A Novel Nanocomposite Membrane Combining BN Nanosheets and GO for Effective Removal of Antibiotic in Water
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
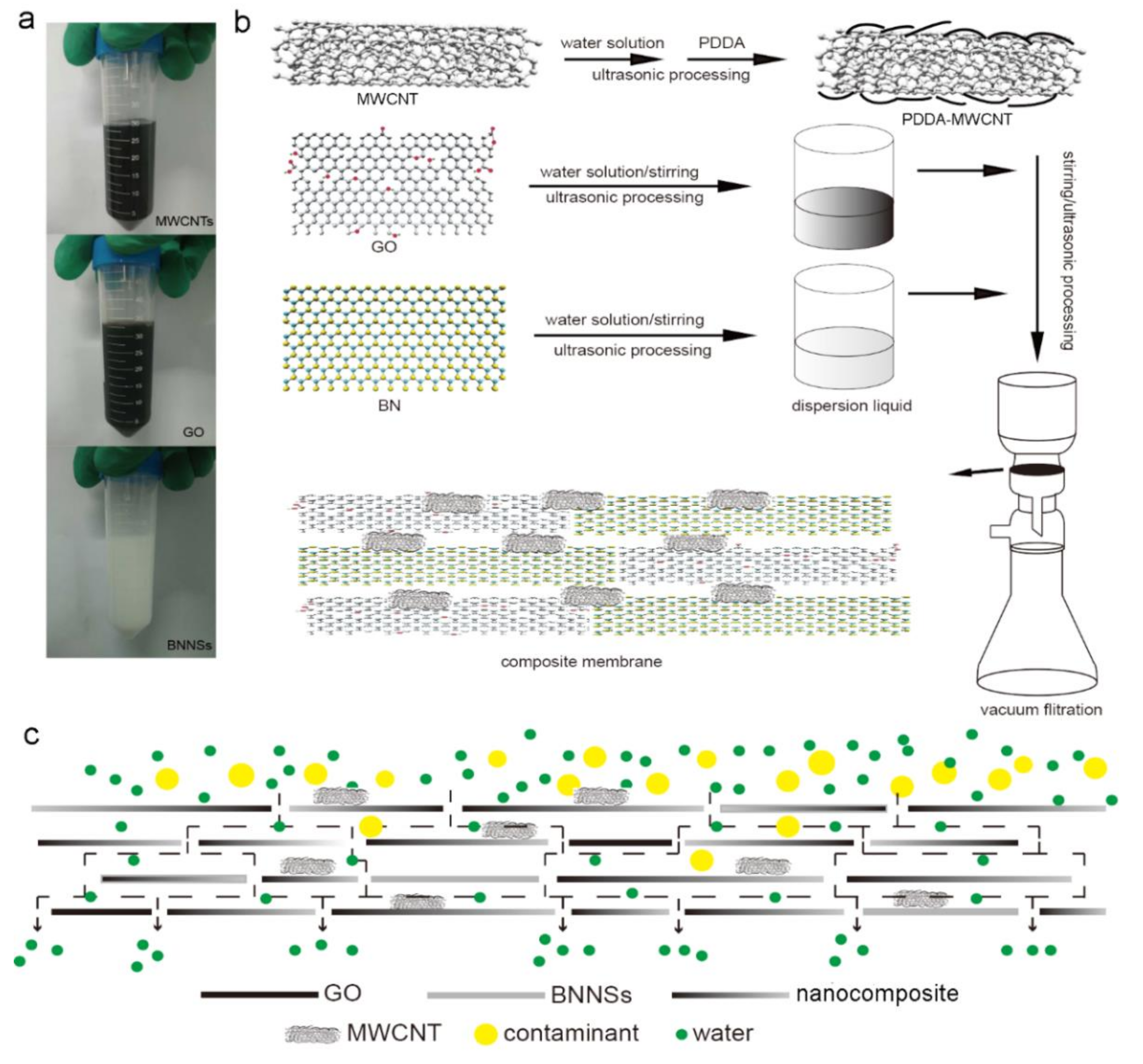
2.2. Fabrication of Graphene Oxide (GO) Dispersion
2.3. Fabrication of Polydiallyldimethylammonium Chloride-Multi-Walled Carbon Nanotubes (PDDA-MWCNTs) Dispersion
2.4. Fabrication of Boron Nitride Nanosheets (BNNSs) Dispersion
2.5. Fabrication of the Membrane
2.6. Filtration Experiment
2.7. Characterization
3. Results and Discussion
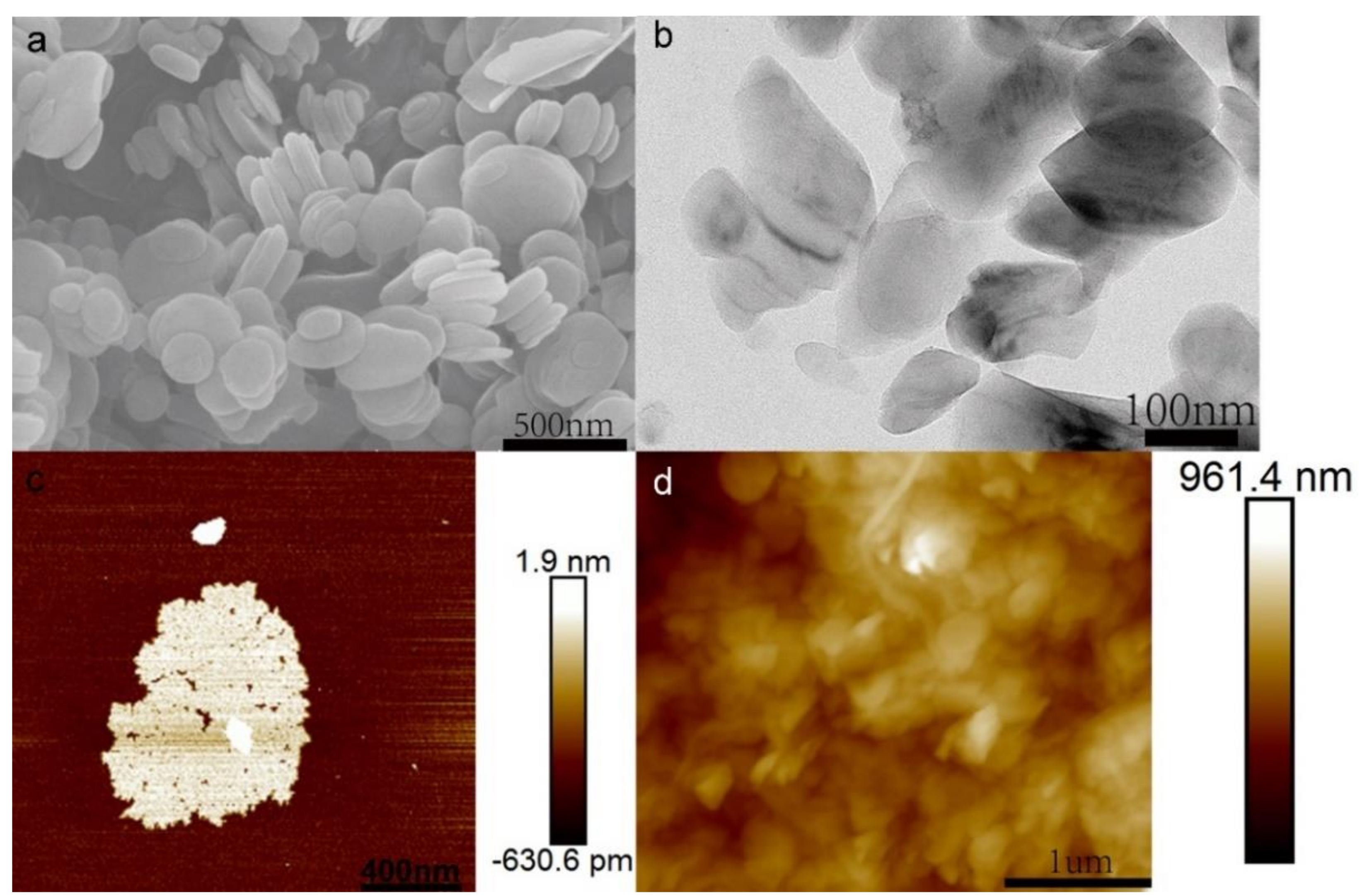
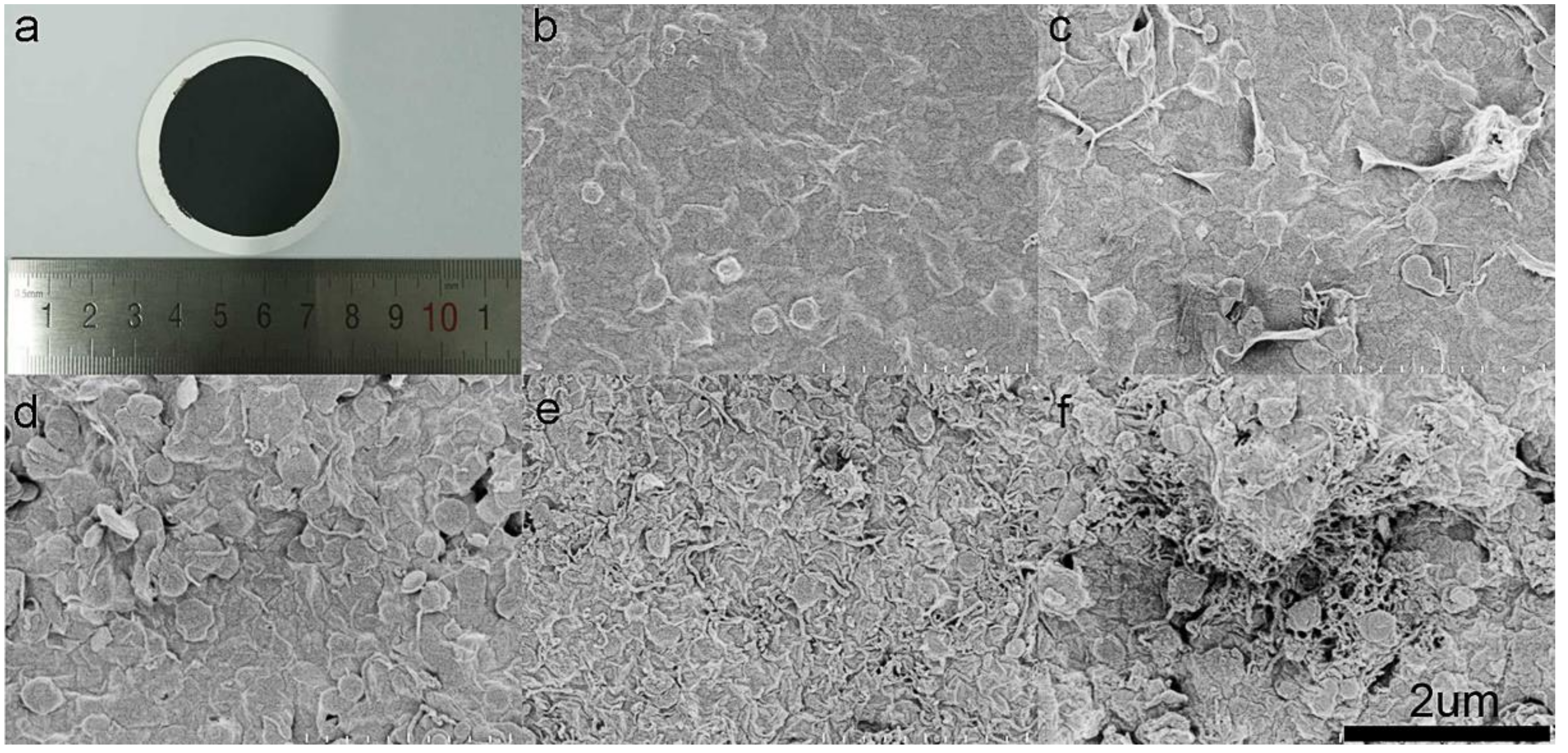
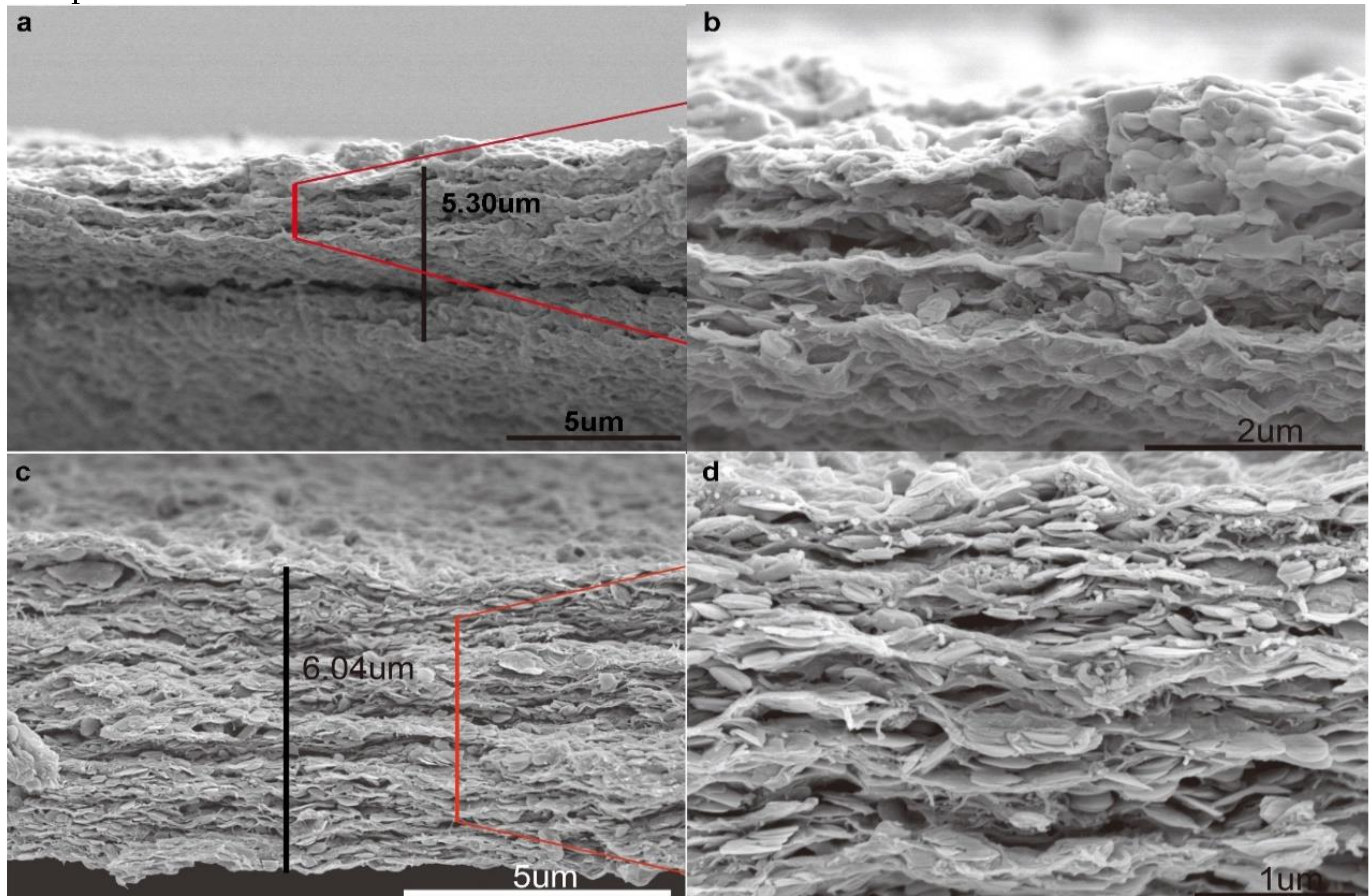
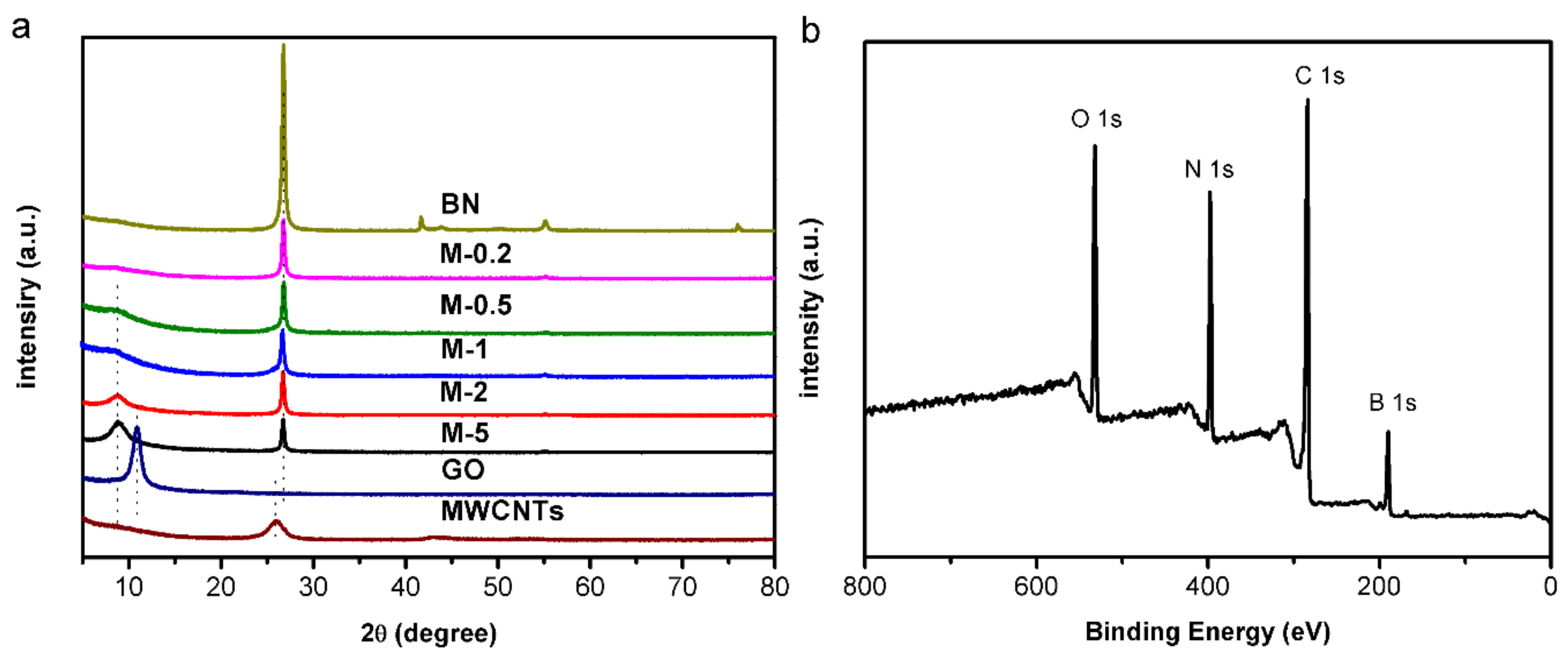
3.1. Characterization and Mechanism
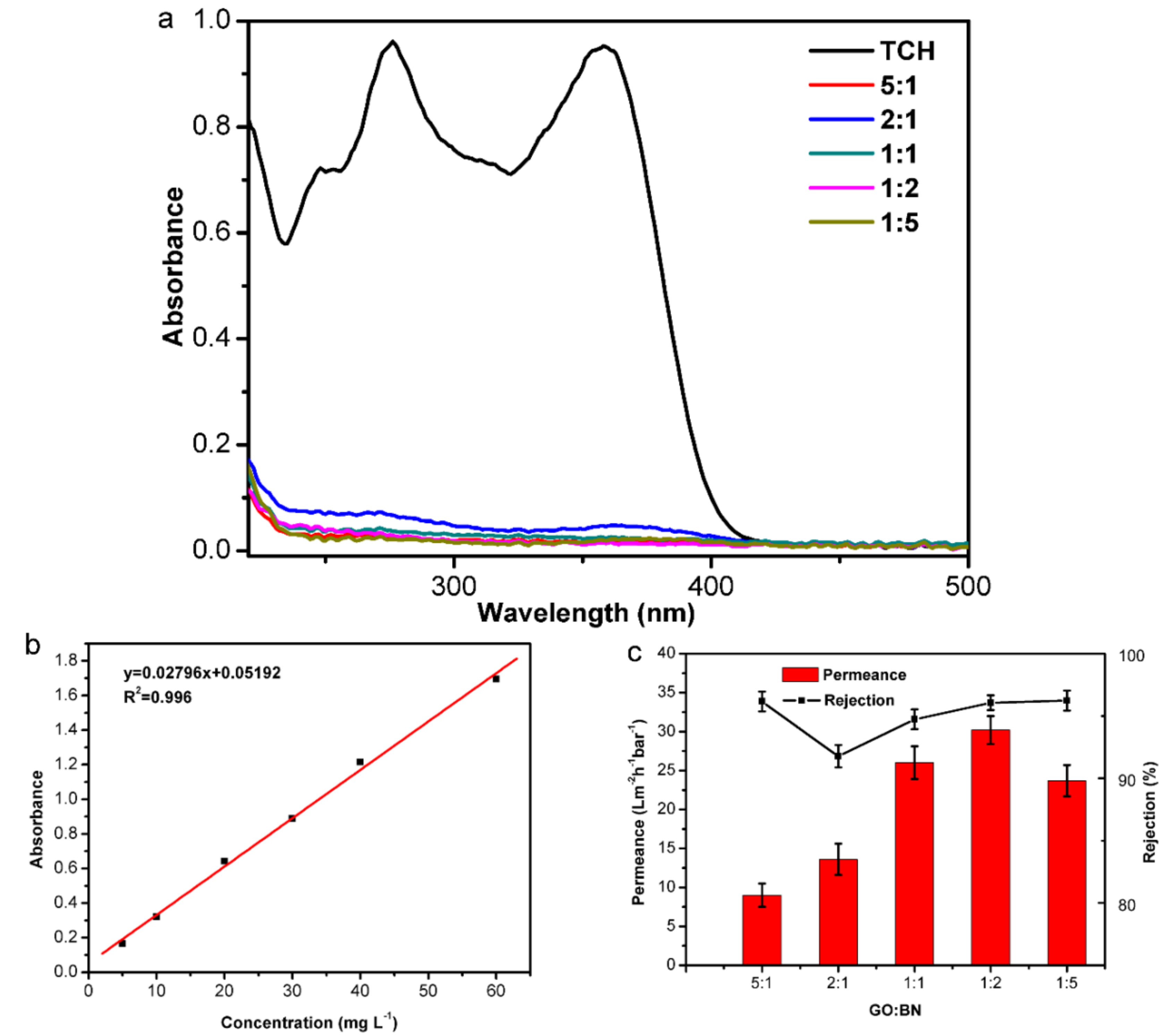
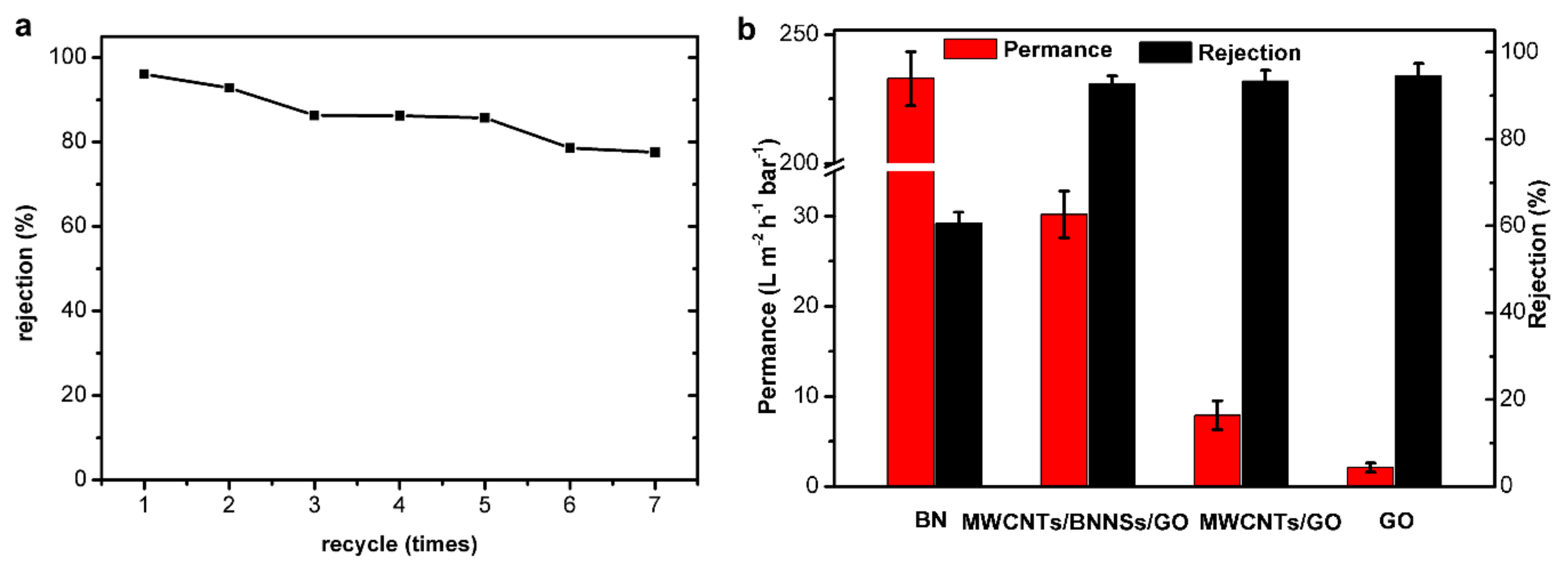
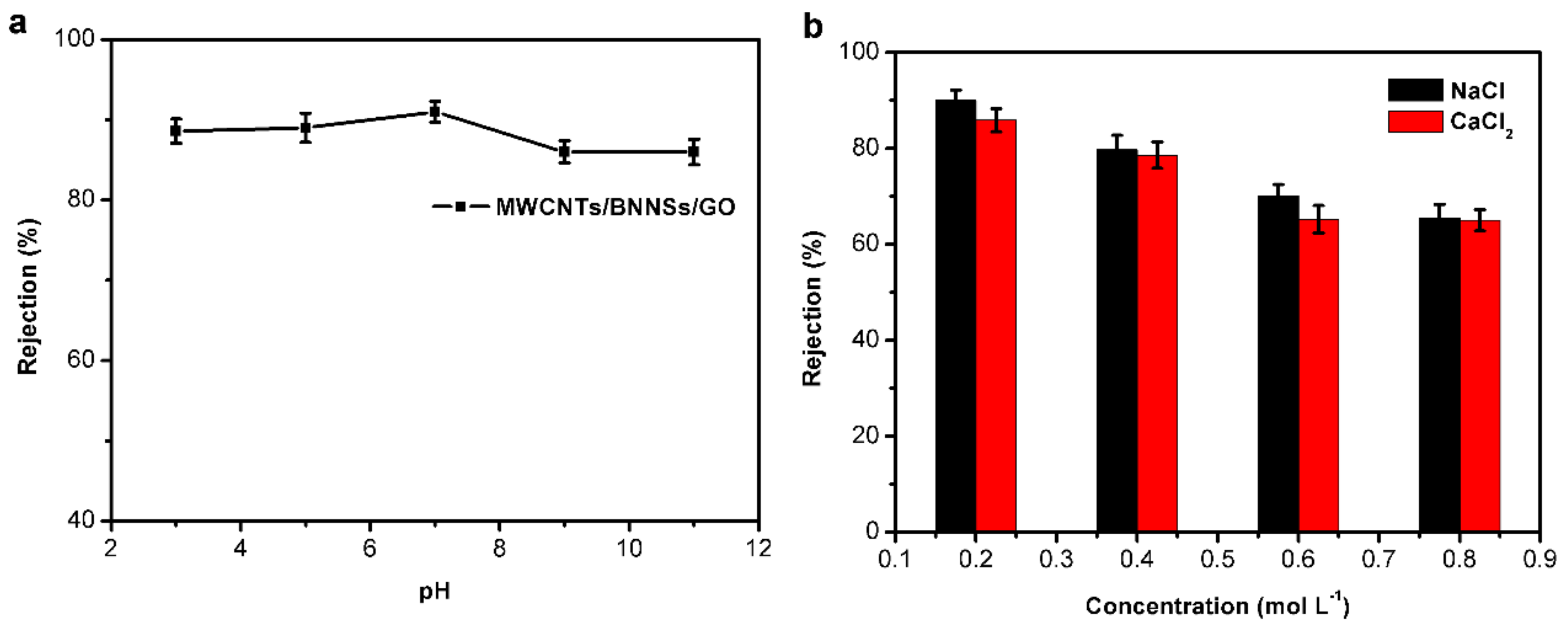
3.2. Membrane Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arenas, N.E.; Melo, V.M. Producción pecuaria y emergencia de antibiótico resistencia en Colombia: Revisión sistemática. Infectio 2018, 22, 110–119. [Google Scholar] [CrossRef]
