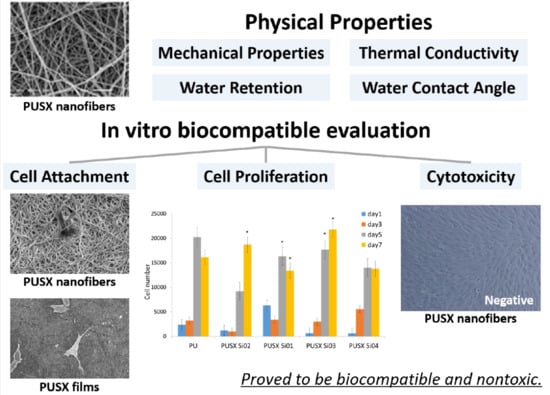
Physical Properties and In Vitro Biocompatible Evaluation of Silicone-Modified Polyurethane Nanofibers and Films
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
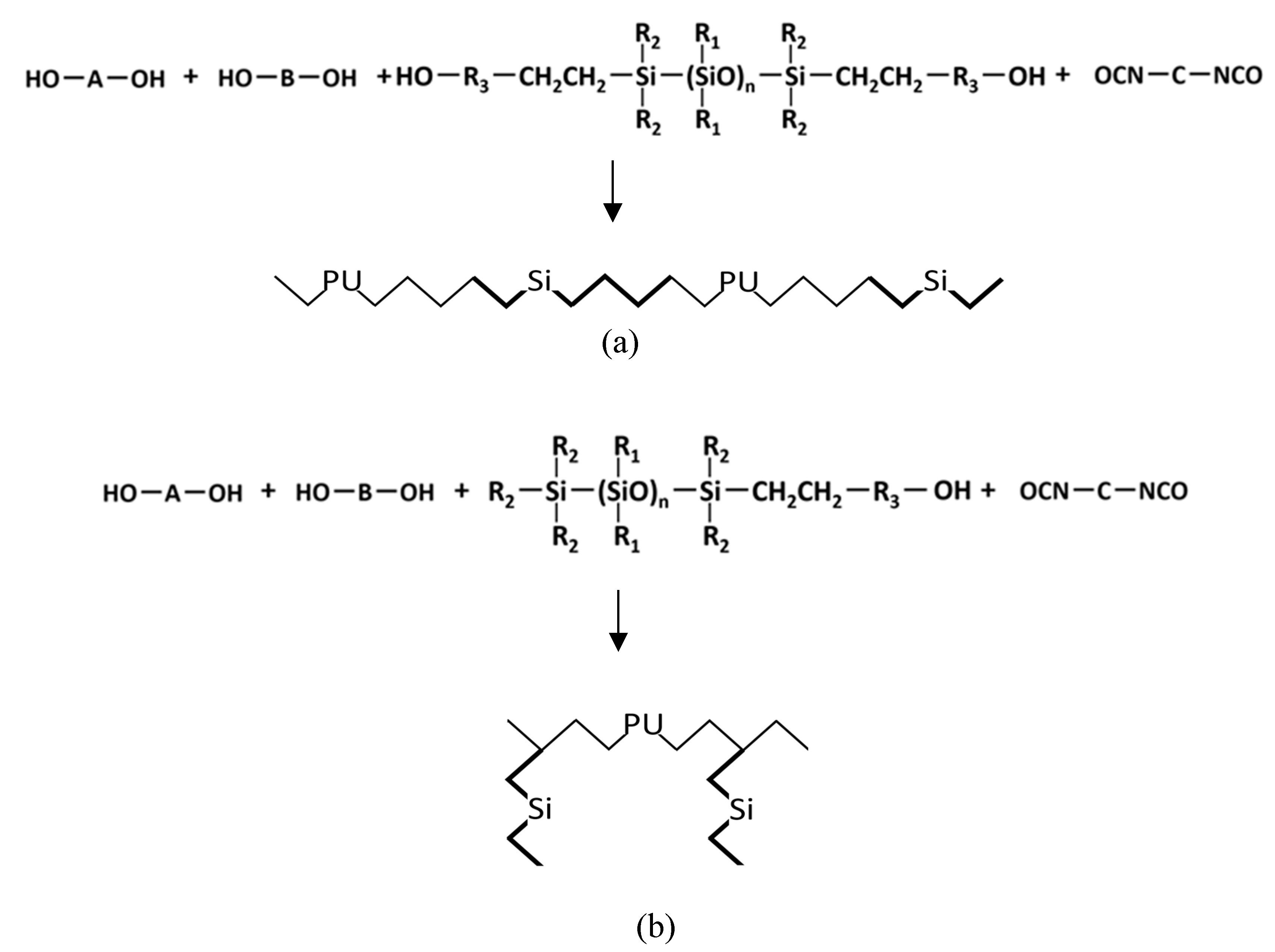
2.2. Electrospinning Method of PUSX Nanofibers
2.3. Physical Properties
2.4. In Vitro Biocompatible Evaluation
2.4.1. Cell Culture Studies
