Effect of Salinity on Silica Nanoparticle Adsorption Kinetics and Mechanisms for Fluid/Rock Interaction with Calcite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorption Experiments
2.2. Surface Forces
3. Results and Discussions
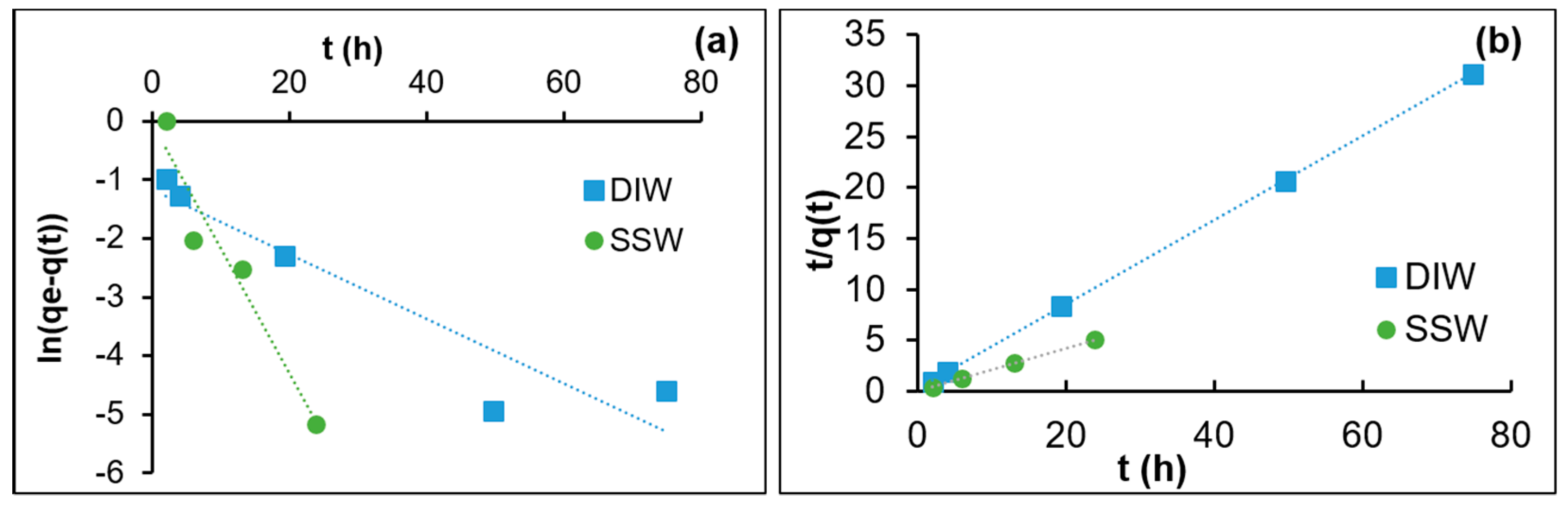
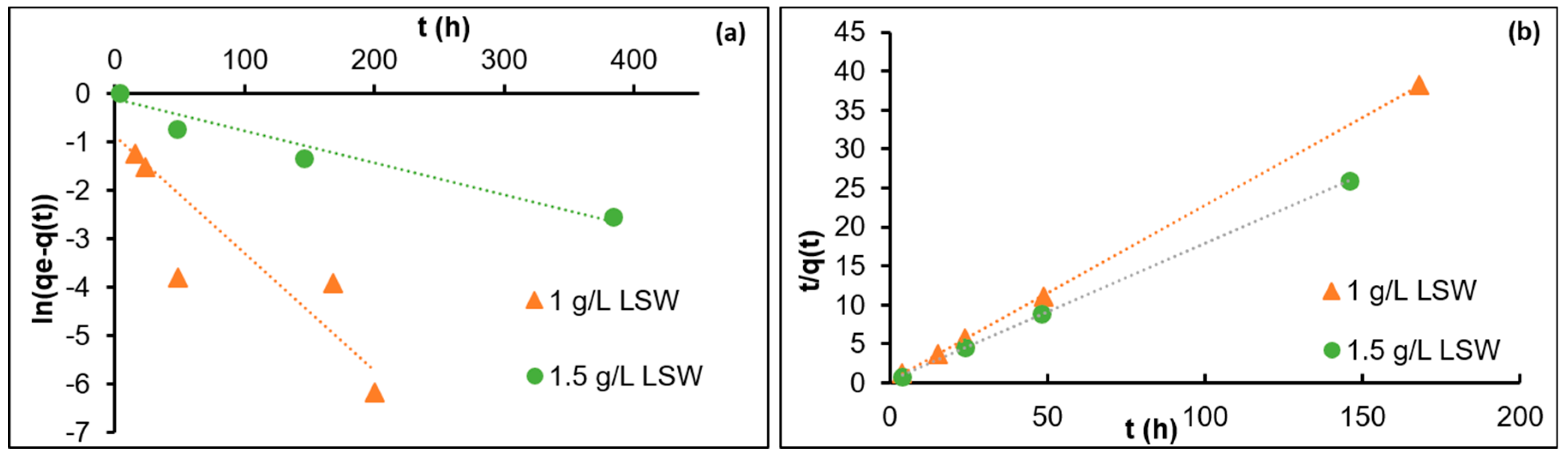
3.1. Adsorption Kinetics
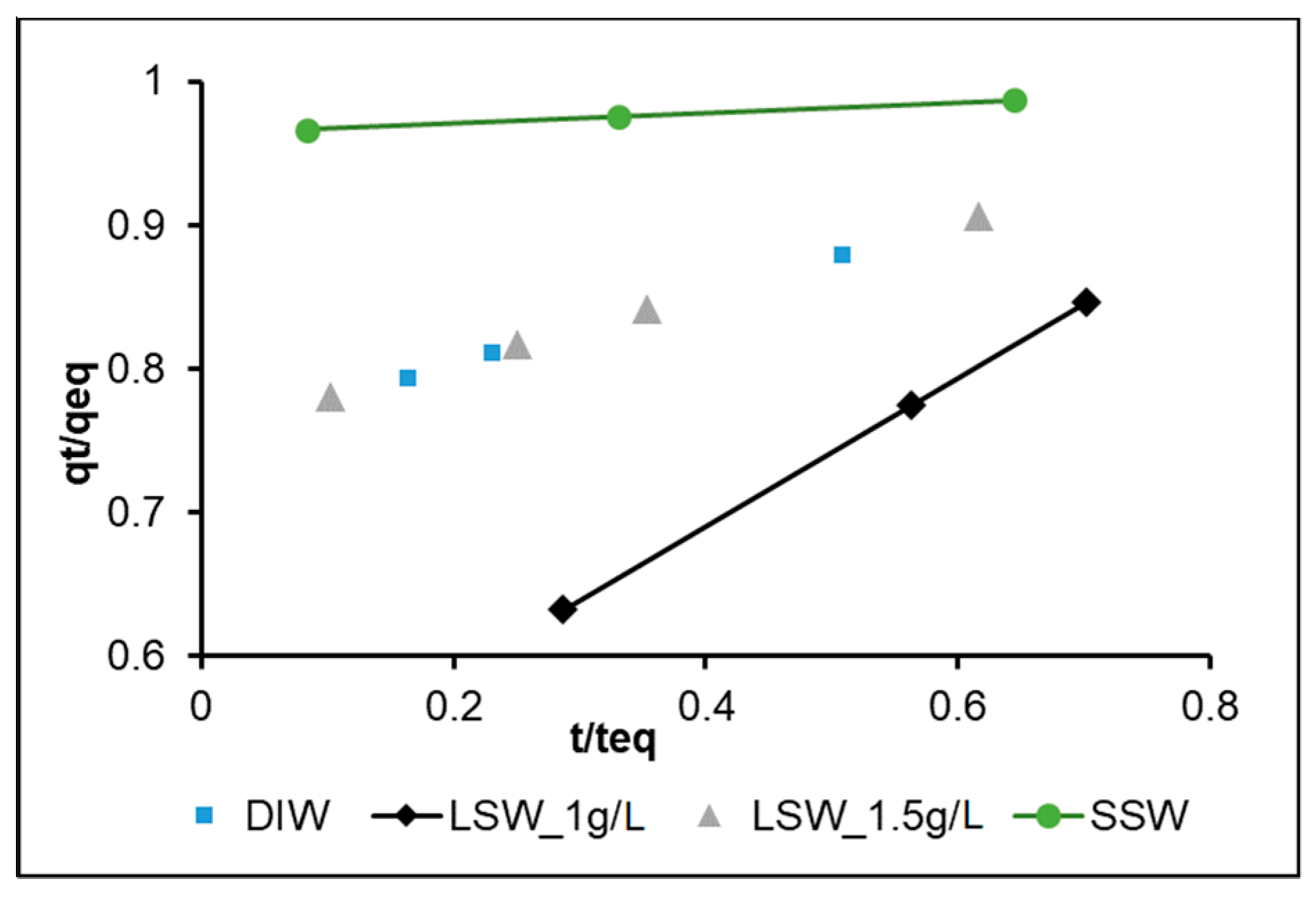