- Pellerito, A.; Ameen, S.M.; Micali, M.; Caruso, G. Antimicrobial Substances for Food Packaging Products: The Current Situation. J. AOAC Int. 2018, 101. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events With Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef]
- Fridkin, S.; Baggs, J.; Fagan, R.; Magill, S.; Pollack, L.A.; Malpiedi, P.; Slayton, R.; Khader, K.; Rubin, M.A.; Jones, M.; et al. Vital Signs: Improving Antibiotic Use Among Hospitalized Patients. MMWR-Morb. Mortal. Wkly. Rep. 2014, 63, 194–200. [Google Scholar] [PubMed]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, L.Y.; Ashbolt, N.; Wang, X.L.; Cui, Y.X.; Zhu, X.; Xu, Y.; Yang, Y.; Mao, D.Q.; Luo, Y. Arctic antibiotic resistance gene contamination, a result of anthropogenic activities and natural origin. Sci. Total Environ. 2018, 621, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.I. The First Case of Multidrug-resistant NDM−1-harboring Enterobacteriaceae in Taiwan: Here Comes the Superbacteria! J. Chin. Med. Assoc. 2010, 73, 557–558. [Google Scholar] [CrossRef]
- Tong, S.; Pan, J.; Lu, S.; Tang, J. Patient compliance with antimicrobial drugs: A Chinese survey. Am. J. Infect. Control 2018, 46, E25–E29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; He, Y.-W.; Chen, M.; Gao, M.; Qiu, T.-L.; Wang, X.