gasification through an SCMD
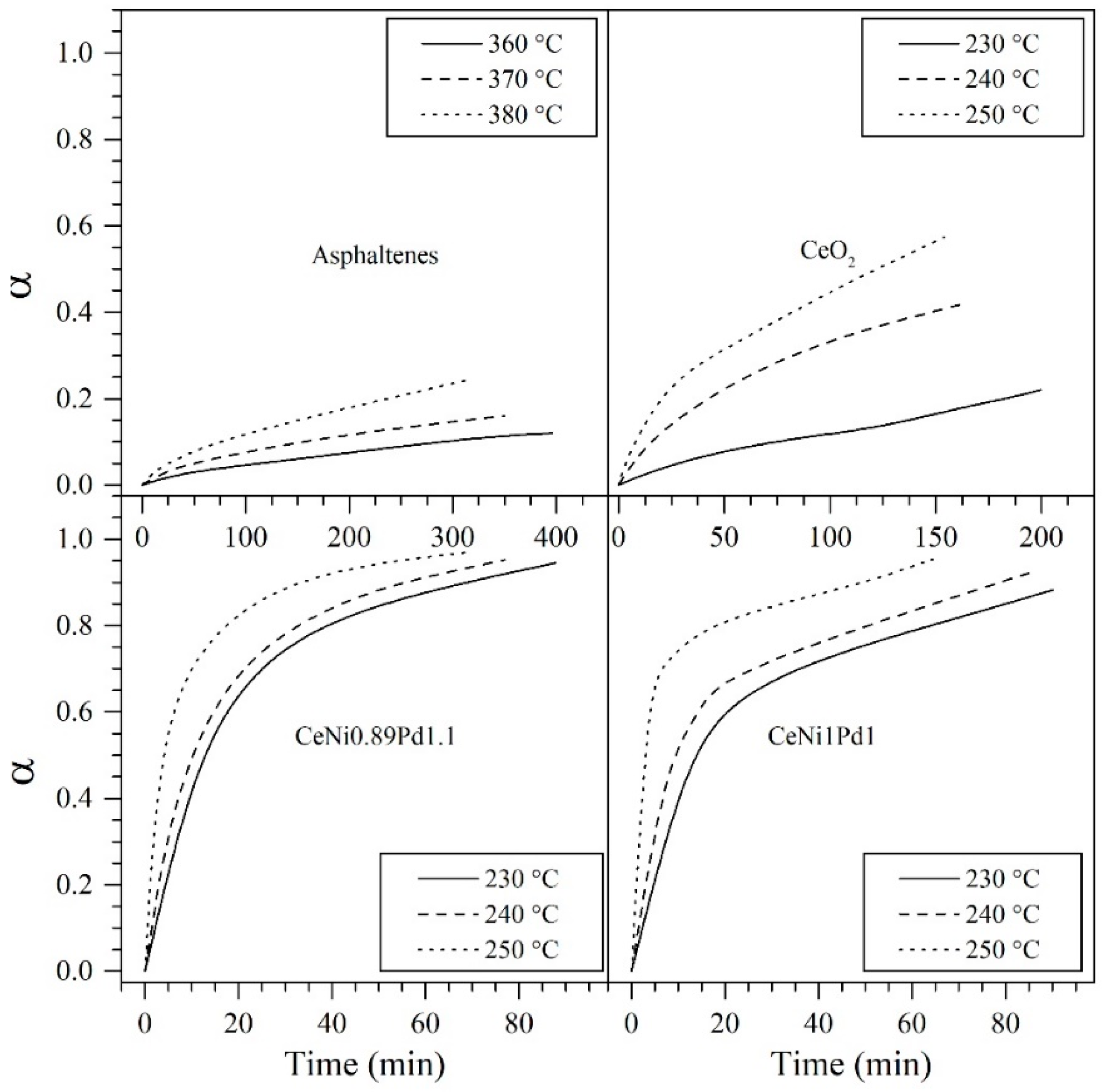
4.5.1. Isothermal Conversion of Asphaltenes Adsorbed on Nanoparticles from SCMD
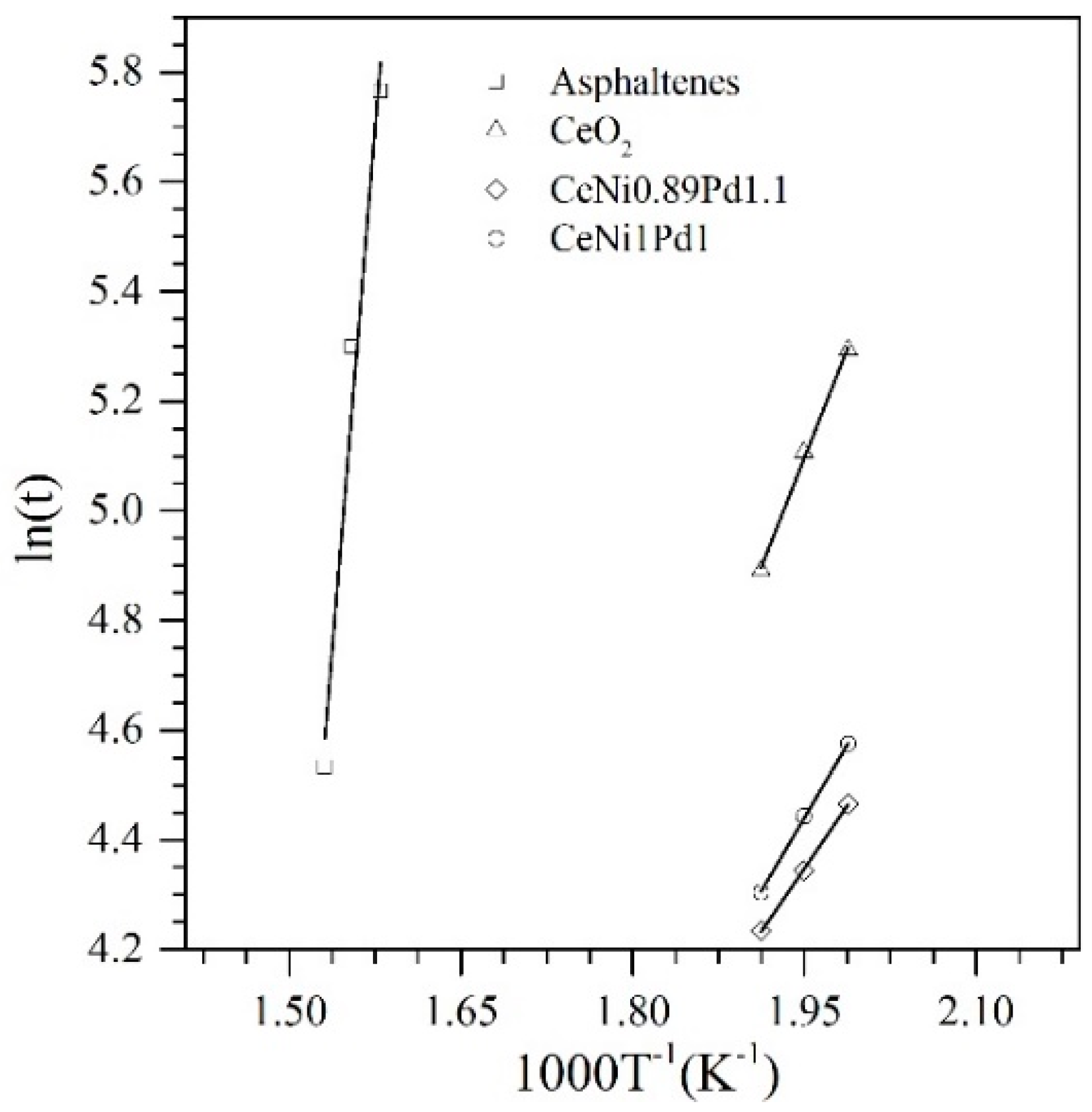
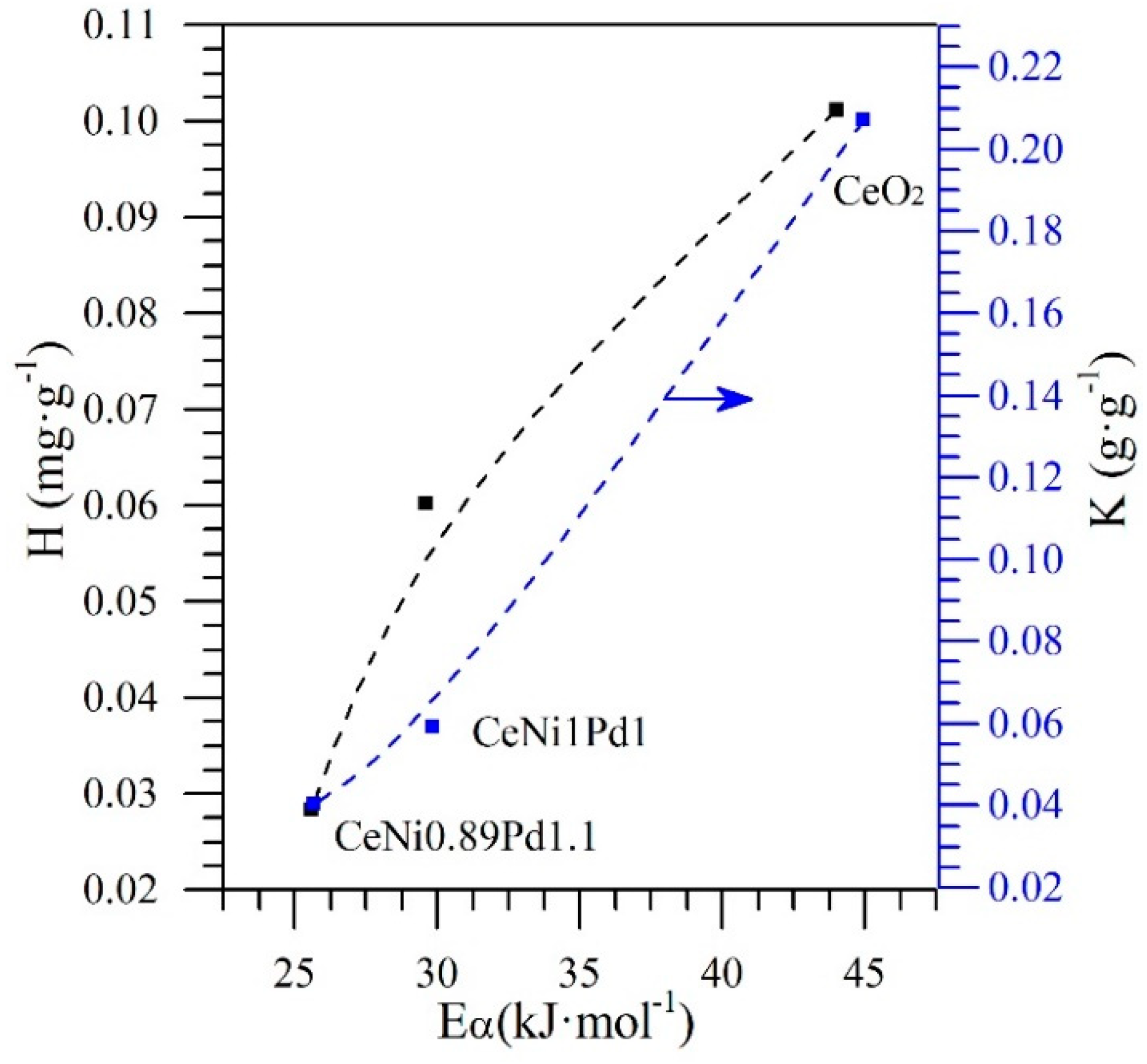
4.5.2. Effective Activation Energy and Kinetics of the Catalytic Steam Gasification of Asphaltenes in the Presence and Absence of Nanoparticles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gill, S.; Tsolakis, A.; Dearn, K.; Rodríguez-Fernández, J. Combustion characteristics and emissions of Fischer–Tropsch diesel fuels in IC engines. Prog. Energy Combust. Sci. 2011, 37, 503–523. [Google Scholar] [CrossRef]
- Mitra-Kirtley, S.; Mullins, O.C. Sulfur chemical moieties in carbonaceous materials. In Asphaltenes, Heavy Oils, and Petroleomics; Springer: Berlin/Heidelberg, Germany, 2007; pp. 157–188. [Google Scholar]
- Lv, Y.; Tang, D.; Xu, H.; Luo, H. Production characteristics and the key factors in high-rank coalbed methane fields: A case study on the Fanzhuang Block, Southern Qinshui Basin, China. Int. J. Coal Geol. 2012, 96, 93–108. [Google Scholar] [CrossRef]
- Gray, M.R.; McCaffrey, W.C. Role of chain reactions and olefin formation in cracking, hydroconversion, and coking of petroleum and bitumen fractions. Energy Fuels 2002, 16, 756–766. [Google Scholar] [CrossRef]
- Groenzin, H.; Mullins, O.C. Molecular size and structure of asphaltenes from various sources. Energy Fuels 2000, 14, 677–684. [Google Scholar] [CrossRef]
- Calemma, V.; Iwanski, P.; Nali, M.; Scotti, R.; Montanari, L. Structural characterization of asphaltenes of different origins. Energy Fuels 1995, 9, 225–230. [Google Scholar] [CrossRef]
- Piro, G.; Canonico, L.B.; Galbariggi, G.; Bertero, L.; Carniani, C. Asphaltene adsorption onto formation rock: An approach to asphaltene formation damage prevention. SPE Prod. Facil. 1996, 11, 156–160. [Google Scholar] [CrossRef]
- Yi, S.; Babadagli, T.; Li, H.A. Use of nickel nanoparticles for promoting aquathermolysis reaction during cyclic steam stimulation. SPE J. 2018, 23, 145–156. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. In situ upgrading of Athabasca bitumen using multimetallic ultradispersed nanocatalysts in an oil sands packed-bed column: Part 1. Produced liquid quality enhancement. Energy Fuels 2013, 28, 1338–1350. [Google Scholar] [CrossRef]