2.4.2. Toxicity Evaluation
2.5. Statistical Analysis
3. Results and Discussions
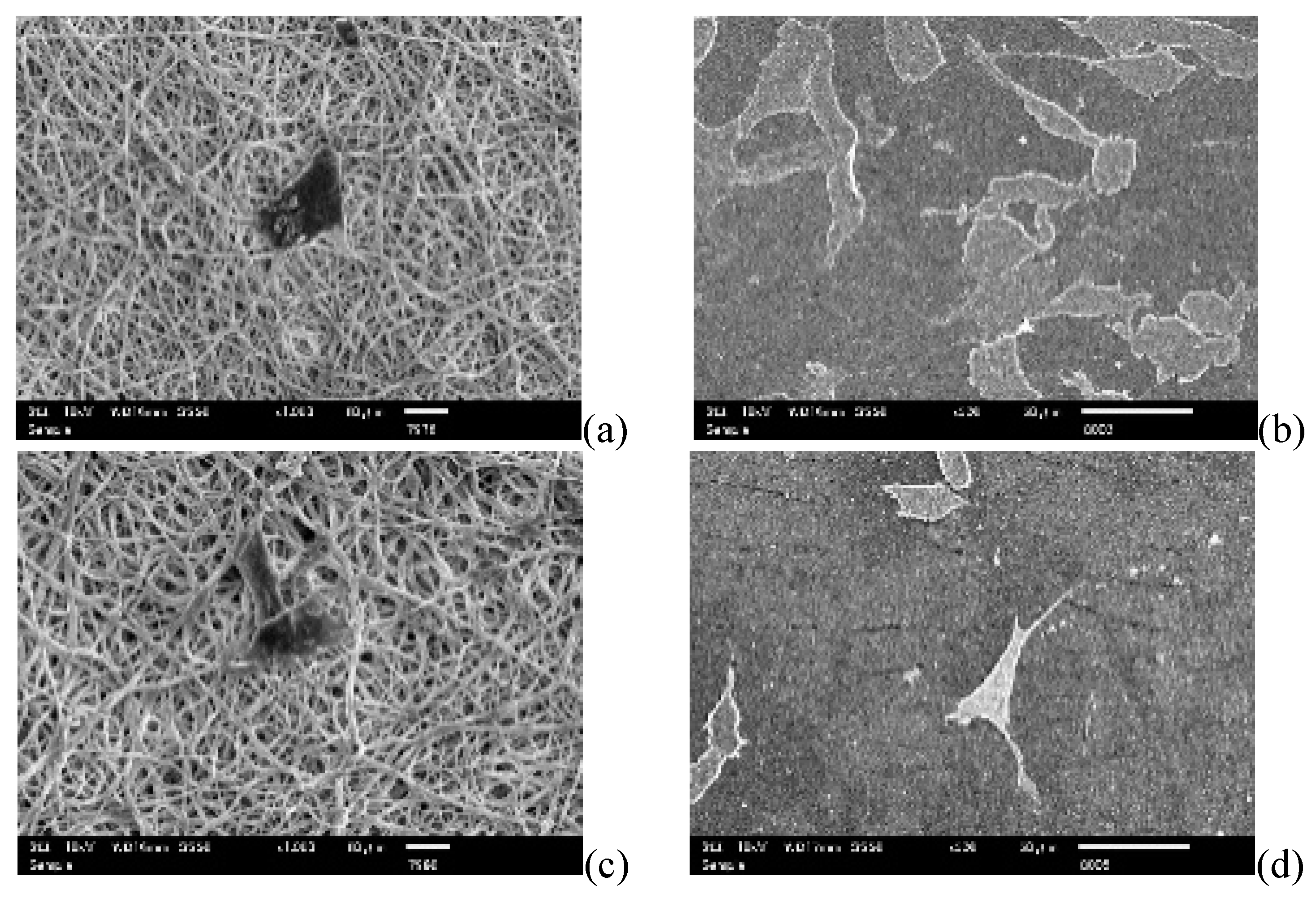
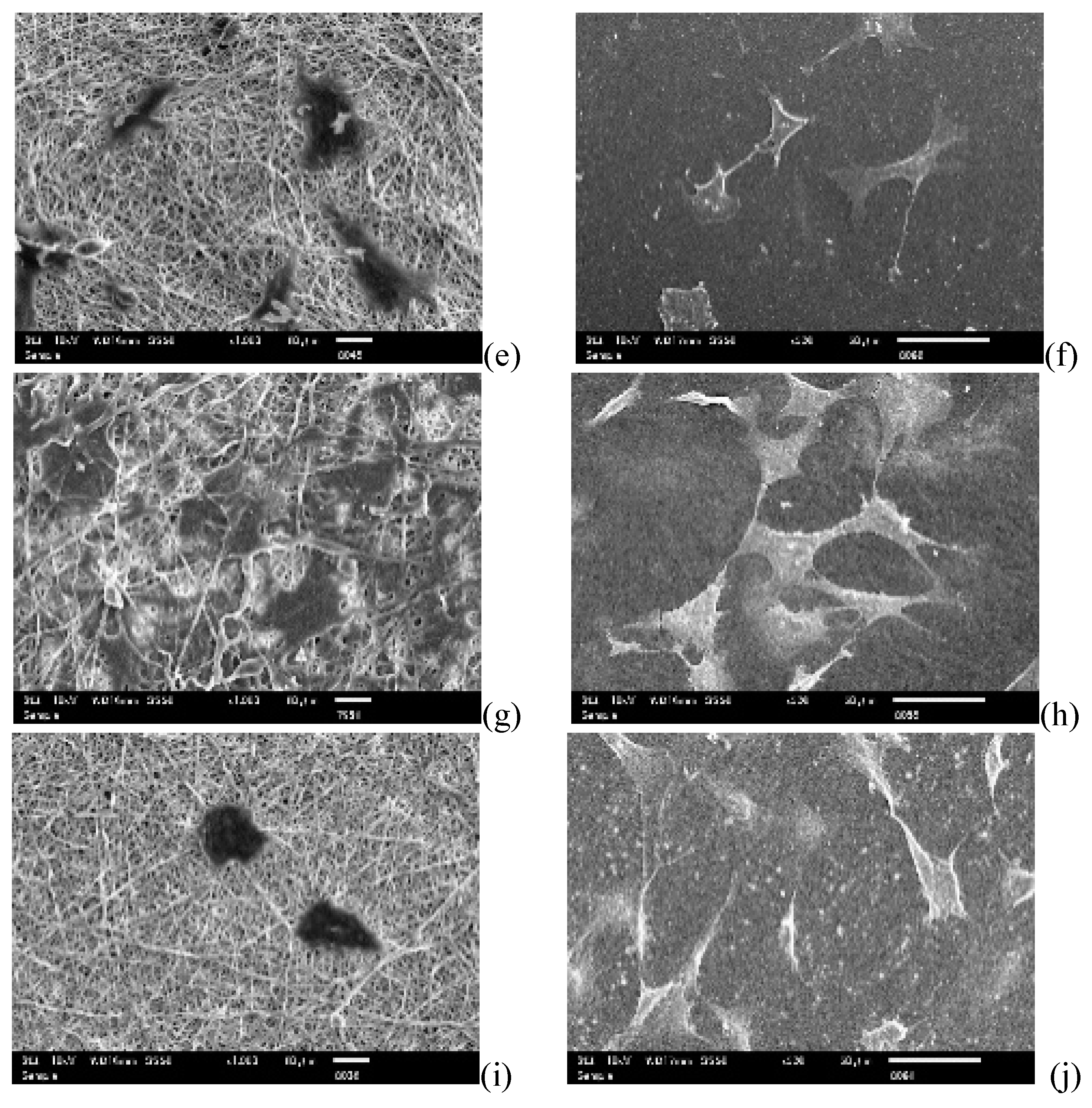
3.1. Morphology of PUSX Nanofibers Prepared for Physical and Biocompatability Evaluations
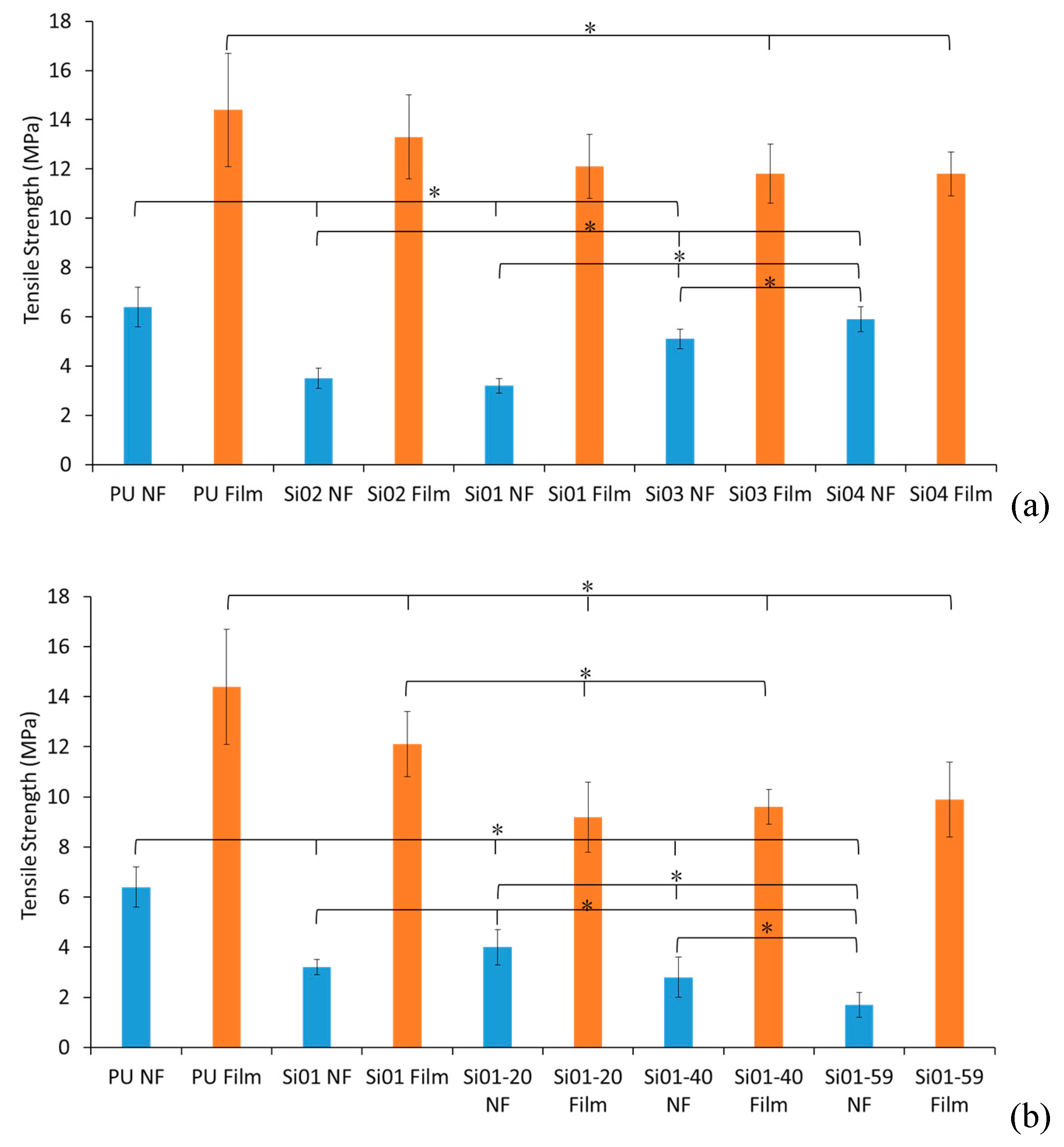
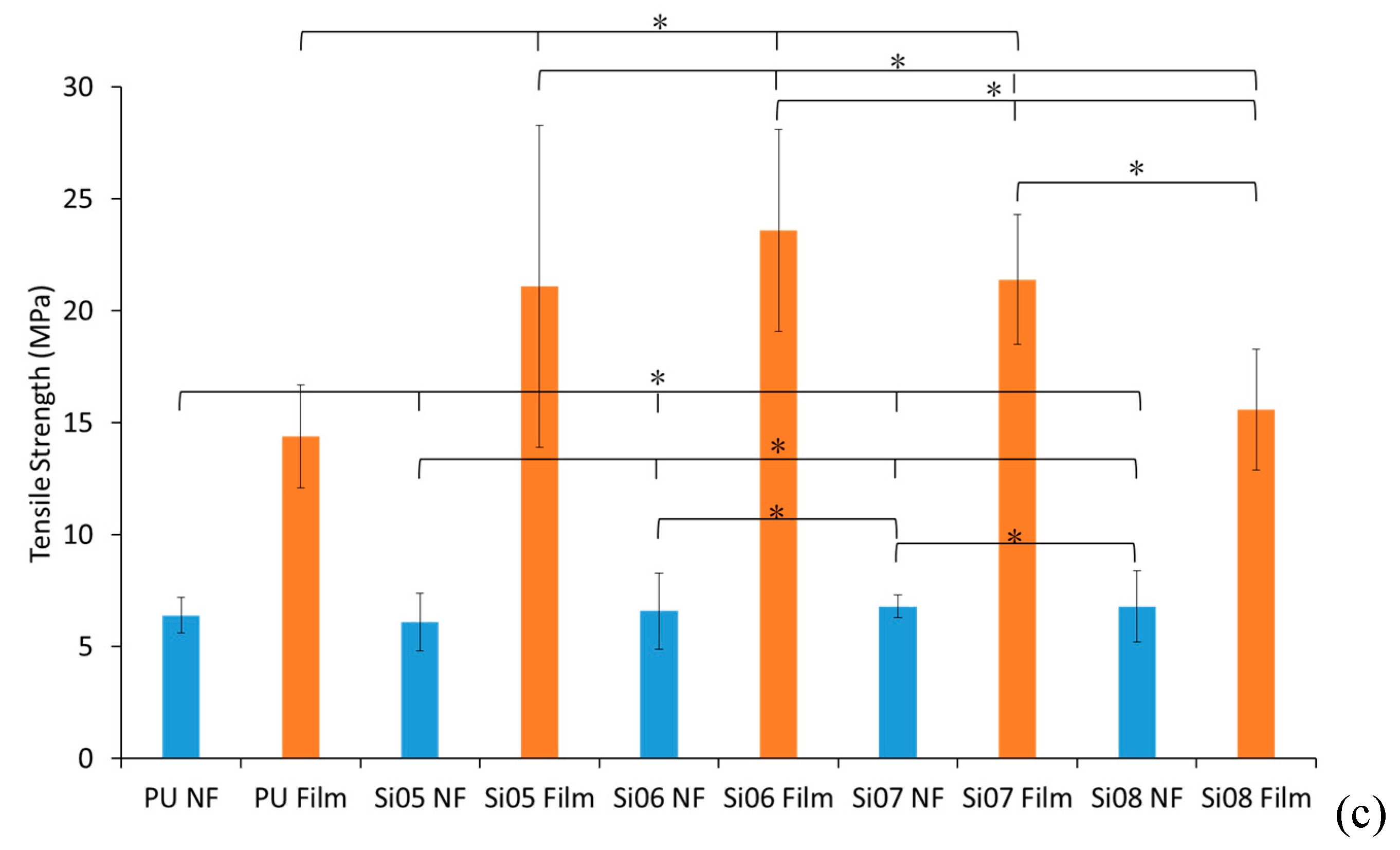
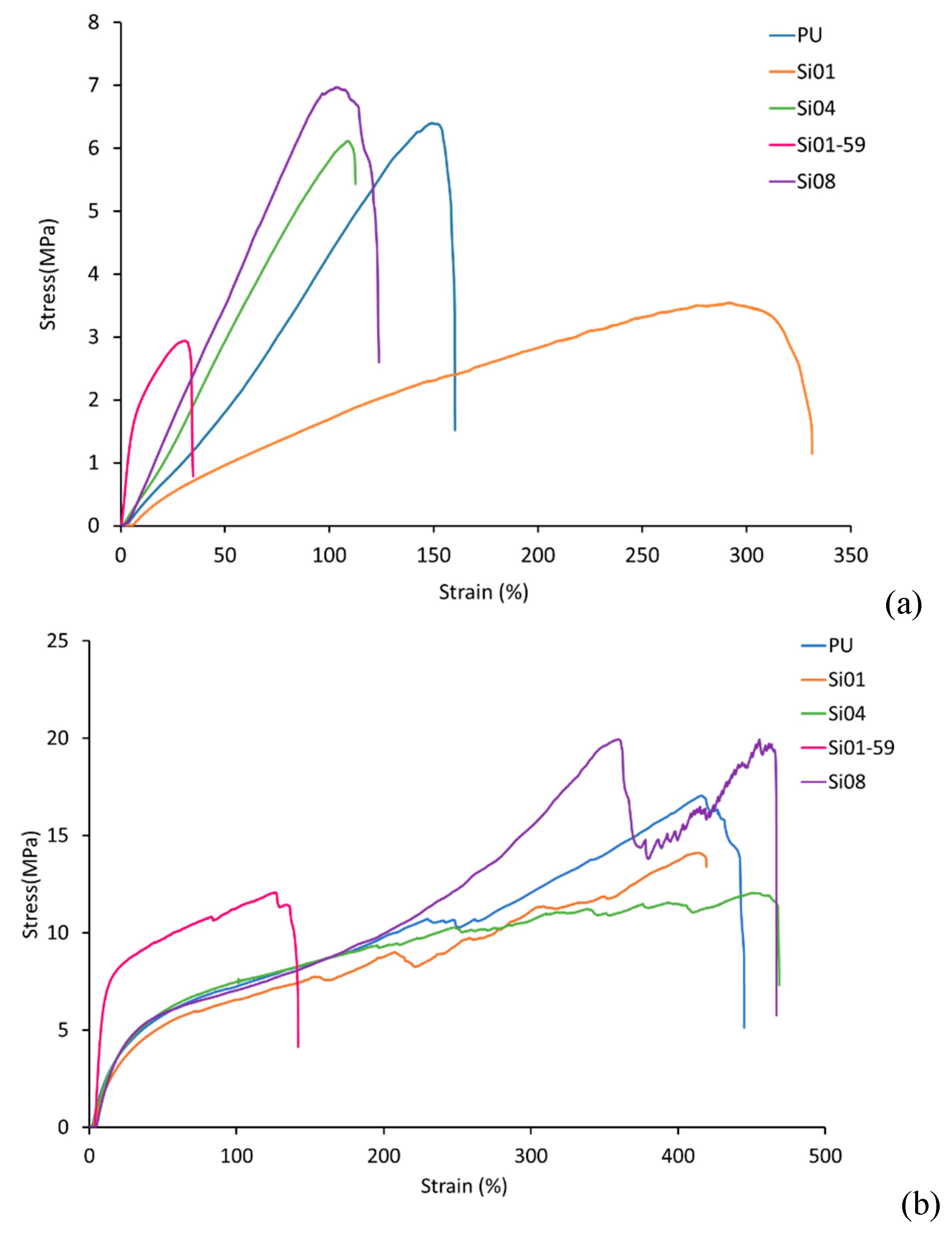
3.2. Mechanical Properties
