Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bilayer Systems Preparation
2.3. Characterization Techniques
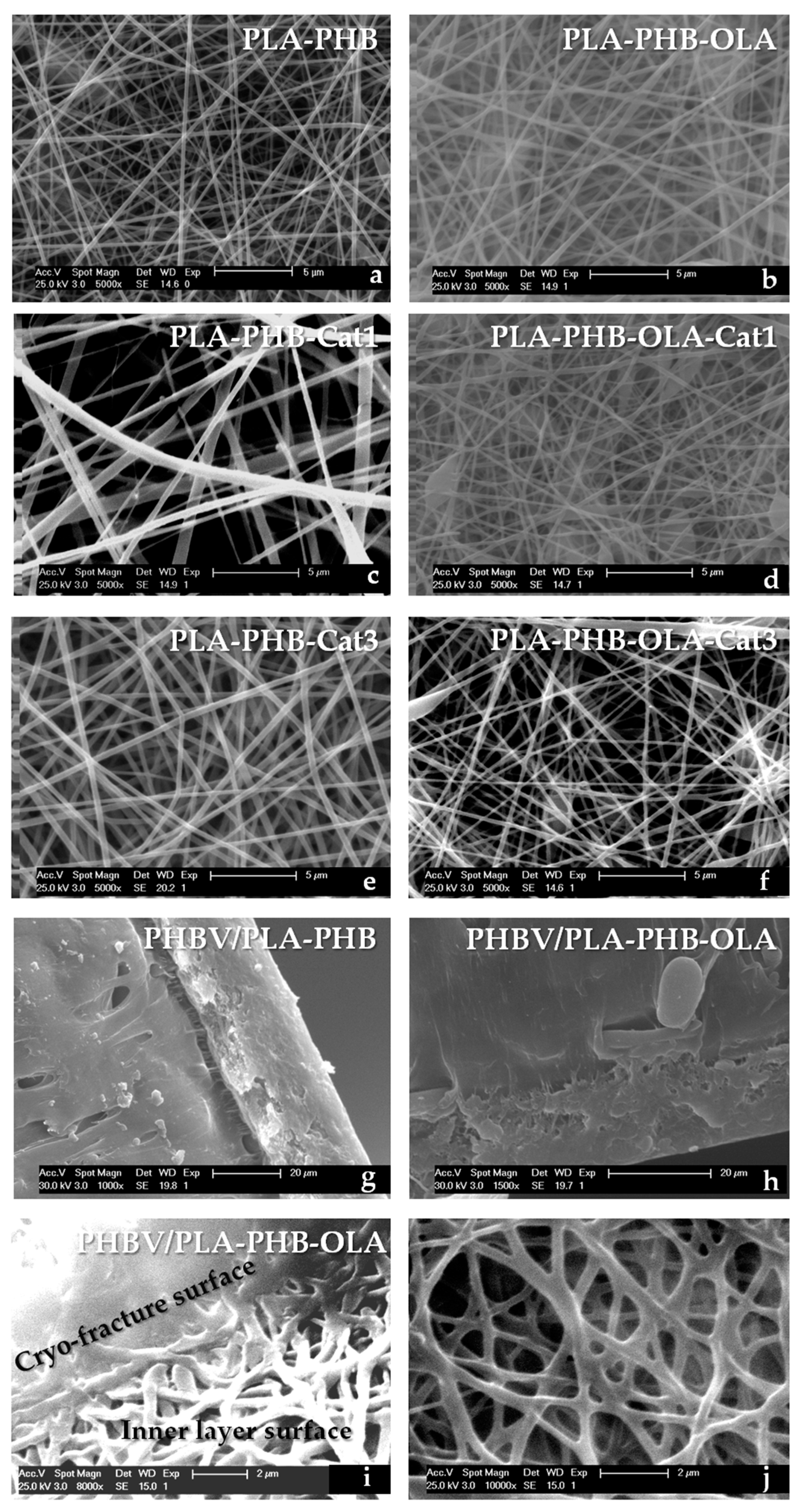
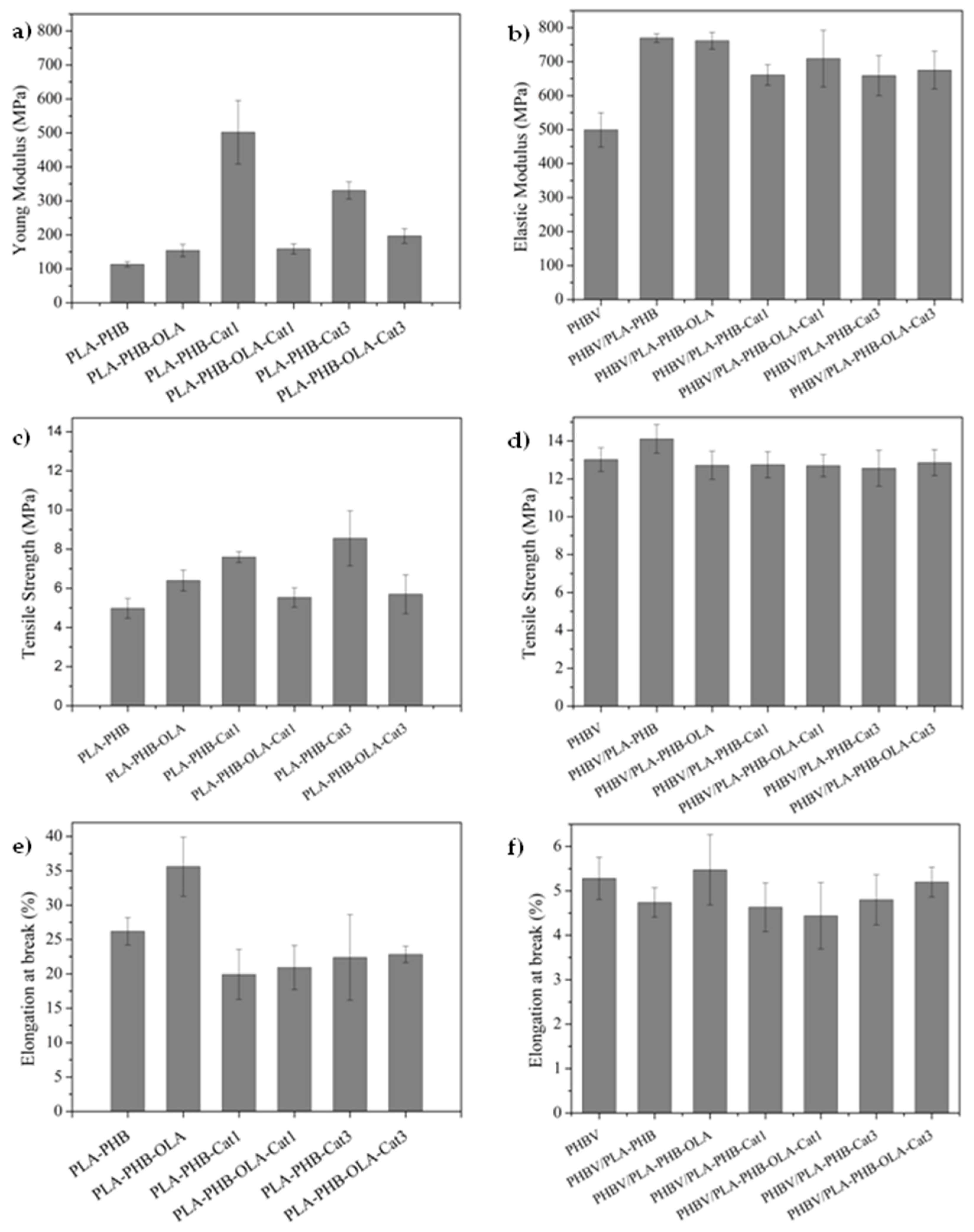
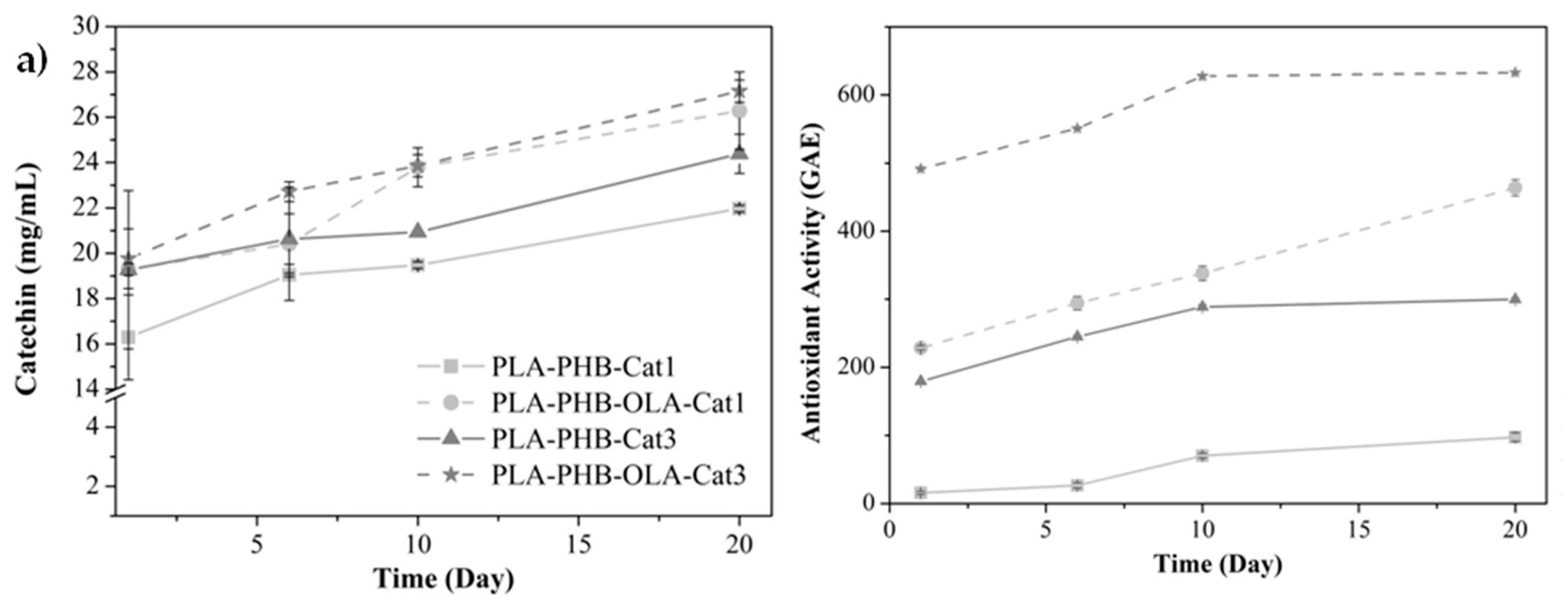
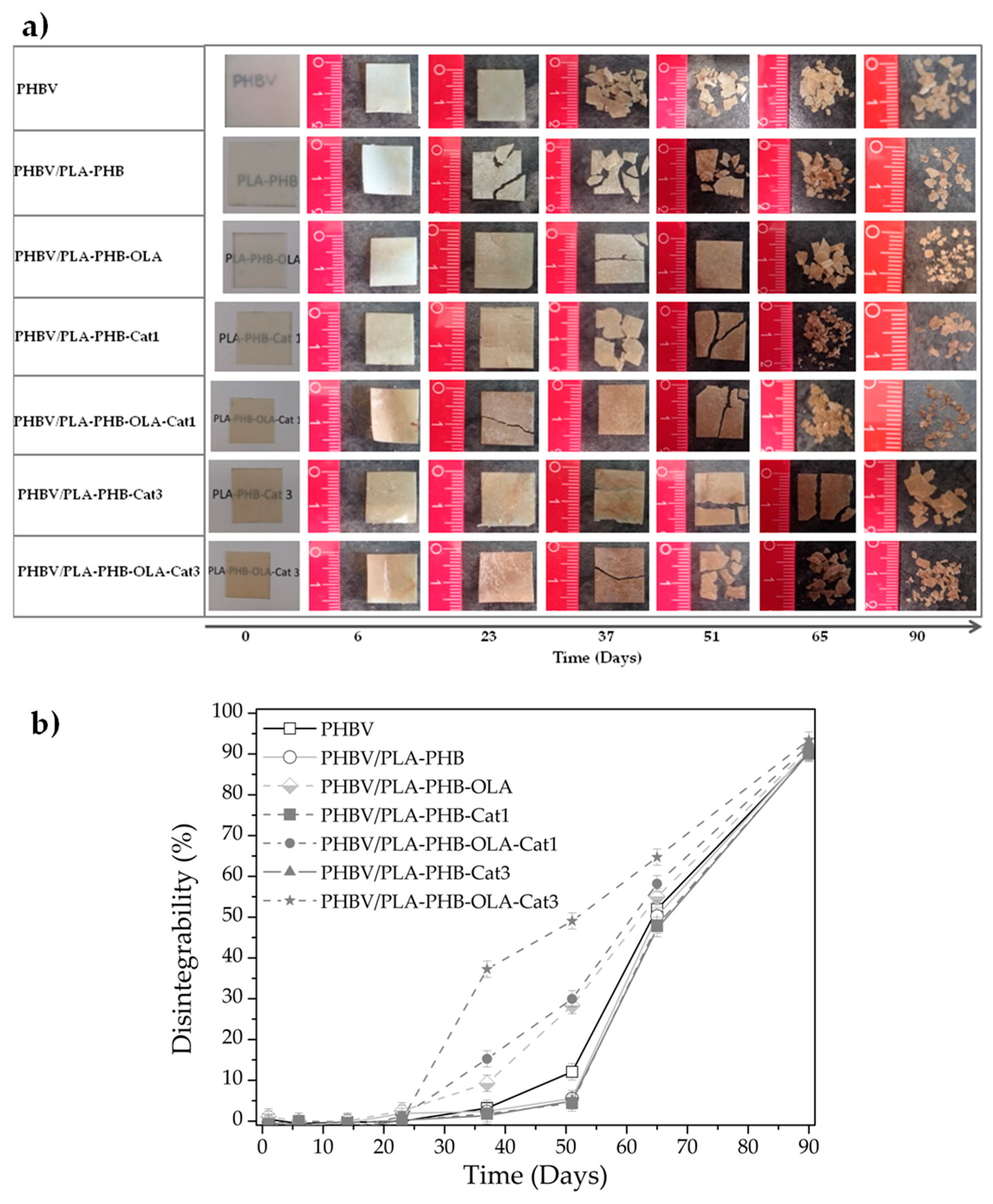
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vera, P.; Canellas, E.; Nerín, C. New antioxidant multilayer packaging with nanoselenium to enhance the shelf-life of market food products. Nanomaterials 2018, 8, 837. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Garcia, A.; Sonseca, A.; Arrieta, M.P.; Yusef, M.; López, D.; Gimenez, E.; Kenny, J.M.; Peponi, L. Electrospun fibers based on biopolymers. In Advanced Surface Engineering Materials; Tiwari, A., Wang, R., Wei, B., Eds.; Scrivener Publishing LLC: Salem, MA, USA, 2016; pp. 385–438. [Google Scholar]
- Castro-López, M.M.; López de Dicastillo, C.; López-Vilariño, J.M.; González-Rodríguez, M.A.V. Improving the capacity of polypropylene to be used in antioxidant active films: Incorporation of plasticizer and natural antioxidants. J. Agric. Food Chem. 2013, 61, 8462–8470. [Google Scholar]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of the electrohydrodynamic process to develop active/bioactive bilayer films for food packaging applications. Food Hydrocoll. 2016, 55, 11–18. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Castro-López, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized poly(lactic acid)-poly(hydroxybutyrate) (PLA-PHB) blends incorporated with catechin intended for active food-packaging applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Effect of chitosan and catechin addition on the structural, thermal, mechanical and disintegration properties of plasticized electrospun PLA-PHB biocomposites. Polym. Degrad. Stab. 2016, 132, 145–156. [Google Scholar] [CrossRef]
- Castro Mayorga, J.L.; Fabra Rovira, M.J.; Cabedo Mas, L.; Sánchez Moragas, G.; Lagarón Cabello, J.M. Antimicrobial nanocomposites and electrospun coatings based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018, 135, 45673. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Torres-Giner, S.; Pardo-Figuerez, M.; Álvarez-Láinez, M.; Lagaron, J.M. Superhydrophobic bilayer coating based on annealed electrospun ultrathin poly(ε-caprolactone) fibers and electrosprayed nanostructured silica microparticles for easy emptying packaging applications. Coatings 2018, 8, 173. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun PLA-PHB blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Echegoyen, Y.; Teruel-Juanes, R.; Badia, J.; Ribes-Greus, A.; Lagaron, J. Electrospun poly (ethylene-co-vinyl alcohol)/graphene nanoplatelets composites of interest in intelligent food packaging applications. Nanomaterials 2018, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Gimenez, E.; Lagarón, J.M. Characterization of the morphology and thermal properties of zein prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 2008, 22, 601–614. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Lopez-Rubio, A. Nanotechnology for bioplastics: Opportunities, challenges and strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Burgos, N.; Peltzer, M.A.; López, J.; Peponi, L. Nanocellulose-based polymeric blends for food packaging applications. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; Puglia, D., Fortunati, E., Kenny, J.M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 205–252. [Google Scholar]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer structures based on annealed electrospun biopolymer coatings of interest in water and aroma barrier fiber-based food packaging applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fabra, M.J.; Castro-Mayorga, J.L.; Bourbon, A.I.; Pastrana, L.M.; Vicente, A.A.; Lagaron, J.M. Use of electrospinning to develop antimicrobial biodegradable multilayer systems: Encapsulation of cinnamaldehyde and their physicochemical characterization. Food Bioprocess Technol. 2016, 9, 1874–1884. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Patiño, C.; Galotto, M.J.; Palma, J.L.; Alburquenque, D.; Escrig, J. Novel antimicrobial titanium dioxide nanotubes obtained through a combination of atomic layer deposition and electrospinning technologies. Nanomaterials 2018, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López de Dicastillo, C.; Garrido, L.; Roa, K.; Galotto, M.J. Electrospun pva fibers loaded with antioxidant fillers extracted from durvillaea antarctica algae and their effect on plasticized PLA bionanocomposites. Eur. Polym. J. 2018, 103, 145–157. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Fabra, M.; Cabedo, L.; Lagaron, J. On the use of the electrospinning coating technique to produce antimicrobial polyhydroxyalkanoate materials containing in situ-stabilized silver nanoparticles. Nanomaterials 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Sessini, V.; Peponi, L. Biodegradable poly(ester-urethane) incorporated with catechin with shape memory and antioxidant activity for food packaging. Eur. Polym. J. 2017, 94, 111–124. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Fernández-Torres, A.; Peponi, L. Humidity-activated shape memory effect on plasticized starch-based biomaterials. Carbohydr. Polym. 2018, 179, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sessini, V.; Arrieta, M.P.; Kenny, J.M.; Peponi, L. Processing of edible films based on nanoreinforced gelatinized starch. Polym. Degrad. Stab. 2016, 132, 157–168. [Google Scholar] [CrossRef]
- Han, J.; Shin, S.H.; Park, K.M.; Kim, K.M. Characterization of physical, mechanical, and antioxidant properties of soy protein-based bioplastic films containing carboxymethylcellulose and catechin. Food Sci. Biotechnol. 2015, 24, 939–945. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined effect of poly(hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Burgos, N.; Armentano, I.; Fortunati, E.; Dominici, F.; Luzi, F.; Fiori, S.; Cristofaro, F.; Visai, L.; Jiménez, A.; Kenny, J.M. Functional properties of plasticized bio-based poly(lactic acid)_poly(hydroxybutyrate) (PLA_PHB) films for active food packaging. Food Bioprocess Technol. 2017, 10, 770–780. [Google Scholar] [CrossRef]
- Requena, R.; Jiménez, A.; Vargas, M.; Chiralt, A. Poly[(3-hydroxybutyrate)-co-(3-hydroxyvalerate)] active bilayer films obtained by compression moulding and applying essential oils at the interface. Polym. Int. 2016, 65, 883–891. [Google Scholar] [CrossRef]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.S. Processing and characterization of microcellular PHBV/PBAT blends. Polym. Eng. Sci. 2010, 50, 1440–1448. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Lee, J.; Pilla, S.; Clemons, C.; Gong, S.; Turng, L.-S. Processing and characterization of solid and microcellular PHBV/PBAT blend and its RWF/nanoclay composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 982–990. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Sustainable green composites: Value addition to agricultural residues and perennial grasses. ACS Sustain. Chem. Eng. 2013, 1, 325–333. [Google Scholar] [CrossRef]
- Fiori, S.; Ara, P. Method for Plasticizing Lactic Acid Polymers. Patent WO 2009/092825, 25 January 2008. [Google Scholar]