3.2. Intraparticle Diffusion Model (IPD)
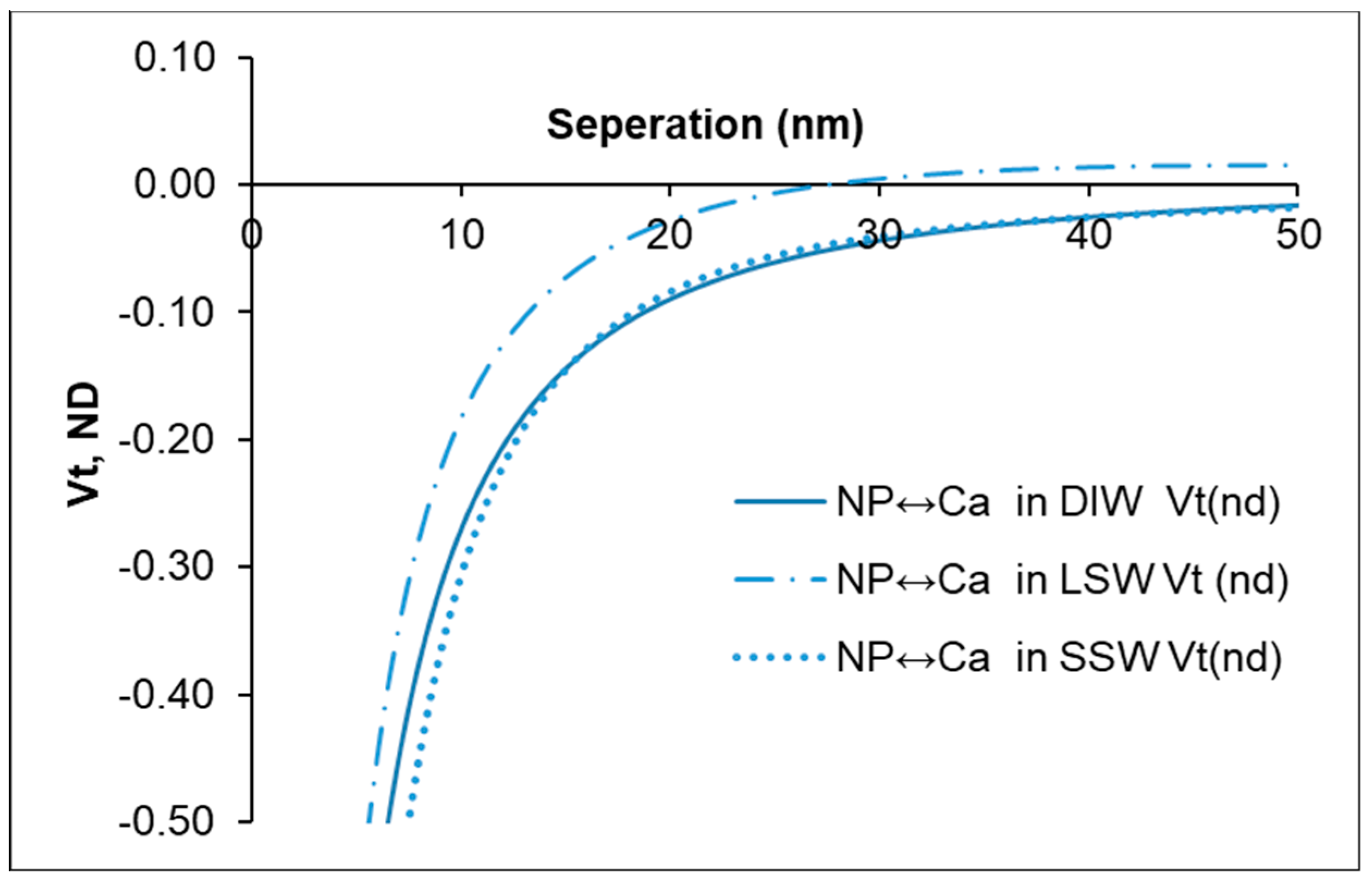
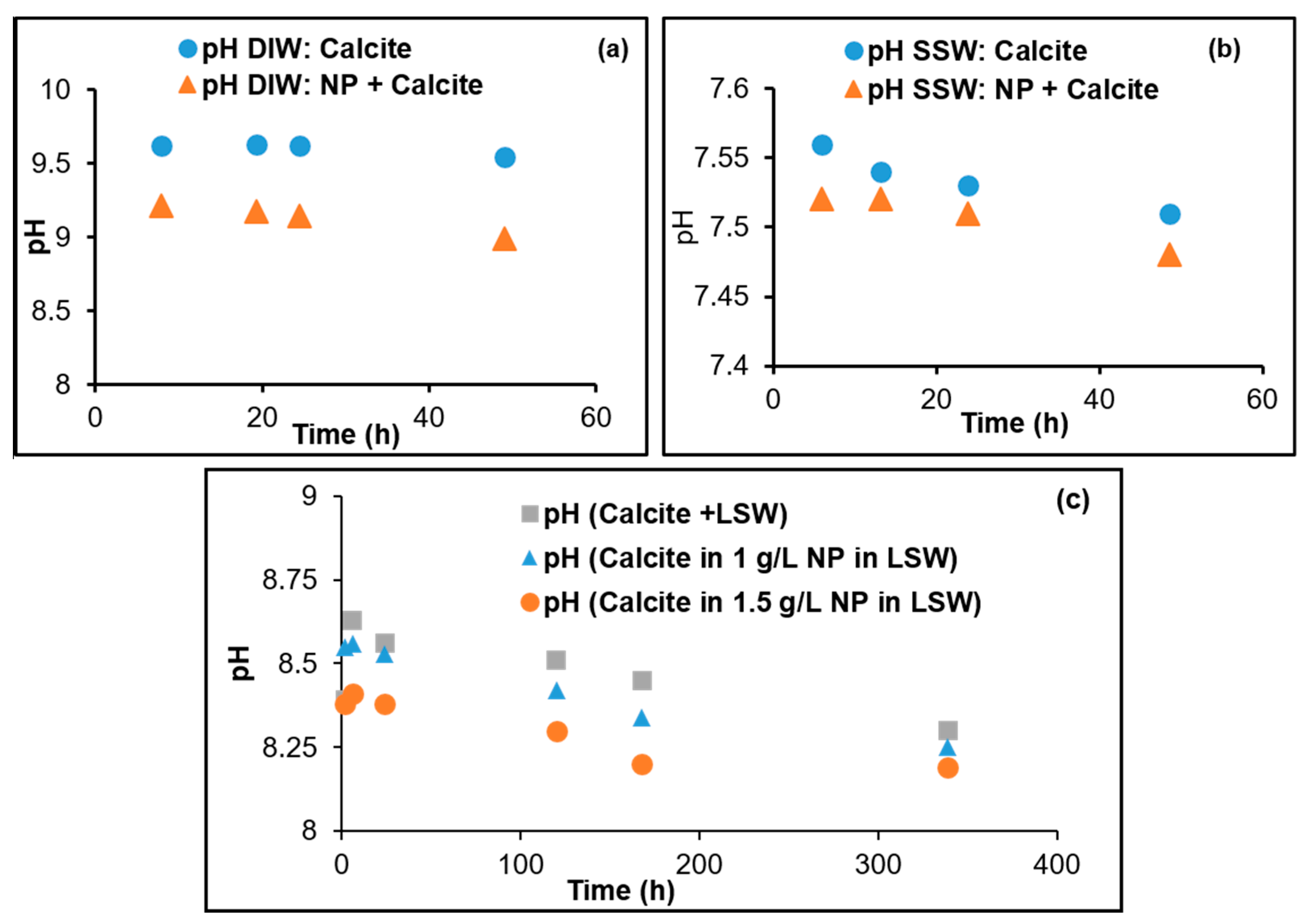
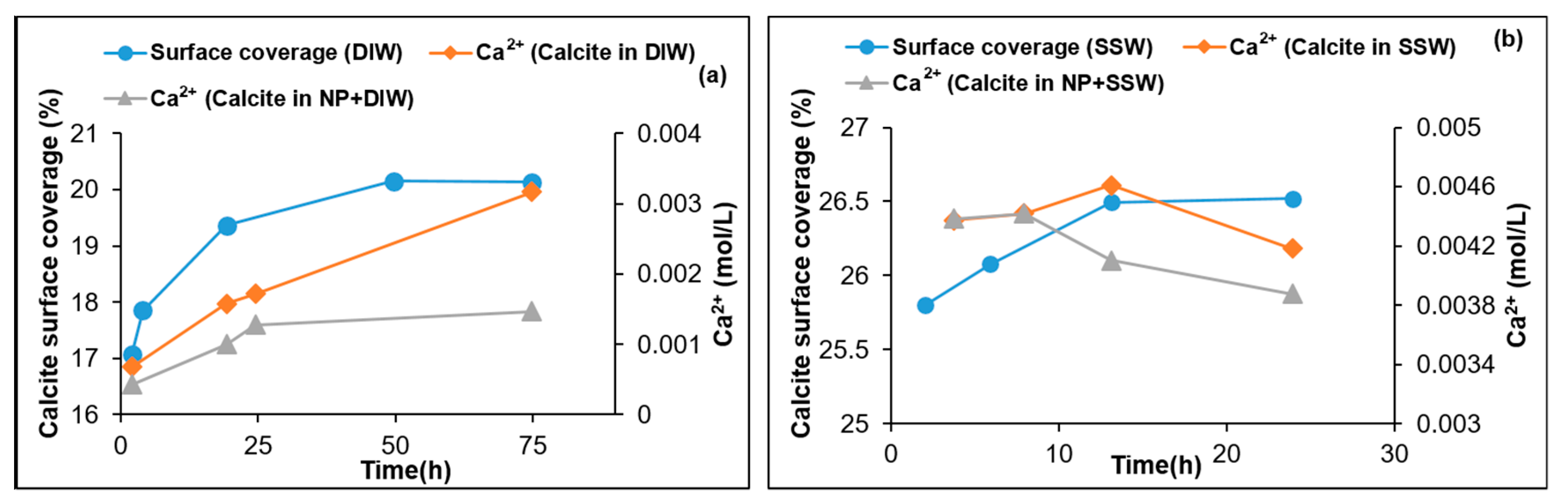