- Sarathi, P.S.; Olsen, D.K. Practical Aspects of Steam Injection Processes: A Handbook for Independent Operators; National Inst. for Petroleum and Energy Research: Bartlesville, OK, USA, 1992. [Google Scholar]
- Barillas, J.; Júnior, T.D.; Mata, W. Improved oil recovery process for heavy oil: A review. Braz. J. Petrol. Gás 2008, 2, 45–54. [Google Scholar]
- Benavides Nieves, L.D.; Pinilla Najar, L.A. Evaluación de la Viabilidad Técnica de la Inyección de vapor en Yacimientos de Crudo pesado, Mediante un Modelo Analítico; Fundación Universidad de América: Bogota, Colombia, 2017. [Google Scholar]
- Nassar, N.N.; Franco, C.A.; Montoya, T.; Cortés, F.B.; Hassan, A. Effect of oxide support on Ni–Pd bimetallic nanocatalysts for steam gasification of n-C7 asphaltenes. Fuel 2015, 156, 110–120. [Google Scholar] [CrossRef]
- Franco, C.; Cardona, L.; Lopera, S.; Mejía, J.; Cortés, F. Heavy oil upgrading and enhanced recovery in a continuous steam injection process assisted by nanoparticulated catalysts. In Proceedings of the SPE Improved oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Franco, C.A. Synthesis and Application of Supported Metallic and Multi-Metallic Oxides Nanoparticles for In-Situ Upgrading and Inhibition of Formation Damage. Ph.D. Thesis, Universidad Nacional de Colombia-Sede Medellín, Medellín, Colombia, 2015. [Google Scholar]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Franco, C.A.; Zabala, R.; Cortés, F.B. Nanotechnology applied to the enhancement of oil and gas productivity and recovery of Colombian fields. J. Petrol. Sci. Eng. 2017, 157, 39–55. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- Betancur, S.; Carmona, J.C.; Nassar, N.N.; Franco, C.A.; Cortés, F.B. Role of particle size and surface acidity of silica gel nanoparticles in inhibition of formation damage by asphaltene in oil reservoirs. Ind. Eng. Chem. Res. 2016, 55, 6122–6132. [Google Scholar] [CrossRef]