- Mujica-Garcia, A.; Navarro-Baena, I.; Kenny, J.M.; Peponi, L. Influence of the processing parameters on the electrospinning of biopolymeric fibers. J. Renew. Mater. 2014, 2, 23–34. [Google Scholar] [CrossRef]
- European Commission. No. 10/2011 of 14 january 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, L12, 1–89. [Google Scholar]
- López de Dicastillo, C.; Castro-López, M.M.; Lasagabaster, A.; Vilariño, J.M.L.; Rodríguez, M.V.G. Interaction and release of catechin from anhydride maleic-grafted polypropylene films. ACS Appl. Mater Interfaces. 2013, 5, 3281–3289. [Google Scholar]
- UNE-EN ISO. Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test; UNE-EN ISO-20200:2015; AENOR: Madrid, España, 2016. [Google Scholar]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Biodegradable electrospun bionanocomposite fibers based on plasticized PLA–PHB blends reinforced with cellulose nanocrystals. Ind. Crop. Prod. 2016, 93, 290–301. [Google Scholar] [CrossRef]
- Weast, R.C.; Astle, M.J.; Beyer, W.H. Crc Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1989; Volume 1990. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Calvao, P.S.; Chenal, J.M.; Gauthier, C.; Demarquette, N.R.; Bogner, A.; Cavaille, J.Y. Understanding the mechanical and biodegradation behaviour of poly (hydroxybutyrate)/rubber blends in relation to their morphology. Polym. Int. 2012, 61, 434–441. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Burgos, N.; Tolaguera, D.; Fiori, S.; Jiménez, A. Synthesis and characterization of lactic acid oligomers: Evaluation of performance as poly(lactic acid) plasticizers. J. Polym. Environ. 2014, 22, 227–235. [Google Scholar] [CrossRef]