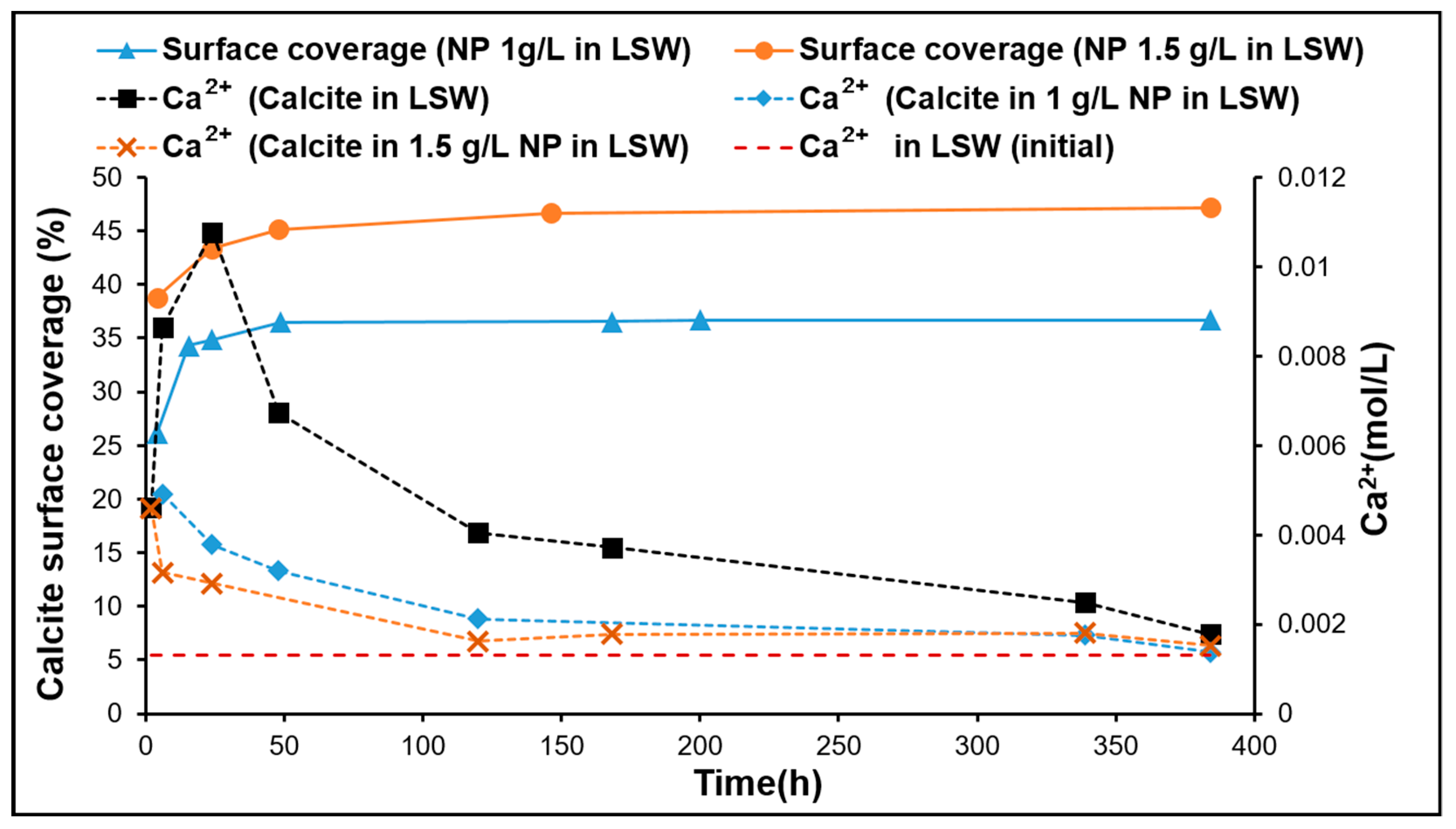
3.3. Fluid/Mineral Interaction
4. Conclusions
- The adsorption of silica nanoparticles on calcite is best described with a pseudo-second-order model.