- Cortés, F.B.; Montoya, T.; Acevedo, S.; Nassar, N.N.; Franco, C.A. Adsorption-desorption of n-c7 asphaltenes over micro-and nanoparticles of silica and its impact on wettability alteration. CT&F-Ciencia Tecnología y Futuro 2016, 6, 89–106. [Google Scholar]
- López, D.; Giraldo, L.J.; Salazar, J.P.; Zapata, D.M.; Ortega, D.C.; Franco, C.A.; Cortés, F.B. Metal Oxide Nanoparticles Supported on Macro-Mesoporous Aluminosilicates for Catalytic Steam Gasification of Heavy Oil Fractions for On-Site Upgrading. Catalysts 2017, 7, 319. [Google Scholar] [CrossRef]
- Nassar, N.N.; Betancur, S.; Acevedo, S.c.; Franco, C.A.; Cortés, F.B. Development of a population balance model to describe the influence of shear and nanoparticles on the aggregation and fragmentation of asphaltene aggregates. Ind. Eng. Chem. Res. 2015, 54, 8201–8211. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene adsorption, a literature review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. In-situ upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. SPE Reserv. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Thermogravimetric studies on catalytic effect of metal oxide nanoparticles on asphaltene pyrolysis under inert conditions. J. Therm. Anal. Calorim. 2012, 110, 1327–1332. [Google Scholar] [CrossRef]
- Amanam, U.U.; Kovscek, A.R. Analysis of the effects of copper nanoparticles on in-situ combustion of extra heavy-crude oil. J. Petrol. Sci. Eng. 2017, 152, 406–415. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Luna, G.; Pereira-Almao, P. Kinetics of the catalytic thermo-oxidation of asphaltenes at isothermal conditions on different metal oxide nanoparticle surfaces. Catal. Today 2013, 207, 127–132. [Google Scholar] [CrossRef]
- Franco, C.; Patiño, E.; Benjumea, P.; Ruiz, M.A.; Cortés, F.B. Kinetic and thermodynamic equilibrium of asphaltenes sorption onto nanoparticles of nickel oxide supported on nanoparticulated alumina. Fuel 2013, 105, 408–414. [Google Scholar] [CrossRef]