3.2.1. Tensile Strength
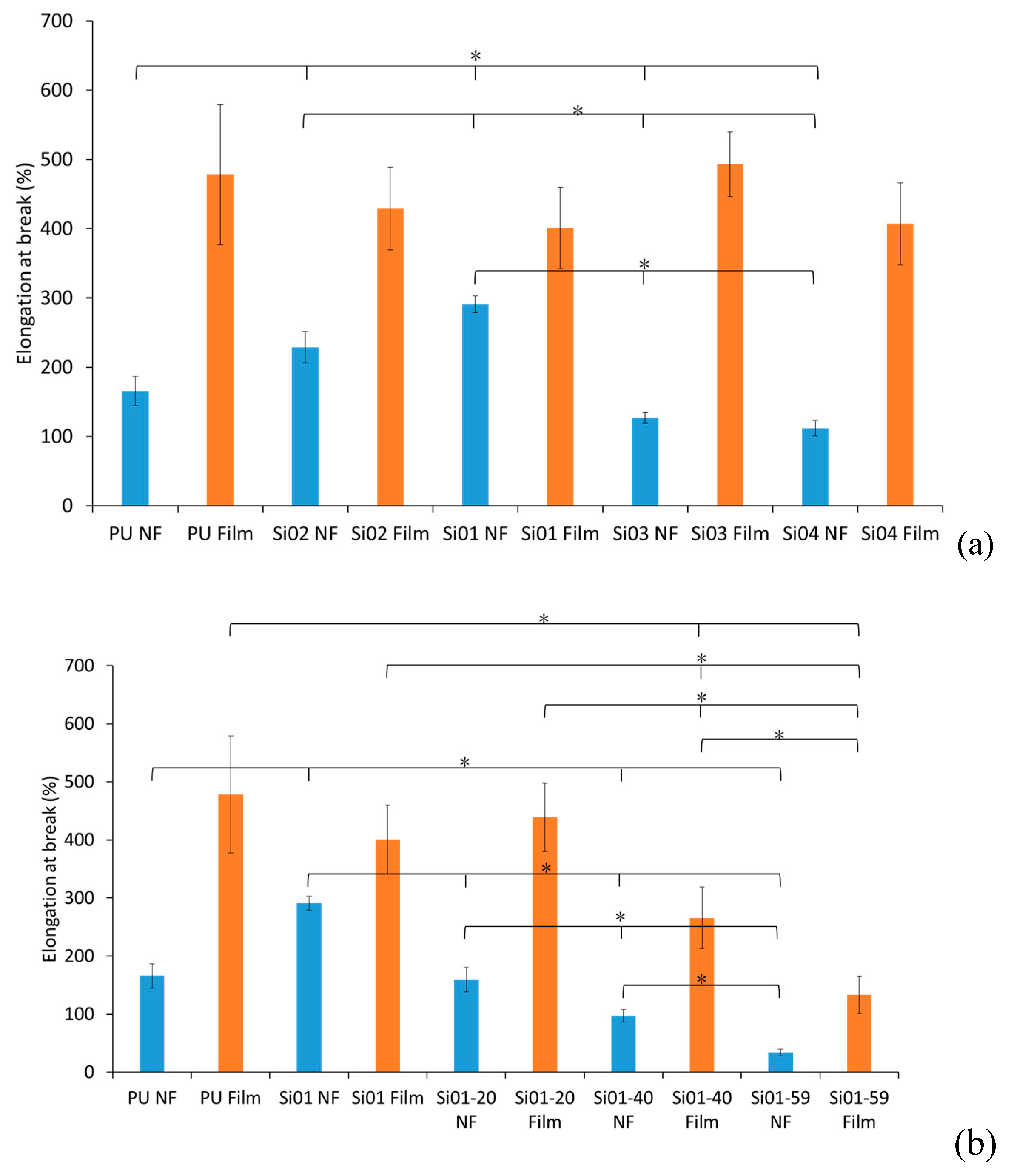
3.2.2. Elongation at Break
3.2.3. Young’s Modulus
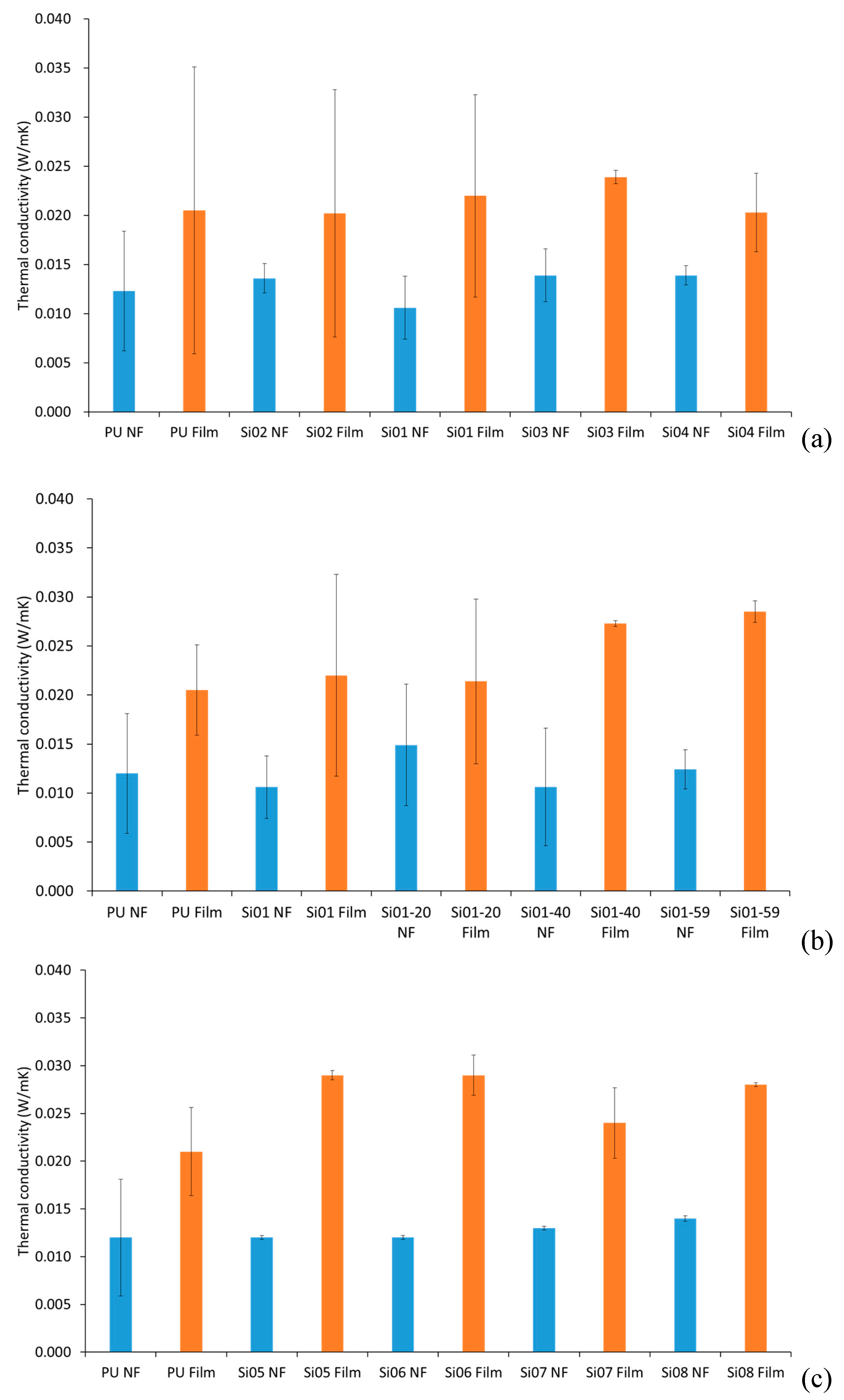
3.3. Thermal Conductivity
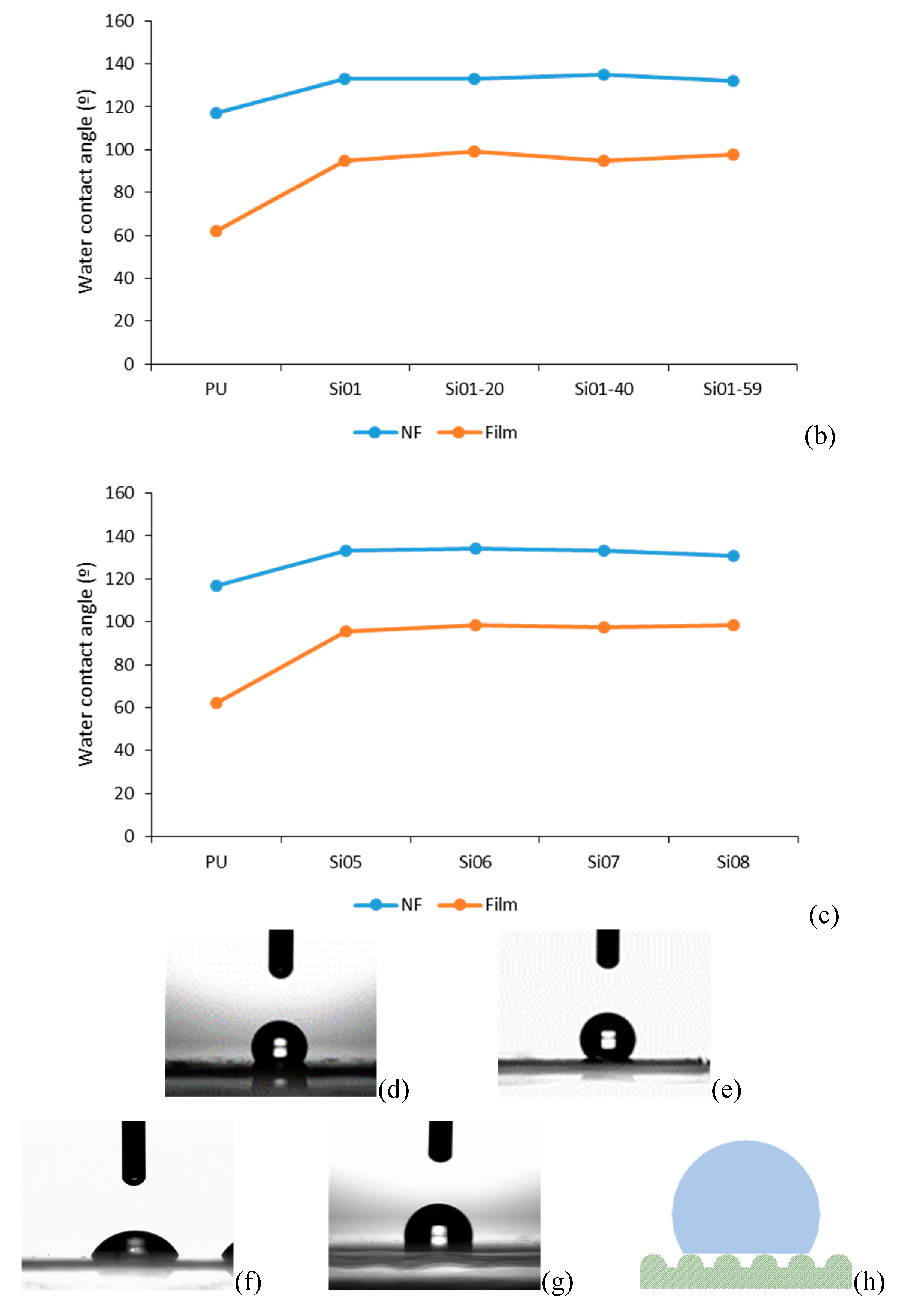
3.4. Water Retention and Water Contact Angle
3.4.1. Water Retention
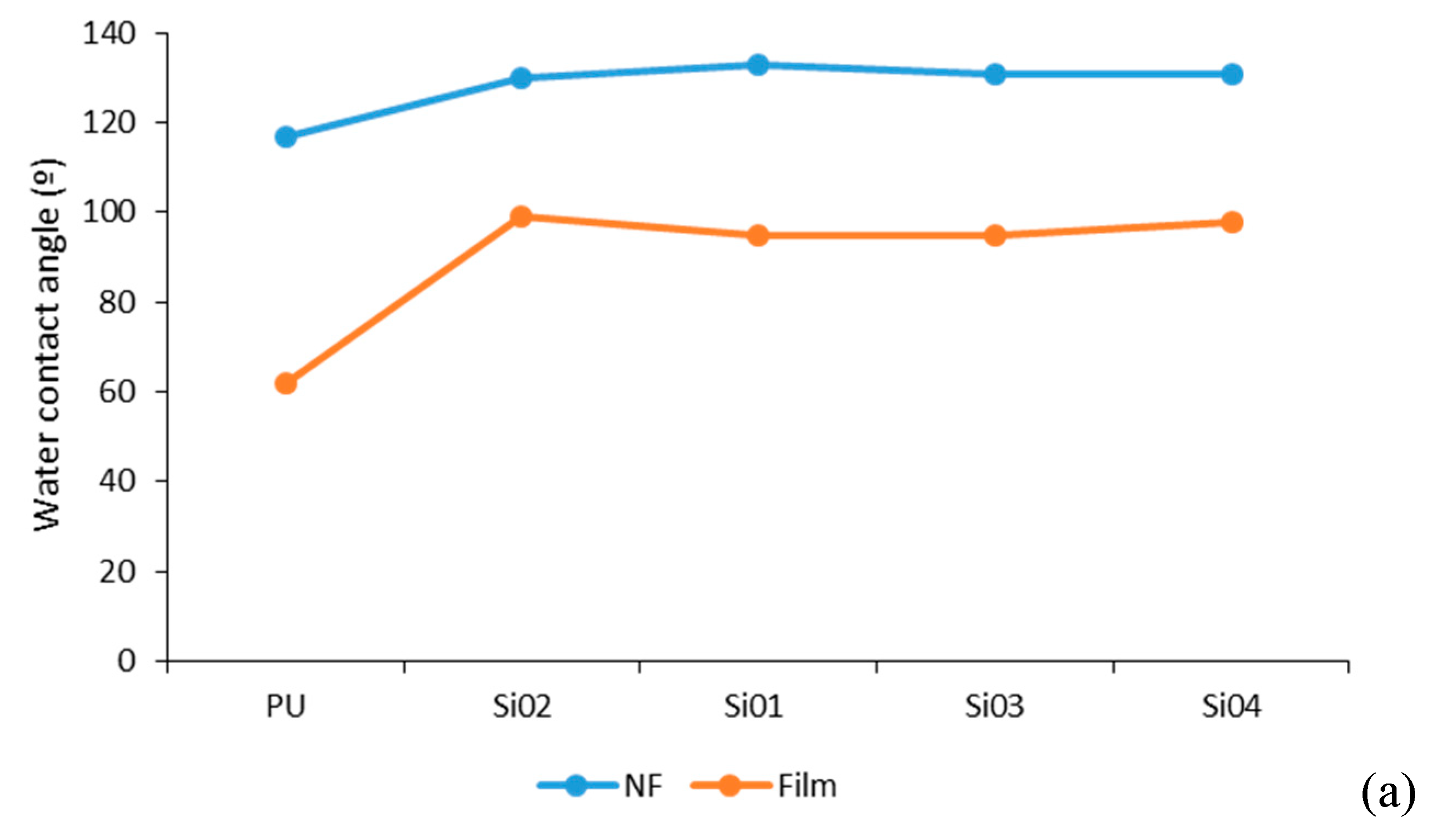
3.4.2. Water Contact Angle
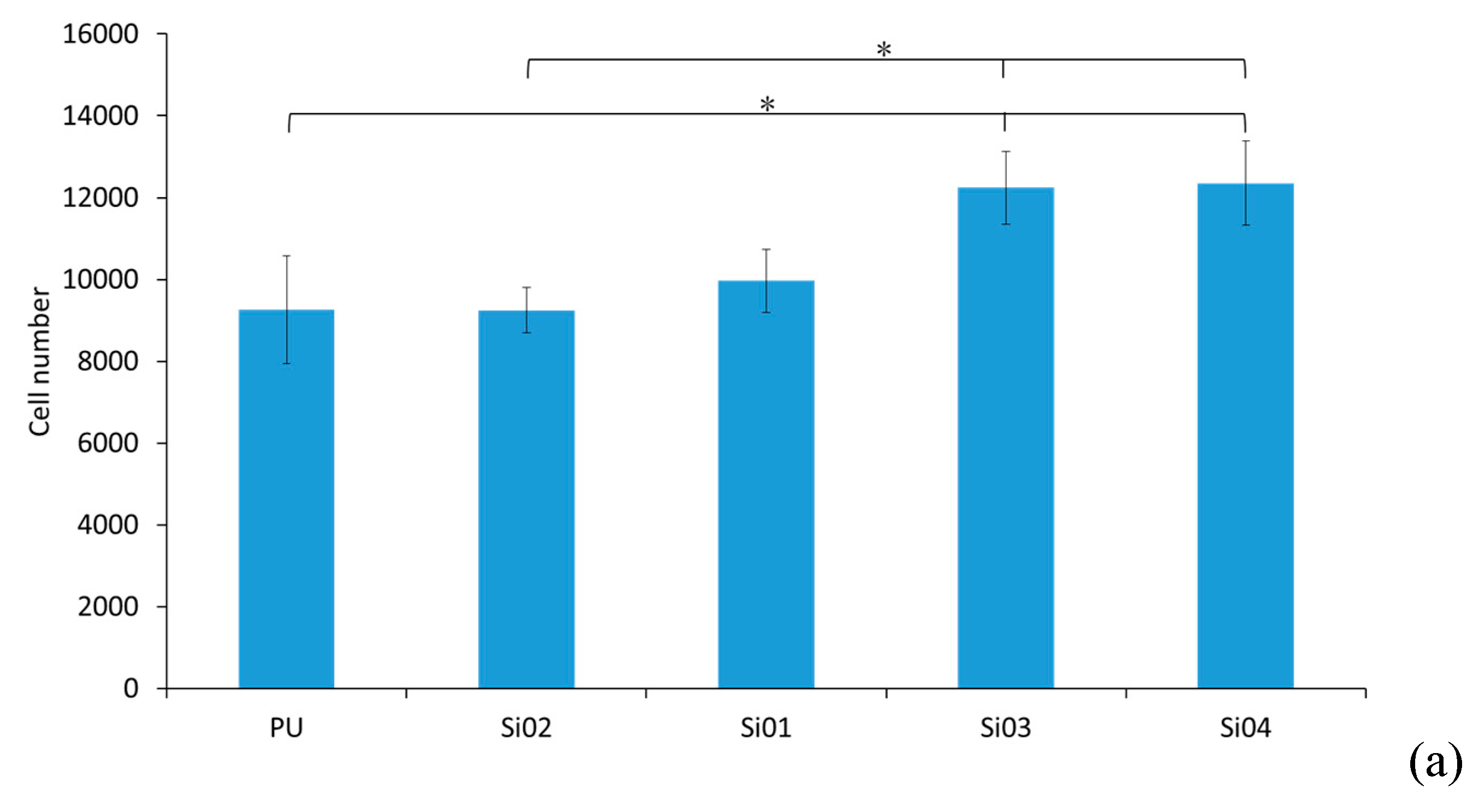