- Both the rate of adsorption and the level of equilibrium adsorption increase substantially as the salinity increases from DIW to SSW.
- The reduction by half of Ri in LSW as the nanoparticle concentration increases from 1 to 1.5 g/L may be explained by repulsive forces among the nanoparticles as they diffuse from the bulk fluid towards the calcite surface. This may also explain the lower adsorption rate observed for LSW with nanoparticles at 1.5 g/L during the investigation of adsorption kinetic order. The almost equal Ri in both DIW and LSW (1.5 g/L) supports the above hypothesis; where the presence of salt ions (in the LSW) acts as a barrier reducing the adsorption rate, and in the absence of salt ions (in the DIW), the repulsive forces among nanoparticles reduce the adsorption rate.
- The estimation of the surface forces based on the DLVO theory showed that with nanoparticles in LSW, the interaction between nanoparticles and calcite mineral is less attractive in comparison with SSW and DIW.
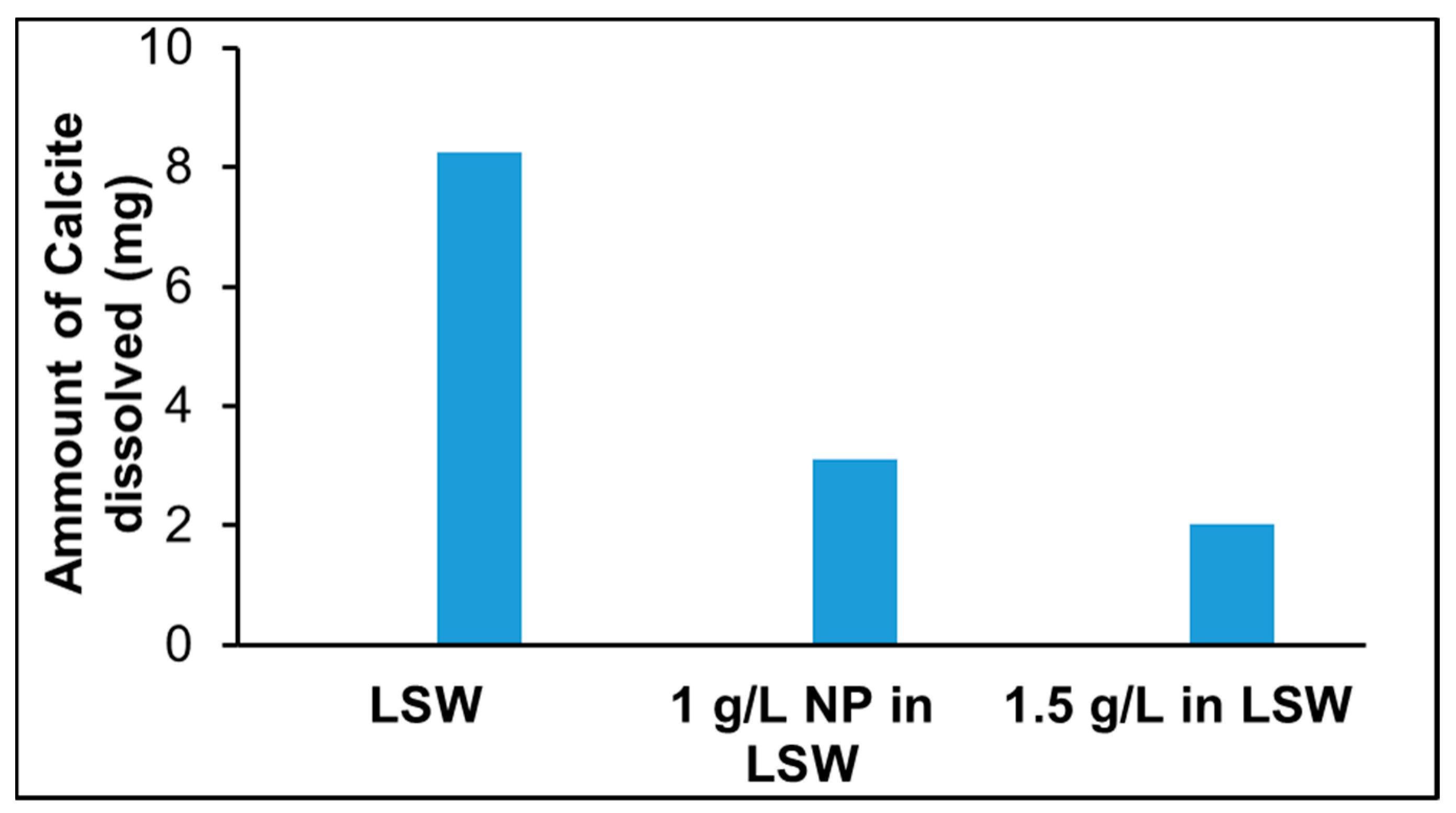
- Adsorption of silica nanoparticles reduces calcite dissolution. This was confirmed by the Ca2+ ion concentration, pH, and lower dissolution observed at increased nanoparticle concentrations. Mass balance based on the analyzed Ca2+ profile demonstrates the increased dissolution reduction with increasing nanoparticle concentration. This is an important outcome especially when LSW is a candidate for EOR in chalk fields, where less formation damage/dissolution of chalk is expected when silica nanoparticles are combined with LSW.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayatollahi, S.; Zerafat, M.M. In Nanotechnology-assisted eor techniques: New solutions to old challenges. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Zhang, H.; Nikolov, A.; Wasan, D. Enhanced oil recovery (eor) using nanoparticle dispersions: Underlying mechanism and imbibition experiments. Energy Fuels 2014, 28, 3002–3009. [Google Scholar] [CrossRef]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. J. Pet. Sci. Eng. 2016, 142, 85–100. [Google Scholar] [CrossRef]
- Chávez-Miyauchi, T.S.E.; Firoozabadi, A.; Fuller, G.G. Nonmonotonic elasticity of the crude oil–brine interface in relation to improved oil recovery. Langmuir 2016, 32, 2192–2198. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, A.; Mohammadi, A. Environmentally responsive surface-modified silica nanoparticles for enhanced oil recovery. J. Nanoparticle Res. 2016, 18, 1–19. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. A stabilizer that enhances the oil recovery process using silica-based nanofluids. Transp. Porous Media 2015, 108, 679–696. [Google Scholar] [CrossRef]
- Ogolo, N.; Olafuyi, O.; Onyekonwu, M. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar]
- Shahrabadi, A.; Bagherzadeh, H.; Roostaie, A.; Golghanddashti, H. Experimental investigation of hlp nanofluid potential to enhance oil recovery: A mechanistic approach. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Ortega, D.J.S.; Kim, H.B.; James, L.A.; Johansen, T.E.; Zhang, Y. The effectiveness of silicon dioxide SiO2 nanoparticle as an enhanced oil recovery agent in ben nevis formation, hebron field, offshore eastern canada. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, UAE, 7–10 November 2016. [Google Scholar]
- Haroun, M.R.; Alhassan, S.; Ansari, A.A.; Al Kindy, N.A.M.; Abou Sayed, N.; Kareem, A.; Ali, B.; Sarma, H.K. Smart nano-eor process for abu dhabi carbonate reservoirs. In Proceedings of the Abu Dhabi International Petroleum Conference and Exhibition, Abu Dhabi, UAE, 11–14 November 2012. [Google Scholar]
- Agista, M.; Guo, K.; Yu, Z. A state-of-the-art review of nanoparticles application in petroleum with a focus on enhanced oil recovery. Appl. Sci. 2018, 8, 871. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Li, S.; Torsæter, O. A coreflood investigation of nanofluid enhanced oil recovery. J. Pet. Sci. Eng. 2013, 111, 128–138. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohammadi, S.; Ghazanfari, M.H.; Kharrat, R.; Masihi, M. Monitoring wettability alteration by silica nanoparticles during water flooding to heavy oils in five-spot systems: A pore-level investigation. Exp. Therm. Fluid Sci. 2012, 40, 168–176. [Google Scholar] [CrossRef]