- Cortés, F.B.; Mejía, J.M.; Ruiz, M.A.; Benjumea, P.; Riffel, D.B. Sorption of asphaltenes onto nanoparticles of nickel oxide supported on nanoparticulated silica gel. Energy Fuels 2012, 26, 1725–1730. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Pereira-Almao, P.; Cortés, F.B. Adsorption and subsequent oxidation of colombian asphaltenes onto nickel and/or palladium oxide supported on fumed silica nanoparticles. Energy Fuels 2013, 27, 7336–7347. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Metal oxide nanoparticles for asphaltene adsorption and oxidation. Energy Fuels 2011, 25, 1017–1023. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira Almao, P. Enhanced heavy oil recovery by in situ prepared ultradispersed multimetallic nanoparticles: A study of hot fluid flooding for Athabasca bitumen recovery. Energy Fuels 2013, 27, 2194–2201. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Comparative oxidation of adsorbed asphaltenes onto transition metal oxide nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 145–149. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Almao, P.P. Nanoparticle technology for heavy oil in-situ upgrading and recovery enhancement: Opportunities and challenges. Appl. Energy 2014, 133, 374–387. [Google Scholar] [CrossRef]
- Nassar, N.N. Asphaltene adsorption onto alumina nanoparticles: Kinetics and thermodynamic studies. Energy Fuels 2010, 24, 4116–4122. [Google Scholar] [CrossRef]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.B.; Lopera, S.H.; Franco, C.A. Heavy Oil Upgrading and Enhanced Recovery in a Steam Injection Process Assisted by NiO-and PdO-Functionalized SiO2 Nanoparticulated Catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Cardona Rojas, L. Efecto de nanopartículas en procesos con inyección de vapor a diferentes calidades. Master’s Thesis, Universidad Nacional de Colombia-Sede Medellín, Medellín, Colombia, March 2018. [Google Scholar]
- Jacobs, G.; Ricote, S.; Graham, U.M.; Patterson, P.M.; Davis, B.H. Low temperature water gas shift: Type and loading of metal impacts forward decomposition of pseudo-stabilized formate over metal/ceria catalysts. Catal. Today 2005, 106, 259–264. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dejhosseini, M.; Aida, T.; Watanabe, M.; Takami, S.; Hojo, D.; Aoki, N.; Arita, T.; Kishita, A.; Adschiri, T. Catalytic cracking reaction of heavy oil in the presence of cerium oxide nanoparticles in supercritical water. Energy Fuels 2013, 27, 4624–4631. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Vitale, G. Comparing kinetics and mechanism of adsorption and thermo-oxidative decomposition of Athabasca asphaltenes onto TiO2, ZrO2, and CeO2 nanoparticles. Appl. Catal. A Gen. 2014, 484, 161–171. [Google Scholar] [CrossRef]
- Sánchez Gil, J.J. Síntesis y Estudios de Catalizadores Nanoestructurados de Óxido de Cerio soportado sobre Óxido de Magnesio con baja cantidad en Lantánido. Master’s Thesis, Universidad de Cádiz, Cádiz, Spanish, January 2013. [Google Scholar]
- Li, Y.; Fu, Q.; Flytzani-Stephanopoulos, M. Low-temperature water-gas shift reaction over Cu-and Ni-loaded cerium oxide catalysts. Appl. Catal. B Environ. 2000, 27, 179–191. [Google Scholar] [CrossRef]
- Bunluesin, T.; Gorte, R.; Graham, G. Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh: Implications for oxygen-storage properties. Appl. Catal. B Environ. 1998, 15, 107–114. [Google Scholar] [CrossRef]
- Meunier, F.; Reid, D.; Goguet, A.; Shekhtman, S.; Hardacre, C.; Burch, R.; Deng, W.; Flytzani-Stephanopoulos, M. Quantitative analysis of the reactivity of formate species seen by DRIFTS over a Au/Ce (La) O2 water–gas shift catalyst: First unambiguous evidence of the minority role of formates as reaction intermediates. J. Catal. 2007, 247, 277–287. [Google Scholar] [CrossRef]
- Alamolhoda, S.; Vitale, G.; Hassan, A.; Nassar, N.N.; Almao, P.P. Synergetic effects of cerium and nickel in Ce-Ni-MFI catalysts on low-temperature water-gas shift reaction. Fuel 2019, 237, 361–372. [Google Scholar] [CrossRef]
- Ancheyta, J.; Centeno, G.; Trejo, F.; Marroquin, G.; Garcia, J.; Tenorio, E.; Torres, A. Extraction and characterization of asphaltenes from different crude oils and solvents. Energy Fuels 2002, 16, 1121–1127. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Montoya, T.; Ruíz, M.A.; Cortés, F.B. Influence of asphaltene aggregation on the adsorption and catalytic behavior of nanoparticles. Energy Fuels 2015, 29, 1610–1621. [Google Scholar] [CrossRef]