-M. Pollution Characteristics of Antibiotic Resistant Bacteria from Atmospheric Environment of Animal Feeding Operations. Huan Jing Ke Xue 2016, 37, 4531–4537. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.M.; Sobsey, M.D. Antibiotic-Resistant Enteric Bacteria in Environmental Waters. Water 2016, 8, 561. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Elbahri, M. Graphene membranes for water desalination. NPG Asia Mater. 2017, 9, e427. [Google Scholar] [CrossRef]
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; Grossman, J.C. Water Desalination across Nanoporous Graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, Z.; Hua, W.X.; Liu, D.; Tao, T.; Rahman, M.M.; Lei, W.W.; Huang, S.M.; Chen, Y. Functionalized Boron Nitride Nanosheets/Graphene Interlayer for Fast and Long-Life Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 6. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.R.; Santos, E.J.G.; Scullion, D.; Qian, D.; Zhang, R.; Yang, Z.; Huang, S.M.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Commun. 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Hirunpinyopas, W.; Prestat, E.; Worrall, S.D.; Haigh, S.J.; Dryfe, R.A.W.; Bissett, M.A. Desalination and Nanofiltration through Functionalized Laminar MoS2 Membranes. ACS Nano 2017, 11, 11082–11090. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.P.; Tong, R.; Zhou, X.B.; Gong, Y.; Zhang, Z.P.; Ji, Q.Q.; Zhang, Y.; Fang, Q.Y.; Gu, L.; Wang, X.N.; et al. Temperature-Mediated Selective Growth of MoS2/WS2 and WS2/MoS2 Vertical Stacks on Au Foils for Direct Photocatalytic Applications. Adv. Mater. 2016, 28, 10664. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, Y.T.; Xie, X.M.; Ye, X.Y. Facile and Green Production of Impurity-Free Aqueous Solutions of WS2 Nanosheets by Direct Exfoliation in Water. Small 2016, 12, 6703–6713. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, M.; Xu, W.L.; Zhou, F.; Yoon, Y.; Yu, M. Graphene Oxide: A Novel 2-Dimensional Material in Membrane Separation for Water Purification. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Wang, Y. Visible active N-doped TiO2/reduced graphene oxide for the degradation of tetracycline hydrochloride. Chem. Phys. Lett. 2018, 691, 408–414. [Google Scholar] [CrossRef]
- Jiao, S.; Xu, Z. Non-Continuum Intercalated Water Diffusion Explains Fast Permeation through Graphene Oxide Membranes. ACS Nano 2017, 11, 11152–11161. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Jiang, G.; Garvey, C.J.; Wang, Y.; Simon, G.P.; Liu, J.Z.; Li, D. Ion transport in complex layered graphene-based membranes with tuneable interlayer spacing. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Yanan, L.; Yanlei, S.; Jingyuan, G.; Jialin, C.; Runnan, Z.; Mingrui, H.; Kang, G.; Linjie, Z.; Zhongyi, J. 2D Heterostructure Membranes with Sunlight-Driven Self-Cleaning Ability for Highly Efficient Oil-Water Separation. Adv. Funct. Mater. 2018, 28, 1706545. [Google Scholar] [CrossRef]
- Jingjing, Y.; Dian, G.; Guihua, L.; Gaofeng, Z.; Qiyan, W.; Yelei, Z.; Guojuan, L.; Ping, W.; Evgeny, V.; Zheng, P.; et al. Self-Assembly of Thiourea-Crosslinked Graphene Oxide Framework Membranes toward Separation of Small Molecules. Adv. Mater. 2018, 30, 1705775. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, M.; Xie, W.; Sun, L.; Chen, Y.; Lei, W. Porous BN/TiO2 hybrid nanosheets as highly efficient visible-light-driven photocatalysts. Appl. Catal. B-Environ. 2017, 207, 72–78. [Google Scholar] [CrossRef]