- Li, S.; Torsæter, O. Experimental investigation of the influence of nanoparticles adsorption and transport on wettability alteration for oil wet berea sandstone. In Proceedings of the SPE Middle East Oil & Gas Show and Conference, Manama, Bahrain, 8–11 March 2015. [Google Scholar]
- Lovell, C.E.; Scott, J.; Amal, R. Ni-sio2 catalysts for the carbon dioxide reforming of methane: Varying support properties by flame spray pyrolysis. Molecules 2015, 20, 4594–4609. [Google Scholar] [CrossRef]
- Walcarius, A.; Mercier, L.J.J. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Di Credico, B.; Bellobono, I.R.; Arienzo, M.; Fumagalli, D.; Redaelli, M.; Scotti, R.; Morazzoni, F. Efficacy of the reactive oxygen species generated by immobilized TiO2 in the photocatalytic degradation of diclofenac. Int. J. Photoenergy 2015, 2015, 919217. [Google Scholar] [CrossRef]
- Zhang, T.; Murphy, M.J.; Yu, H.; Bagaria, H.G.; Yoon, K.Y.; Nielson, B.M.; Bielawski, C.W.; Johnston, K.P.; Huh, C.; Bryant, S.L. Investigation of nanoparticle adsorption during transport in porous media. SPE J. 2015, 20, 667–677. [Google Scholar] [CrossRef]
- Zhang, T.; Murphy, M.; Yu, H.; Huh, C.; Bryant, S.L. Mechanistic model for nanoparticle retention in porous media. Transp. Porous Media 2016, 115, 387–406. [Google Scholar] [CrossRef]
- Li, S.; Torsæter, O. The impact of nanoparticles adsorption and transport on wettability alteration of water wet berea sandstone. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Muscat, Oman, 26–28 January 2015. [Google Scholar]
- Abhishek, R.; Hamouda, A.A.; Murzin, I. Adsorption of silica nanoparticles and its synergistic effect on fluid/rock interactions during low salinity flooding in sandstones. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 397–406. [Google Scholar] [CrossRef]
- Abhishek, R.; Hamouda, A.A. Effect of various silica nanofluids: Reduction of fines migrations and surface modification of berea sandstone. Appl. Sci. 2017, 7, 1216. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Wang, S.; Barifcani, A.; Lebedev, M.; Iglauer, S. Effect of temperature and sio 2 nanoparticle size on wettability alteration of oil-wet calcite. Fuel 2017, 206, 34–42. [Google Scholar] [CrossRef]
- Roustaei, A.; Bagherzadeh, H. Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J. Pet. Explor. Prod. Technol. 2015, 5, 27–33. [Google Scholar] [CrossRef]
- Abhishek, R.; Kumar, G.S.; Sapru, R. Wettability alteration in carbonate reservoirs using nanofluids. Pet. Sci. Technol. 2015, 33, 794–801. [Google Scholar] [CrossRef]
- Abhishek, R.; Bagalkot, N.; Kumar, G.S. Effect of transverse forces on velocity of nanoparticles through a single fracture in a fractured petroleum reservoir. Int. J. OilGas Coal Technol. 2016, 12, 379–395. [Google Scholar] [CrossRef]
- Nwidee, L.N.; Al-Anssari, S.; Barifcani, A.; Sarmadivaleh, M.; Lebedev, M.; Iglauer, S. Nanoparticles influence on wetting behaviour of fractured limestone formation. J. Pet. Sci. Eng. 2017, 149, 782–788. [Google Scholar] [CrossRef]
- Nazari Moghaddam, R.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative study of using nanoparticles for enhanced oil recovery: Wettability alteration of carbonate rocks. Energy Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Barifcani, A.; Wang, S.; Iglauer, S. Wettability alteration of oil-wet carbonate by silica nanofluid. J. Colloid Interface Sci. 2016, 461, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Monfared, A.D.; Ghazanfari, M.; Jamialahmadi, M.; Helalizadeh, A. Adsorption of silica nanoparticles onto calcite: Equilibrium, kinetic, thermodynamic and dlvo analysis. Chem. Eng. J. 2015, 281, 334–344. [Google Scholar] [CrossRef]