- Choi, S.; Byun, D.H.; Lee, K.; Kim, J.-D.; Nho, N.S. Asphaltene precipitation with partially oxidized asphaltene from water/heavy crude oil emulsion. J. Petrol. Sci. Eng. 2016, 146, 21–29. [Google Scholar] [CrossRef]
- Lozano, M.M.; Franco, C.A.; Acevedo, S.A.; Nassar, N.N.; Cortés, F.B. Effects of resin I on the catalytic oxidation of n-C 7 asphaltenes in the presence of silica-based nanoparticles. RSC Adv. 2016, 6, 74630–74642. [Google Scholar] [CrossRef]
- Franco, C.A.; Zabala, R.D.; Zapata, J.; Mora, E.; Botero, O.; Candela, C.; Castillo, A. Inhibited gas stimulation to mitigate condensate banking and maximize recovery in cupiagua field. SPE Prod. Oper. 2013, 28, 154–167. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Z.; Feng, C.; Hu, K.; Li, M.; Li, Y.; Shimizu, K.; Chen, N.; Sugiura, N. Application of simplex-centroid mixture design in developing and optimizing ceramic adsorbent for As (V) removal from water solution. Microporous Mesoporous Mater. 2010, 131, 115–121. [Google Scholar] [CrossRef]
- Talu, O.; Meunier, F. Adsorption of associating molecules in micropores and application to water on carbon. AIChE J. 1996, 42, 809–819. [Google Scholar] [CrossRef]
- Montoya, T.; Coral, D.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. A Novel Solid–Liquid Equilibrium Model for Describing the Adsorption of Associating Asphaltene Molecules onto Solid Surfaces Based on the “Chemical Theory”. Energy Fuels 2014, 28, 4963–4975. [Google Scholar] [CrossRef]
- Cao, A.; Lu, R.; Veser, G. Stabilizing metal nanoparticles for heterogeneous catalysis. Phys. Chem. Chem. Phys. 2010, 12, 13499–13510. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.M.; Auyeung, N.; Rahmatian, N.; Klausner, J.F.; Coker, E.N. Cobalt ferrite in YSZ for use as reactive material in solar thermochemical water and carbon dioxide splitting, part II: Kinetic modeling. JOM 2013, 65, 1682–1693. [Google Scholar] [CrossRef]
- Sharma, A.; Saito, I.; Nakagawa, H.; Miura, K. Effect of carbonization temperature on the nickel crystallite size of a Ni/C catalyst for catalytic hydrothermal gasification of organic compounds. Fuel 2007, 86, 915–920. [Google Scholar] [CrossRef]