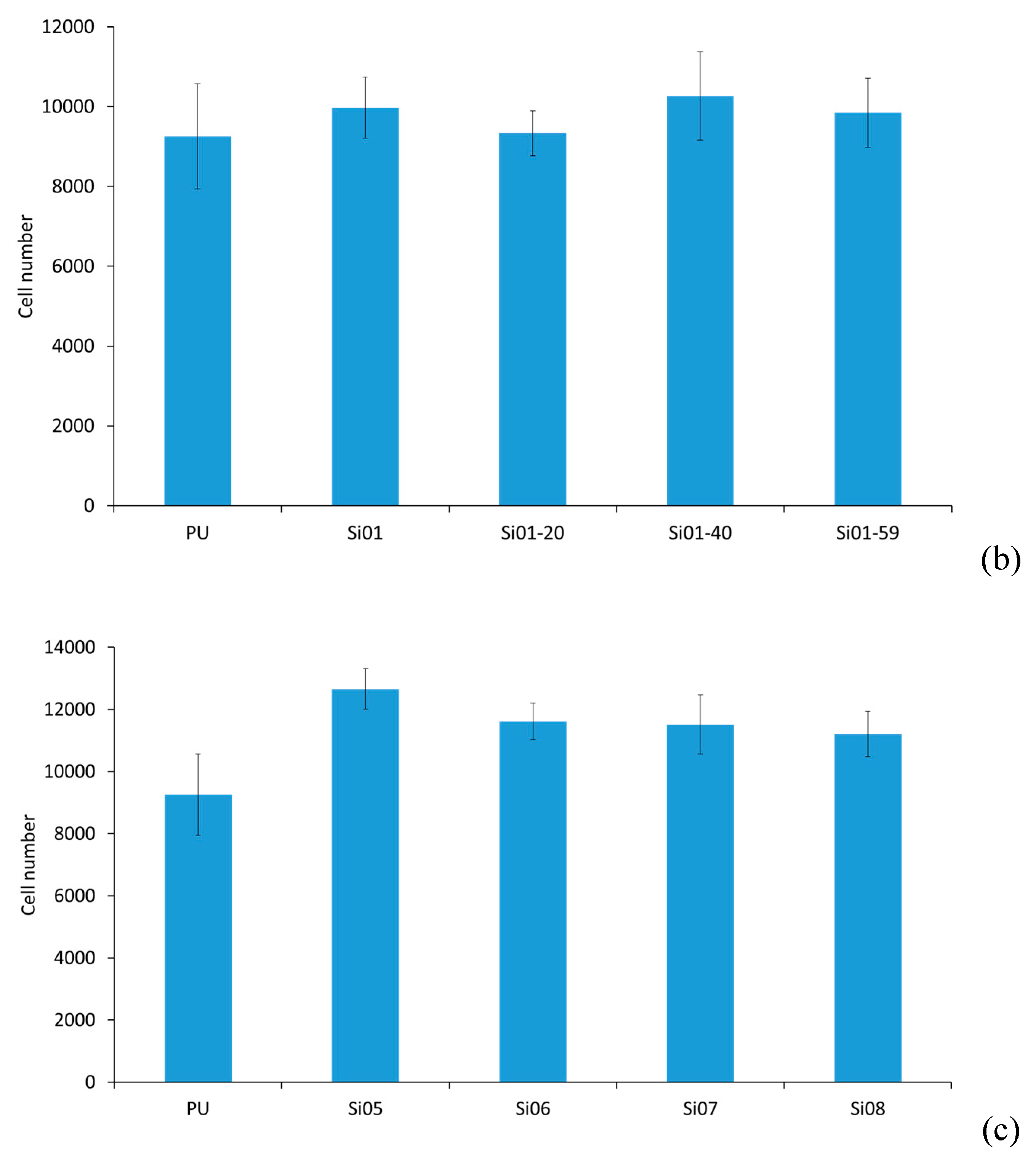
3.5. Cell Culture Studies
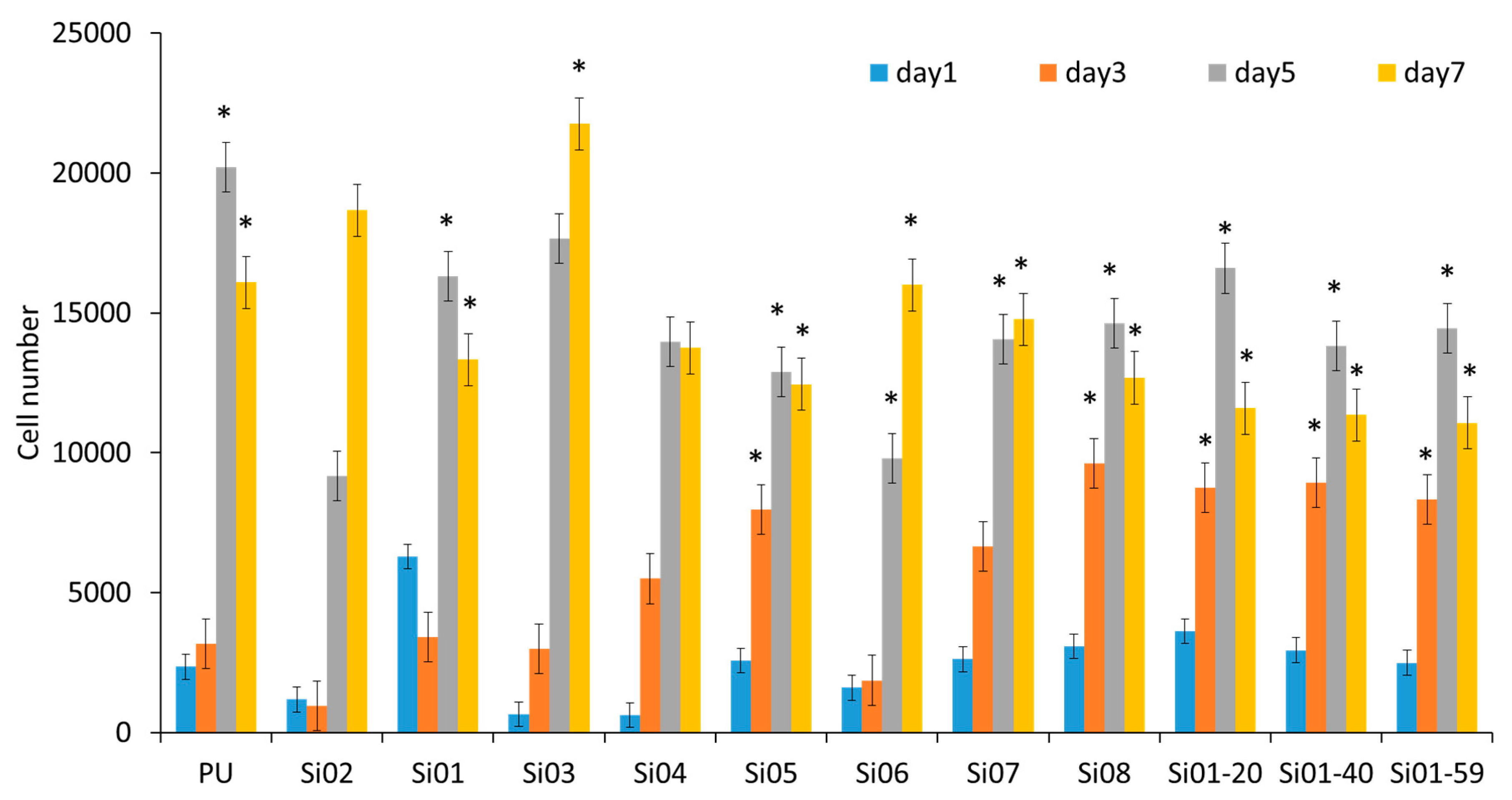
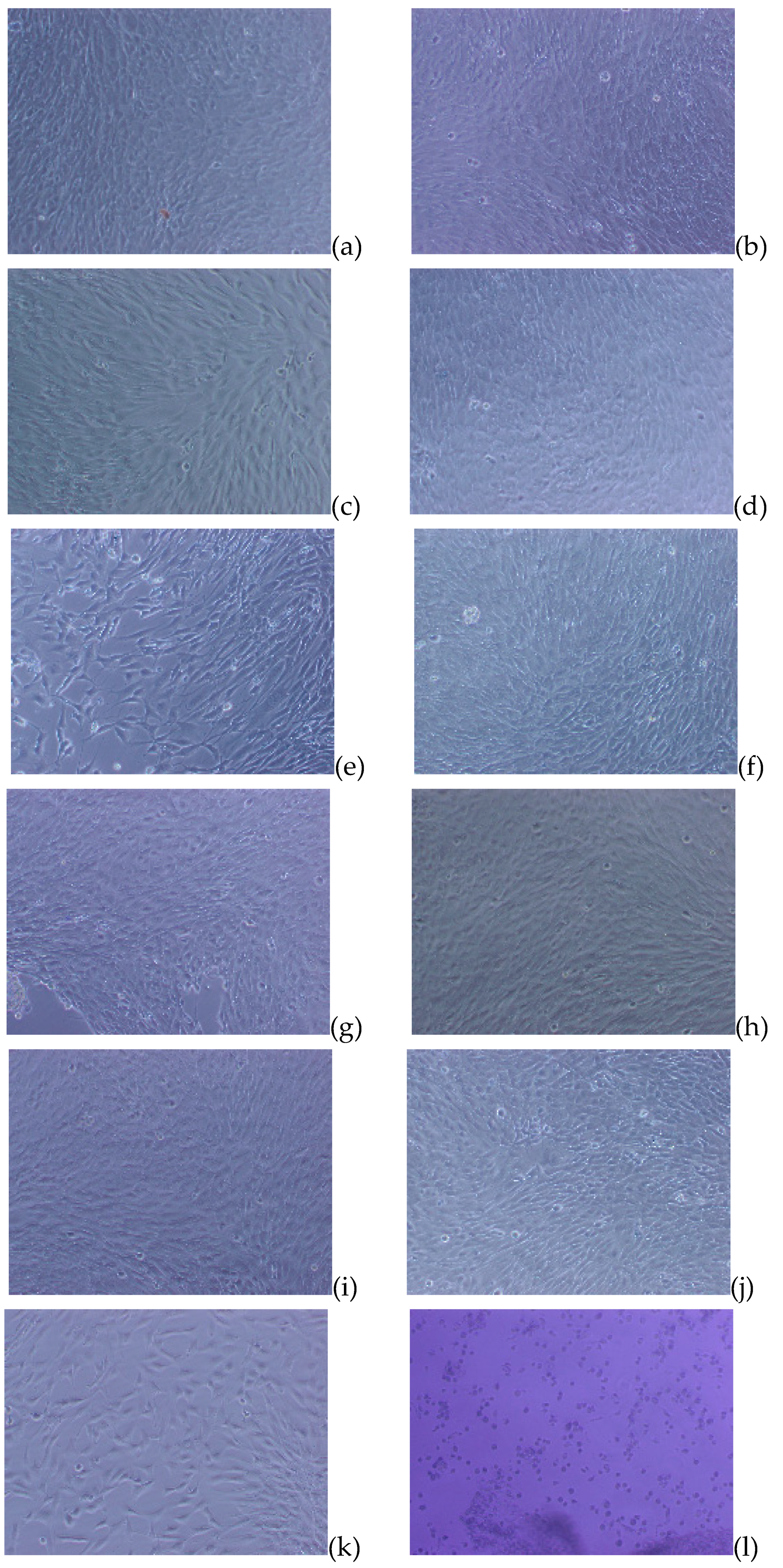
3.5.1. Cell Attachment and Cell Proliferation
3.5.2. Toxicity Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Viness, P.; Clare, D.; Yahya, E.C.; Charu, T.; Lomas, T.; Pradeep, K.; Lisa, C.T.; Valence, M.K.N. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Wang, P.W.; Wei, B.; Mo, X.M.; Cui, F.Z. Electrospun collagen–chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010, 6, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Wenying, L.; Stavros, T.; Younan, X. Electrospun Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar]
- Kiliç, E.; Yakar, A.; Pekel, B.N. Preparation of electrospun polyurethane nanofiber mats for the release of doxorubicine. J. Mater. Sci. Mater. Med. 2018, 29, 8. [Google Scholar] [CrossRef] [PubMed]
- Lionelli, G.T.; Lawrence, W.T. Wound dressing. Surg. Clin. N. Am. 2003, 83, 617–638. [Google Scholar] [CrossRef]
- Thomas, S. Wound management and dressings. Pharm. P 1990, 1, 37–57. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Chuan, Y.; Rino, O.; Mikihisa, K.; Toshihisa, T.; Hatsuhiko, H.; Masaki, T.; Hiromasa, S.; Shota, I.; Yoshitaka, K. Electrospinning of block and graft type silicone modified polyurethane nanofibers. Nanomaterials 2019, 9, 34. [Google Scholar] [CrossRef]
- ISO10993-5, Biological Evaluation of Medical Devices, Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/obp/ui/#iso:std:iso:10993:-5:ed-3:v1:en (accessed on 4 March 2019).