- Abdikheibari, S.; Lei, W.; Dumee, L.F.; Milne, N.; Baskaran, K. Thin film nanocomposite nanofiltration membranes from amine functionalized-boron nitride/polypiperazine amide with enhanced flux and fouling resistance. J. Mater. Chem. A 2018, 6, 12066–12081. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.; Mansouri, J.; Chen, V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Gassara, S.; Cambedouzou, J.; Bechelany, M.; Miele, P. Development of novel h-BNNS/PVA porous membranes via Pickering emulsion templating. Green Chem. 2018, 20, 4319–4329. [Google Scholar] [CrossRef]
- Zhang, L.S.; Fan, W.; Liu, T.X. Flexible hierarchical membranes of WS2 nanosheets grown on graphene-wrapped electrospun carbon nanofibers as advanced anodes for highly reversible lithium storage. Nanoscale 2016, 8, 16387–16394. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.W.; Mochalin, V.N.; Liu, D.; Qin, S.; Gogotsi, Y.; Chen, Y. Boron nitride colloidal solutions, ultralight aerogels and freestanding membranes through one-step exfoliation and functionalization. Nat. Commun. 2015, 6, 8849. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Hanlumyuang, Y.; Liu, Z.; Gong, Y.; Gao, W.; Li, B.; Kono, J.; Lou, J.; Vajtai, R.; Sharma, P.; et al. Boron Nitride-Graphene Nanocapacitor and the Origins of Anomalous Size-Dependent Increase of Capacitance. Nano Lett. 2014, 14, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Gao, T.T.; Zhang, M.; Li, Y.R.; Dai, L.M.; Qu, L.T.; Shi, G.Q. Reduced Graphene Oxide Membranes for Ultrafast Organic Solvent Nanofi ltration. Adv. Mater. 2016, 28, 8669–8674. [Google Scholar] [CrossRef] [PubMed]
- Zereshki, S.; Daraei, P.; Shokri, A. Application of edible paraffin oil for cationic dye removal from water using emulsion liquid membrane. J. Hazard. Mater. 2018, 356, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, J.; Wan, Y. Regenerable biocatalytic nanofiltration membrane for aquatic micropollutants removal. J. Membr. Sci. 2018, 549, 120–128. [Google Scholar] [CrossRef]
- Li, W.; Wu, W.; Li, Z. Controlling Interlayer Spacing of Graphene Oxide Membranes by External Pressure Regulation. Acs Nano 2018, 12, 9309–9317. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Fang, C.; Zhou, F.; Song, Z.; Liu, Q.; Qiao, R.; Yu, M. Self-Assembly: A Facile Way of Forming Ultrathin, High-Performance Graphene Oxide Membranes for Water Purification. Nano Lett. 2017, 17, 2928–2933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Wei, Y.; Xue, J.; Chen, H.; Ding, L.; Caro, J.; Wang, H. Water Transport with Ultralow Friction through Partially Exfoliated g-C3N4 Nanosheet Membranes with Self-Supporting Spacers. Angew. Chem.