- van Oort, E.; Van Velzen, J.; Leerlooijer, K. Impairment by suspended solids invasion: Testing and prediction. SPE Prod. Facil. 1993, 8, 178–184. [Google Scholar] [CrossRef]
- Abhishek, R.; Hamouda, A.; Ayoub, A. Effect of silica nanoparticles on fluid/rock interactions during low salinity water flooding of chalk reservoirs. Appl. Sci. 2018, 8, 1093. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Rezaei Gomari, K.A. Influence of temperature on wettability alteration of carbonate reservoirs. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. [Google Scholar]
- Hamouda, A.; Valderhaug, O.; Munaev, R.; Stangeland, H. Possible mechanisms for oil recovery from chalk and sandstone rocks by low salinity water (lsw). In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Zahid, A.; Shapiro, A.A.; Skauge, A. Experimental studies of low salinity water flooding carbonate: A new promising approach. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012. [Google Scholar]
- Mahani, H.; Keya, A.L.; Berg, S.; Bartels, W.-B.; Nasralla, R.; Rossen, W.R. Insights into the mechanism of wettability alteration by low-salinity flooding (lsf) in carbonates. Energy Fuels 2015, 29, 1352–1367. [Google Scholar] [CrossRef]
- Al-Nofli, K.; Pourafshary, P.; Mosavat, N.; Shafiei, A. Effect of initial wettability on performance of smart water flooding in carbonate reservoirs—an experimental investigation with ior implications. Energies 2018, 11, 1394. [Google Scholar] [CrossRef]
- Wang, X.; Alvarado, V. Kaolinite and silica dispersions in low-salinity environments: Impact on a water-in-crude oil emulsion stability. Energies 2011, 4, 1763. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Gupta, S. Enhancing oil recovery from chalk reservoirs by a low-salinity water flooding mechanism and fluid/rock interactions. Energies 2017, 10, 576. [Google Scholar] [CrossRef]
- Rezaei Gomari, S.; Joseph, N. Study of the effect of clay particles on low salinity water injection in sandstone reservoirs. Energies 2017, 10, 322. [Google Scholar] [CrossRef]
- Omekeh, A.V.; Friis, H.A.; Fjelde, I.; Evje, S. Modeling of ion-exchange and solubility in low salinity water flooding. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; p. 13. [Google Scholar]
- Alagic, E.; Spildo, K.; Skauge, A.; Solbakken, J. Effect of crude oil ageing on low salinity and low salinity surfactant flooding. J. Pet. Sci. Eng. 2011, 78, 220–227. [Google Scholar] [CrossRef]
- Mahani, H.; Keya, A.L.; Berg, S.; Bartels, W.-B.; Nasralla, R.; Rossen, W. Driving mechanism of low salinity flooding in carbonate rocks. In Proceedings of the EUROPEC 2015, Madrid, Spain, 1–4 June 2015. [Google Scholar]
- Patwardhan, S.D.; Singh, D.; Abhishek, R.; Suresh Kumar, G. Modelling of mineral precipitation in fractures with variable aperture. ISH J. Hydraul. Eng. 2017, 23, 203–211. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Maevskiy, E. Oil recovery mechanism (s) by low salinity brines and their interaction with chalk. Energy Fuels 2014, 28, 6860–6868. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Elimelech, M.J.J. Surface element integration: A novel technique for evaluation of dlvo interaction between a particle and a flat plate. J. Colloid Interface Sci. 1997, 193, 273–285. [Google Scholar] [CrossRef]
- Dunphy Guzman, K.A.; Finnegan, M.P.; Banfield, J.F. Influence of surface potential on aggregation and transport of titania nanoparticles. Environ. Sci. Technol. 2006, 40, 7688–7693. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Ghosh, G. Dispersion-equation coefficients for the refractive index and birefringence of calcite and quartz crystals. Opt. Commun. 1999, 163, 95–102. [Google Scholar] [CrossRef]
- Malitson, I. Interspecimen comparison of the refractive index of fused silica. JOSA 1965, 55, 1205–1209. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Temple, S.J.J. Effect of salinity on the refractive index of water: Considerations for archer fish aerial vision. J. Fish Biol. 2007, 70, 1626–1629. [Google Scholar] [CrossRef]