- Stone, H. Electrical conductivity and sintering in iron oxides at high temperatures. J. Mater. Sci. 1968, 3, 321–325. [Google Scholar] [CrossRef]
- Phalnikar, C.; Evans, E.; Baldwin, W. High Temperature Scaling of Cobalt-Chromium Alloys. J. Electrochem. Soc. 1956, 103, 429–438. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of nanoporous materials from adsorption and desorption isotherms. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 11–21. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Cortés, F.B. Nioand pdo supported on fumed silica nanoparticles for adsorption and catalytic steam gasification of colombian c7asphaltenes. In Handbook on Oil Production Research; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 101–145. [Google Scholar]
- Headen, T.; Boek, E.; Jackson, G.; Totton, T.; Muller, E. Simulation of asphaltene aggregation through molecular dynamics: Insights and limitations. Energy Fuels 2017, 31, 1108–1125. [Google Scholar] [CrossRef]
- Bates, M.K.; Jia, Q.; Doan, H.; Liang, W.; Mukerjee, S. Charge-transfer effects in Ni–Fe and Ni–Fe–Co mixed-metal oxides for the alkaline oxygen evolution reaction. ACS Catal. 2015, 6, 155–161. [Google Scholar] [CrossRef]
- Shido, T.; Iwasawa, Y. Reactant-promoted reaction mechanism for water-gas shift reaction on Rh-doped CeO2. J. Catal. 1993, 141, 71–81. [Google Scholar] [CrossRef]
- Vignatti, C.I.; Avila, M.S.; Apesteguia, C.R.; Garetto, T.F. Study of the water-gas shift reaction over Pt supported on CeO2–ZrO2 mixed oxides. Catal. Today 2011, 171, 297–303. [Google Scholar] [CrossRef]
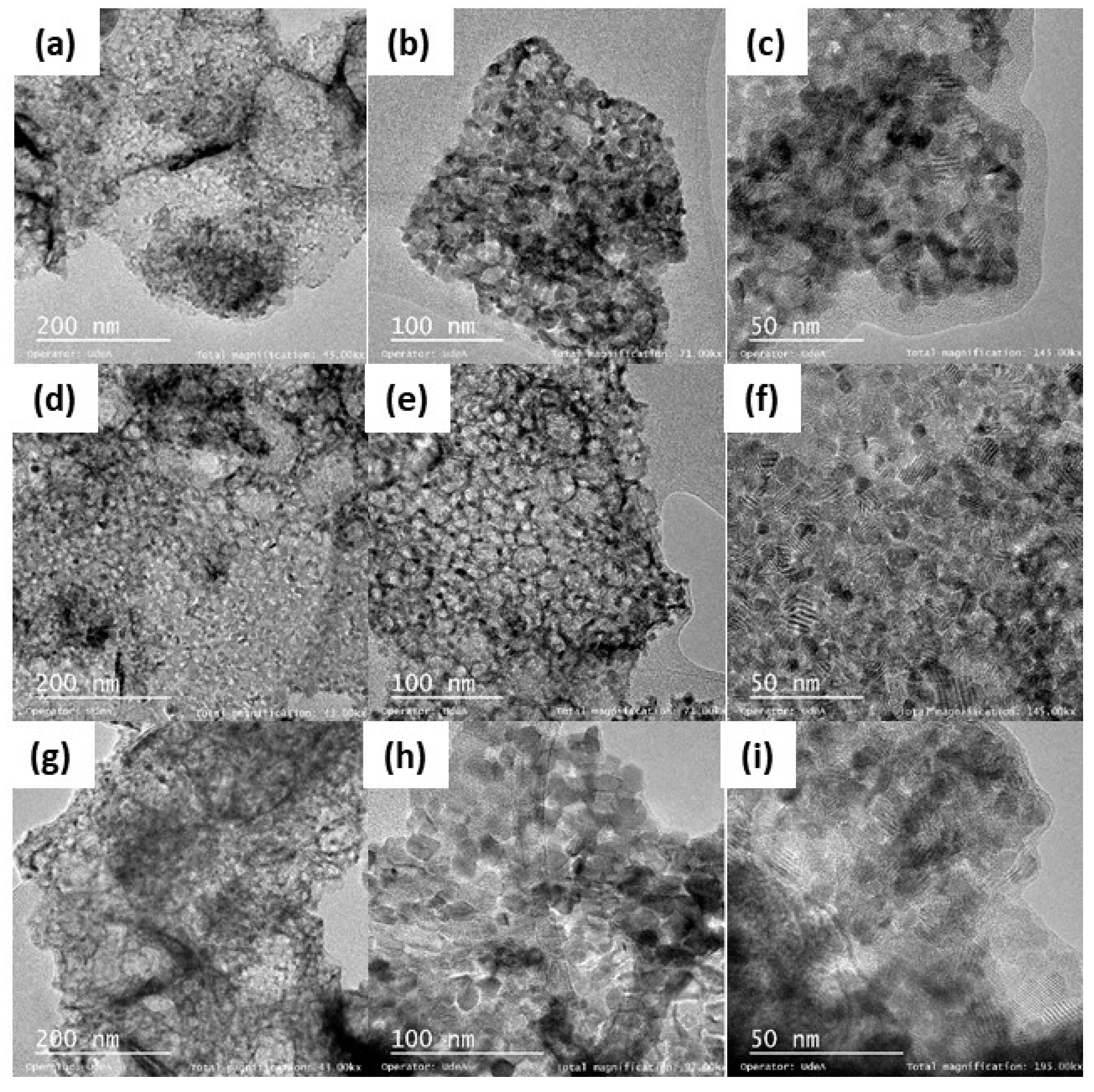

| Sample | Nominal Oxides | Nominal Mass Fraction (%) | Nominal Molar Fraction (%) |
|---|---|---|---|
| CeO2 | CeO2 | 100.0 | 100.0 |
| CeNi1Pd1 | CeO2 | 98.0 | 98.8 |
| NiO | 1.0 | 0.4 | |
| PdO | 1.0 | 0.7 | |
| CeFe1Pd1 | CeO2 | 98.0 | 98.4 |
| Fe2O3 | 1.0 | 0.9 | |
| PdO | 1.0 | 0.7 | |
| CeCo1Pd1 | CeO2 | 98.0 | 97.9 |
| Co3O4 | 1.0 | 1.4 | |
| PdO | 1.0 | 0.7 | |
| CeNi2 | CeO2 | 98.0 | 99.1 |
| NiO | 2.0 | 0.9 | |
| CePd2 | CeO2 | 98.0 | 98.6 |
| PdO | 2.0 | 1.4 | |
| CeNi1 | CeO2 | 99.0 | 99.6 |
| NiO | 1.0 | 0.4 | |
| CePd1 | CeO2 | 99.0 | 99.3 |