- Kang, J.H.; Chung, S.T.; Row, K.H. Comparison of experimental and calculated solubilities of (+)-catechin in green tea. J. Ind. Eng. Chem. 2002, 8, 354–358. [Google Scholar]
- Arrieta, M.P.; Peponi, L. Polyurethane based on PLA and PCL incorporated with catechin: Structural, thermal and mechanical characterization. Eur. Polym. J. 2017, 89, 174–184. [Google Scholar] [CrossRef]
- Samper, M.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The potential of flavonoids as natural antioxidants and UV light stabilizers for polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]

| Formulations | PLA (wt%) | PHB (wt%) | OLA (wt%) | Cat (wt%) | Fibers Diameter (nm) | Dinamic Viscosity (η) (Pa.s) |
|---|---|---|---|---|---|---|
| PLA-PHB | 75 | 25 | - | - | 215 ± 67 | 0.12 ± 0.01 |
| PLA-PHB-OLA | 63.75 | 21.25 | 15 | - | 260 ± 78 | 0.06 ± 0.01 |
| PLA-PHB-Cat1 | 74.25 | 24.75 | - | 1 | 405 ± 143 | 0.13 ± 0.03 |
| PLA-PHB-OLA-Cat1 | 63.0 | 21.0 | 15 | 1 | 228 ± 57 | 0.06 ± 0.02 |
| PLA-PHB-Cat3 | 72.75 | 24.25 | - | 3 | 400 ± 116 | 0.14 ± 0.01 |
| PLA-PHB-OLA-Cat3 | 61.5 | 20.5 | 15 | 3 | 206 ± 57 | 0.07 ± 0.01 |
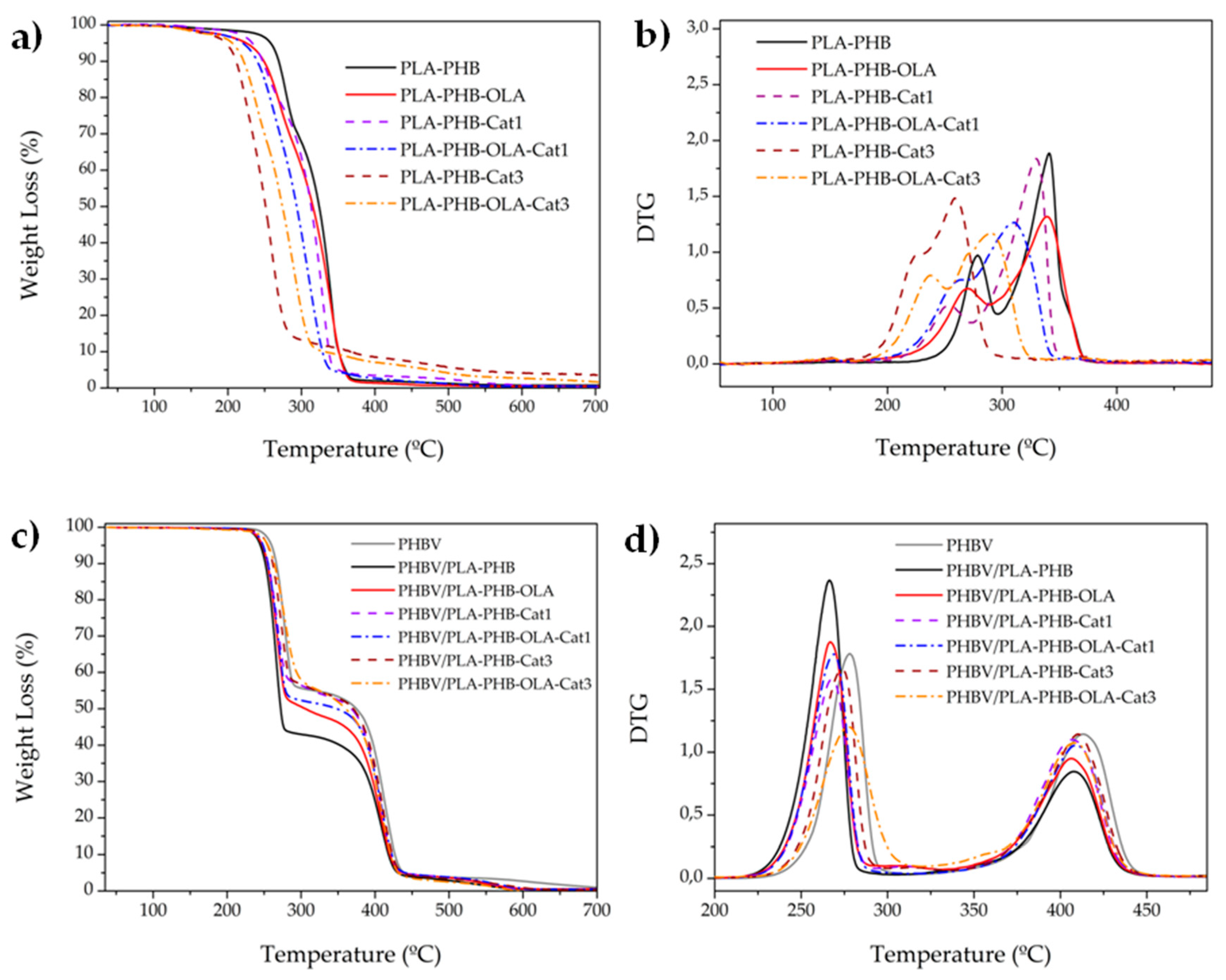
| Electrospun Mats | T0 (°C) | Tmax1 (°C) | Tmax2 (°C) |
|---|---|---|---|
| PLA-PHB | 258.1 | 278.7 | 341.0 |