- Khilar, K.C.; Fogler, H.S. Migrations of Fines in Porous Media; Springer Science & Business Media: New York, NY, USA, 1998; Volume 12. [Google Scholar]
- Ho, Y.-S.; McKay, G.J.P. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.; Morris, J. Removal of biologically-resistant pollutants from waste waters by adsorption. In Proceedings of the First International Conference on Water Pollution Research; Pergamon Press Oxford: Oxford, UK, 1962; pp. 231–266. [Google Scholar]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Iler, R.K. Chemistry of Silica—Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wily: Hoboken, NJ, USA, 1979. [Google Scholar]
- Janusz, W.; Patkowski, J.; Chibowski, S. Competitive adsorption of Ca2+ and Zn(II) ions at monodispersed SiO2/electrolyte solution interface. J. Colloid Interface Sci. 2003, 266, 259–268. [Google Scholar] [CrossRef]
| Ion | Synthetic Seawater (SSW) (mol/L) | Low Salinity Water (LSW) (mol/L) |
|---|---|---|
| HCO3− | 0.002 | 0.0002 |
| Cl− | 0.525 | 0.0525 |
| SO42− | 0.0240 | 0.0024 |
| Mg2+ | 0.045 | 0.0045 |
| Ca2+ | 0.013 | 0.0013 |
| Na+ | 0.450 | 0.045 |
| K+ | 0.010 | 0.0010 |
| Material | Dispersing Fluid | Temperature (°C) | Hydrodynamic Radius (nm) | pH | Zeta-Potential (mV) |
|---|---|---|---|---|---|
| Silica nanoparticles | DIW | 25 | 18.76 | 6.0 | −30.7 |
| Silica nanoparticles | DIW | 50 | 19.29 | - | - |
| Silica nanoparticles | DIW | 80 | 19.7 | - | - |
| Silica nanoparticles | LSW | 25 | 18.96 | 7.2 | −12.1 |
| Silica nanoparticles | LSW | 50 | 19.1 | - | - |
| Silica nanoparticles | LSW | 80 | 19.35 | - | - |
| Silica nanoparticles | SSW | 25 | 28.18 | 7.3 | −6.4 |
| Silica nanoparticles | SSW | 50 | 28.77 | - | - |
| Silica nanoparticles | SSW | 80 | 44.06 | - | - |
| Calcite | DIW | 25 | - | 9.62 | −23.4 |
| Calcite | LSW | 25 | - | 8.39 | −8.0 |
| Calcite | SSW | 25 | - | 7.56 | −3.7 |
| Pseudo-First-Order Model | ||||
| Fluid | Exp qe (mg/g) | R2 | k1: (1/h) | Estimated qe: (mg/g) |
| DIW (nanoparticle conc 1 g/L) | 2.41 | 0.88 | 0.055 | 0.312 |
| SSW (nanoparticle conc 1 g/L) | 4.75 | 0.94 | 0.2132.5 | 0.971 |
| LSW (nanoparticle conc 1 g/L) | 4.4 | 0.9025 | 0.1149 | 1.09319 |
| LSW (nanoparticle conc 1.5 g/L) | 4.75 | 0.9378 | 0.0066 | 0.88923 |
| Pseudo-Second-Order Model | ||||
| Fluid | Exp qe (mg/g) | R2 | k2: (g/mg h) | Estimated qe: (mg/g) |
| DIW (nanoparticle conc 1 g/L) | 2.41 | 0.99 | 0.73 | 2.41955 |
| SSW (nanoparticle conc 1 g/L) | 4.75 | 1 | 2.5 | 4.76644 |
| LSW (nanoparticle conc 1 g/L) | 4.4 | 1 | 0.191 | 4.44 |
| LSW (nanoparticle conc 1.5 g/L) | 4.75 | 0.99 | 0.11 | 5.68 |
| Fluid_Nanoparticle Conc. | C (mg/g) | K (mg/g h0.5) | Ri | teq(hrs)_Adsorption Characterization |
|---|---|---|---|---|
| DIW_1.0 g/L | 1.8 | 0.16 | 0.25 | 49 (hrs)_ Strong initial adsorption |
| LSW_1.0 g/L | 2.13 | 0.51 | 0.52 | 49(hrs)_Strong initial adsorption |
| LSW_1.5 g/L | 4.29 | 0.19 | 0.24 | 49(hrs)_Strong initial adsorption |
| SSW_1.0 g/L | 4.56 | 0.036 | 0.037 | 16(hrs)_near complete initial adsorption |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamouda, A.A.; Abhishek, R. Effect of Salinity on Silica Nanoparticle Adsorption Kinetics and Mechanisms for Fluid/Rock Interaction with Calcite. Nanomaterials 2019, 9, 213. https://doi.org/10.3390/nano9020213
Hamouda AA, Abhishek R. Effect of Salinity on Silica Nanoparticle Adsorption Kinetics and Mechanisms for Fluid/Rock Interaction with Calcite. Nanomaterials. 2019; 9(2):213. https://doi.org/10.3390/nano9020213
Chicago/Turabian StyleHamouda, Aly A., and Rockey Abhishek. 2019. "Effect of Salinity on Silica Nanoparticle Adsorption Kinetics and Mechanisms for Fluid/Rock Interaction with Calcite" Nanomaterials 9, no. 2: 213. https://doi.org/10.3390/nano9020213
APA StyleHamouda, A. A., & Abhishek, R. (2019). Effect of Salinity on Silica Nanoparticle Adsorption Kinetics and Mechanisms for Fluid/Rock Interaction with Calcite. Nanomaterials, 9(2), 213. https://doi.org/10.3390/nano9020213