| PdO | 1.0 | 0.7 | |
| CeNi0.66Pd0.66 | CeO2 | 98.7 | 99.2 |
| NiO | 0.7 | 0.3 | |
| PdO | 0.7 | 0.5 |
| Sample | SBET ± 0.1 m2·g−1 | dp (nm ± 0.2 nm) | Dispersion (%) | ||||
|---|---|---|---|---|---|---|---|
| NiO | Co3O4 | Fe2O3 | PdO | Ni/Co/Fe | Pd | ||
| CeO2 | 67.0 | - | - | - | - | - | - |
| CeNi1Pd1 | 63.8 | 6.4 | - | - | 3.9 | 12.7 | 38.6 |
| CeFe1Pd1 | 64.1 | - | - | 5.4 | 6.9 | 11.2 | 12.8 |
| CeCo1Pd1 | 64.4 | - | 1.9 | - | 6.1 | 18.1 | 20.4 |
| Material | H ± 0.02 (mg·g−1) × 10−2 | K ± 0.08 (g·g−1) | qm ± 0.01 (g·g−1) | RSM (%) |
|---|---|---|---|---|
| CeO2 | 10.12 | 0.21 | 0.13 | 0.02 |
| CePd1 | 7.19 | 0.29 | 0.16 | 0.03 |
| CeNi1 | 7.34 | 0.03 | 0.15 | 0.04 |
| CePd2 | 6.25 | 0.39 | 0.18 | 0.01 |
| CeNi2 | 6.70 | 0.04 | 0.16 | 0.01 |
| CeNi0.66Pd0.66 | 6.61 | 0.05 | 0.16 | 0.02 |
| CeNi1Pd1 | 6.02 | 0.06 | 0.22 | 0.01 |
| CeFe1Pd1 | 6.58 | 0.04 | 0.17 | 0.02 |
| CeCo1Pd | 5.34 | 0.06 | 0.19 | 0.01 |
| Ce Ni0.89Pd1.1 | 2.84 | 0.04 | 0.25 | 0.01 |
| Sample | Temperature °C | (kJ) | Kinetic Rate (min−1) at 50% Conversion |
|---|---|---|---|
| n-C7 asphaltenes (without nanoparticles) | 360 | 211.5 | 0.012 |
| 370 | 0.018 | ||
| 380 | 0.032 | ||
| CeO2 | 230 | 44.0 | 0.013 |
| 240 | 0.021 | ||
| 250 | 0.879 | ||
| CeNi1Pd1 | 230 | 29.6 | 0.0187 |
| 240 | 0.0401 | ||
| 250 | 0.1002 | ||
| CeNi0.89Pd1.1 | 230 | 0.029 | |
| 240 | 25.6 | 0.084 | |
| 250 | 0.179 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures. Nanomaterials 2019, 9, 401. https://doi.org/10.3390/nano9030401
Medina OE, Gallego J, Arias-Madrid D, Cortés FB, Franco CA. Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures. Nanomaterials. 2019; 9(3):401. https://doi.org/10.3390/nano9030401
Chicago/Turabian StyleMedina, Oscar E., Jaime Gallego, Daniela Arias-Madrid, Farid B. Cortés, and Camilo A. Franco. 2019. "Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures" Nanomaterials 9, no. 3: 401. https://doi.org/10.3390/nano9030401
APA StyleMedina, O. E., Gallego, J., Arias-Madrid, D., Cortés, F. B., & Franco, C. A. (2019). Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures. Nanomaterials, 9(3), 401. https://doi.org/10.3390/nano9030401