-Int. Edit. 2017, 56, 8974–8980. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, X.; Wang, J.; Lin, P.; Chen, C.; Zhang, X.; Suffet, I.H. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Zhang, D.; Wang, C.; Liu, H.; Qu, L.; Li, H. A Novel Nanocomposite Membrane Combining BN Nanosheets and GO for Effective Removal of Antibiotic in Water. Nanomaterials 2019, 9, 386. https://doi.org/10.3390/nano9030386
Yang G, Zhang D, Wang C, Liu H, Qu L, Li H. A Novel Nanocomposite Membrane Combining BN Nanosheets and GO for Effective Removal of Antibiotic in Water. Nanomaterials. 2019; 9(3):386. https://doi.org/10.3390/nano9030386
Chicago/Turabian StyleYang, Guohai, Daqing Zhang, Cheng Wang, Hong Liu, Lulu Qu, and Haitao Li. 2019. "A Novel Nanocomposite Membrane Combining BN Nanosheets and GO for Effective Removal of Antibiotic in Water" Nanomaterials 9, no. 3: 386. https://doi.org/10.3390/nano9030386
APA StyleYang, G., Zhang, D., Wang, C., Liu, H., Qu, L., & Li, H. (2019). A Novel Nanocomposite Membrane Combining BN Nanosheets and GO for Effective Removal of Antibiotic in Water. Nanomaterials, 9(3), 386. https://doi.org/10.3390/nano9030386