- Quatela, V.C.; Chow, J. Synthetic facial implants. Facial Plast. Surg. Clin. N. Am. 2008, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.; Kobe, K.; Hyakusoku, H.; Uekusa, K.; Hirakawa, K.; Ohno, Y. Experimental analysis of silicone leakage. J. Nippon Med. Sch. 2009, 76, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Barry, W.; Hansen, D.L.; Burrell, R.E. The comparative efficacy of two antimicrobial barrier dressings: In vitro examination of two controlled release silver dressings. Wounds 1998, 10, 179–188. [Google Scholar]
- Yin, H.Q.; Langford, R.; Burrell, R.E. Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. J. Burn Care Rehabil. 1999, 20, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler, P. Understanding the Effects of Bacterial Communities and Biofilms on Wound Healing. Available online: http://www.worldwidewounds.com/2004/july/Percival/Community-Interactions-Wounds.html (accessed on 4 March 2019).
- Lakshmi, R.L.; Shalumon, K.T.; Sreeja, V.N.; Jayakumar, R.; Nair, S.V. Preparation of Silver Nanoparticles Incorporated Electrospun Polyurethane Nano-fibrous Mat for Wound Dressing. J. Macromol. Sci. A Pure Appl. Chem. 2010, 47, 1012–1018. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Barakat, N.A.; Pichiah, P.B.; Gnanasekaran, G.; Nirmala, R.; Cha, Y.S.; Jung, C.H.; El-Newehy, M.; Kim, H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane–dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr. Polym. 2012, 90, 1786–1793. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. J. Biomater. 2005, 26, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, C.; Ke, Q.; He, C.; Wang, H.; Mo, X. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/collagen compound nanofibers for tissue engineering applications. Colloids Surf. B Biointerfaces 2010, 79, 315–325. [Google Scholar] [CrossRef] [PubMed]


| Sample | PU | Si01 | Si02 | Si03 | Si04 | Si01-20 | Si01-40 | Si01-59 | Si05 | Si06 | Si07 | Si08 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Structure | × | Block Type | Graft Type | |||||||||
| Mw (105) | 1.48 | 1.69 | 1.39 | 1.59 | 1.66 | 1.74 | 2.01 | 2.33 | 1.56 | 1.61 | 1.57 | 1.62 |
| Mn (105) | 0.75 | 0.87 | 0.73 | 0.79 | 0.75 | 0.88 | 1.02 | 1.11 | 0.71 | 0.70 | 0.72 | 0.78 |
| Silicone Concentration (wt%) | 0 | 10 | 10 | 10 | 10 | 20 | 40 | 59 | 10 | 10 | 10 | 10 |
| Silicone Chain Length (n) | × | 20 | 10 | 30 | 50 | 20 | 20 | 20 | 10 | 25 | 30 | 120 |
| Sample | PU | Si01 | Si02 | Si03 |
|---|---|---|---|---|
| SEM |  |  |  |  |
| Average Diameter (nm) | 720 | 636 | 690 | 548 |
| Sample | Si04 | Si01-20 | Si01-40 | Si01-59 |
| SEM |  |  |  |  |
| Average Diameter (nm) | 440 | 531 | 402 | 471 |
| Sample | Si05 | Si06 | Si07 | Si08 |
| SEM |  |  |  |  |
| Average Diameter (nm) | 564 | 544 | 456 | 456 |
| Block-Type PUSX with Different Silicone Chain Lengths | PU | Si02 | Si01 | Si03 | Si04 |
|---|---|---|---|---|---|
| Nanofiber Water Retention (%) | 280.0 ± 40.0 | 27.0 ± 11.0 | 19.0 ± 8.1 | 11.0 ± 4.1 | 12.0 ± 2.5 |
| Film Water Retention (%) | 2.3 ± 1.5 | 2.7 ± 1.1 | 1.5 ± 0.2 | 5.8 ± 5.0 | 3.7 ± 1.1 |
| Block-type PUSX with Different Silicone Concentrations | PU | Si01 | Si01-20 | Si01-40 | Si01-59 |
| Nanofiber Water Retention (%) | 280.0 ± 40.0 | 19.0 ± 8.1 | 4.7 ± 3.1 | 2.7 ± 0.9 | 6.9 ± 6.0 |
| Film Water Retention (%) | 2.3 ± 1.5 | 1.5 ± 0.2 | 3.7 ± 0.7 | 2.7 ± 1.0 | 2.9 ± 2.3 |
| Graft-Type PUSX | PU | Si05 | Si06 | Si07 | Si08 |
| Nanofiber Water Retention (%) | 280.0 ± 40.0 | 169.0 ± 23.3 | 196.0 ± 28.8 | 165.0 ± 55.0 | 200.0 ± 13.5 |
| Film Water Retention (%) | 2.3 ± 1.5 | 2.3 ± 2.6 | 6.6 ± 7.5 | 6.8 ± 3.8 | 1.7 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Rozet, S.; Okamoto, R.; Kondo, M.; Tamada, Y.; Tanaka, T.; Hattori, H.; Tanaka, M.; Sato, H.; Iino, S. Physical Properties and In Vitro Biocompatible Evaluation of Silicone-Modified Polyurethane Nanofibers and Films. Nanomaterials 2019, 9, 367. https://doi.org/10.3390/nano9030367
Yin C, Rozet S, Okamoto R, Kondo M, Tamada Y, Tanaka T, Hattori H, Tanaka M, Sato H, Iino S. Physical Properties and In Vitro Biocompatible Evaluation of Silicone-Modified Polyurethane Nanofibers and Films. Nanomaterials. 2019; 9(3):367. https://doi.org/10.3390/nano9030367
Chicago/Turabian StyleYin, Chuan, Sélène Rozet, Rino Okamoto, Mikihisa Kondo, Yasushi Tamada, Toshihisa Tanaka, Hatsuhiko Hattori, Masaki Tanaka, Hiromasa Sato, and Shota Iino. 2019. "Physical Properties and In Vitro Biocompatible Evaluation of Silicone-Modified Polyurethane Nanofibers and Films" Nanomaterials 9, no. 3: 367. https://doi.org/10.3390/nano9030367
APA StyleYin, C., Rozet, S., Okamoto, R., Kondo, M., Tamada, Y., Tanaka, T., Hattori, H., Tanaka, M., Sato, H., & Iino, S. (2019). Physical Properties and In Vitro Biocompatible Evaluation of Silicone-Modified Polyurethane Nanofibers and Films. Nanomaterials, 9(3), 367. https://doi.org/10.3390/nano9030367