| PLA-PHB-OLA | 225.0 | 270.2 | 339.2 |
| PLA-PHB-Cat1 | 232.9 | 253.8 | 329.7 |
| PLA-PHB-OLA-Cat1 | 220.3 | 263.2 | 310.4 |
| PLA-PHB-Cat3 | 197.5 | 270.8 | 344.7 |
| PLA-PHB-OLA-Cat3 | 205.9 | 250.8 | 282.5 |
| Electrospun Mats | T0 (°C) | Tmax1 (°C) | Tmax2 (°C) |
|---|---|---|---|
| PHVB | 260.0 | 278.2 | 413.1 |
| PHBV/PLA-PHB | 244.9 | 266.6 | 407.8 |
| PHBV/PLA-PHB-OLA | 247.5 | 266.9 | 406.3 |
| PHBV/PLA-PHB-Cat1 | 248.5 | 267.8 | 406.7 |
| PHBV/PLA-PHB-OLA-Cat1 | 248.5 | 268.8 | 408.9 |
| PHBV/PLA-PHB-Cat3 | 254.7 | 273.6 | 410.1 |
| PHBV/PLA-PHB-OLA-Cat3 | 255.2 | 277.3 | 408.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta, M.P.; Díez García, A.; López, D.; Fiori, S.; Peponi, L. Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials 2019, 9, 346. https://doi.org/10.3390/nano9030346
Arrieta MP, Díez García A, López D, Fiori S, Peponi L. Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials. 2019; 9(3):346. https://doi.org/10.3390/nano9030346
Chicago/Turabian StyleArrieta, Marina P., Alberto Díez García, Daniel López, Stefano Fiori, and Laura Peponi. 2019. "Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin" Nanomaterials 9, no. 3: 346. https://doi.org/10.3390/nano9030346
APA StyleArrieta, M. P., Díez García, A., López, D., Fiori, S., & Peponi, L. (2019). Antioxidant Bilayers Based on PHBV and Plasticized Electrospun PLA-PHB Fibers Encapsulating Catechin. Nanomaterials, 9(3), 346. https://doi.org/10.3390/nano9030346