Photocatalytic Decomposition of Gaseous HCHO over Ag Modified TiO2 Nanosheets at Ambient Temperature
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Ag/TiO2 NSs
2.2. Characterization Methods
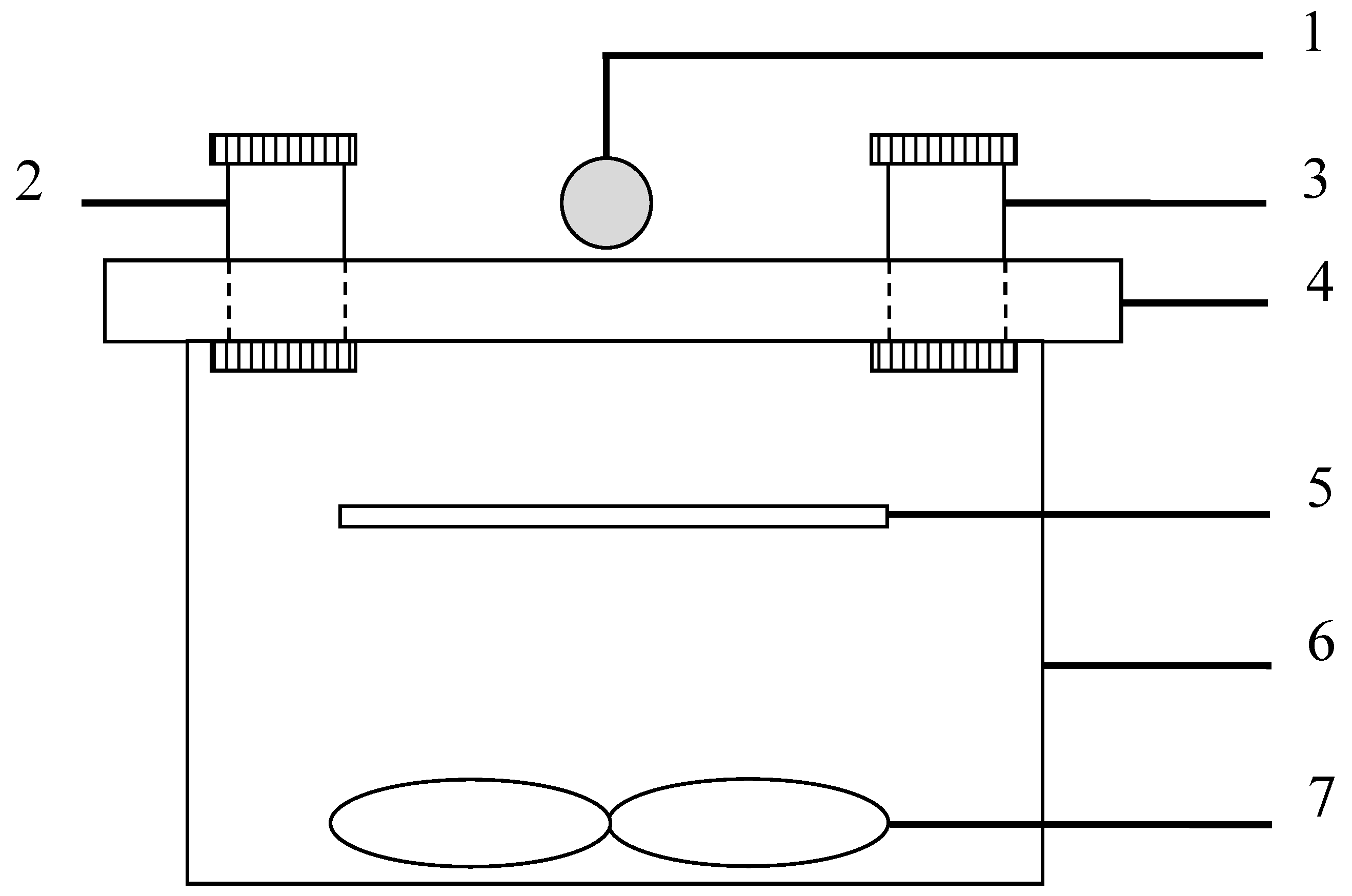
2.3. Degradation Experiment
3. Results and Discussion
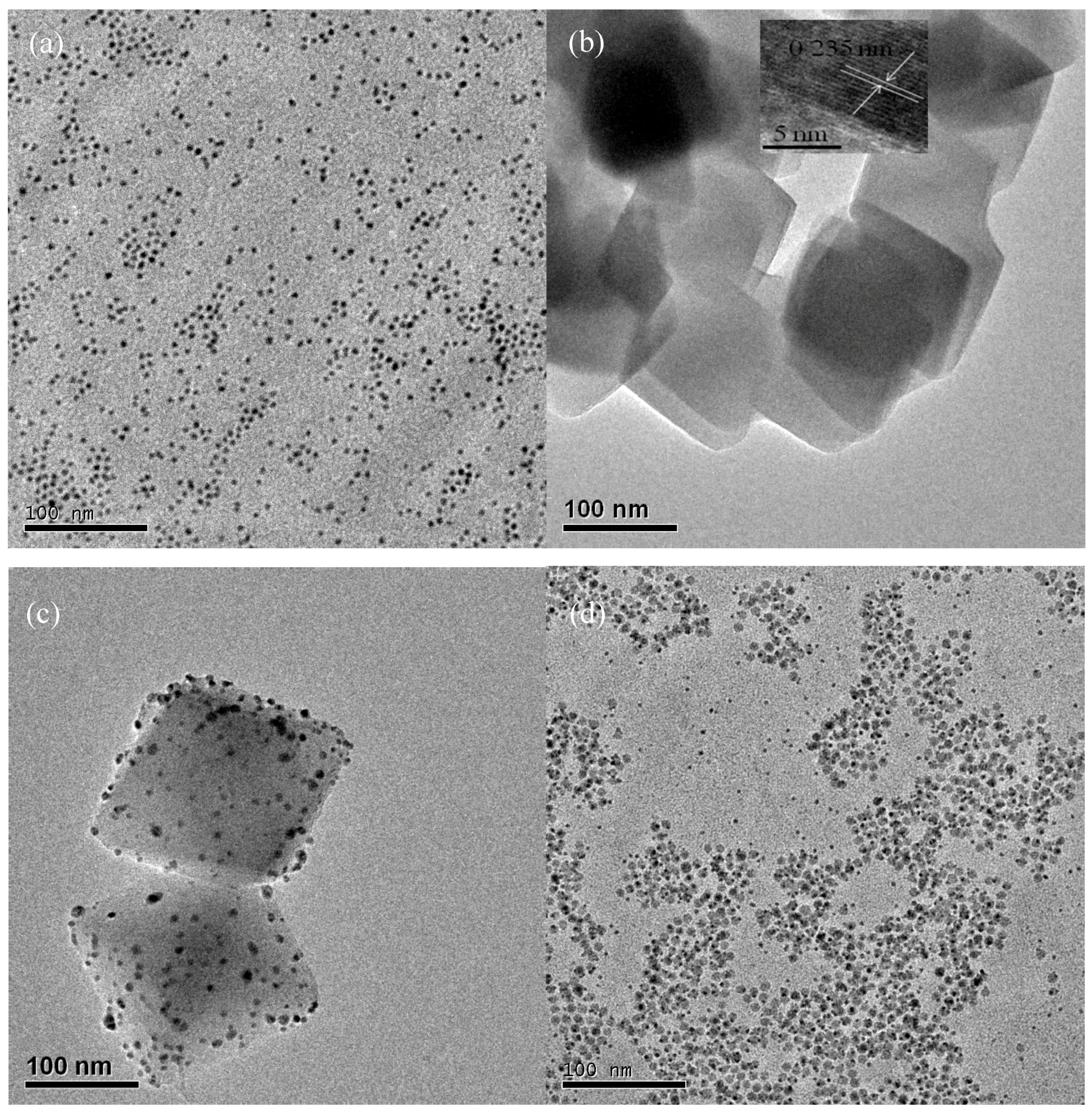
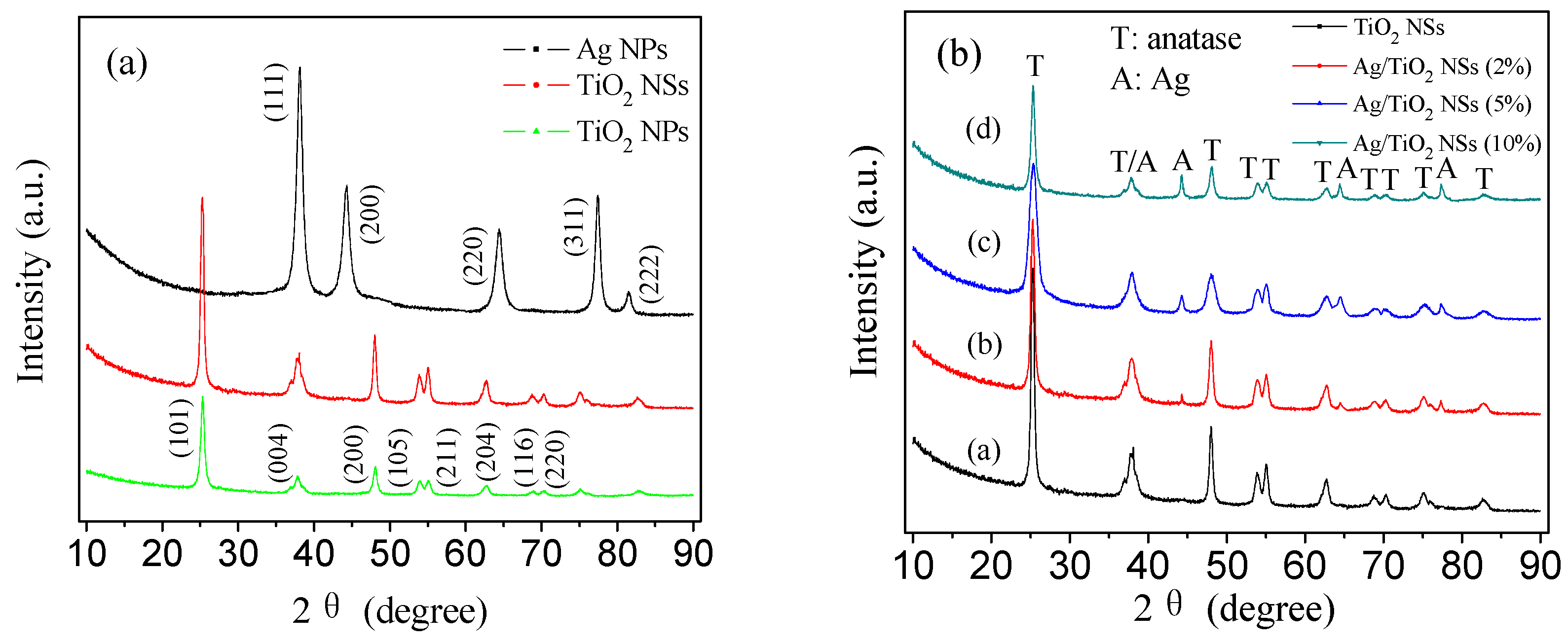
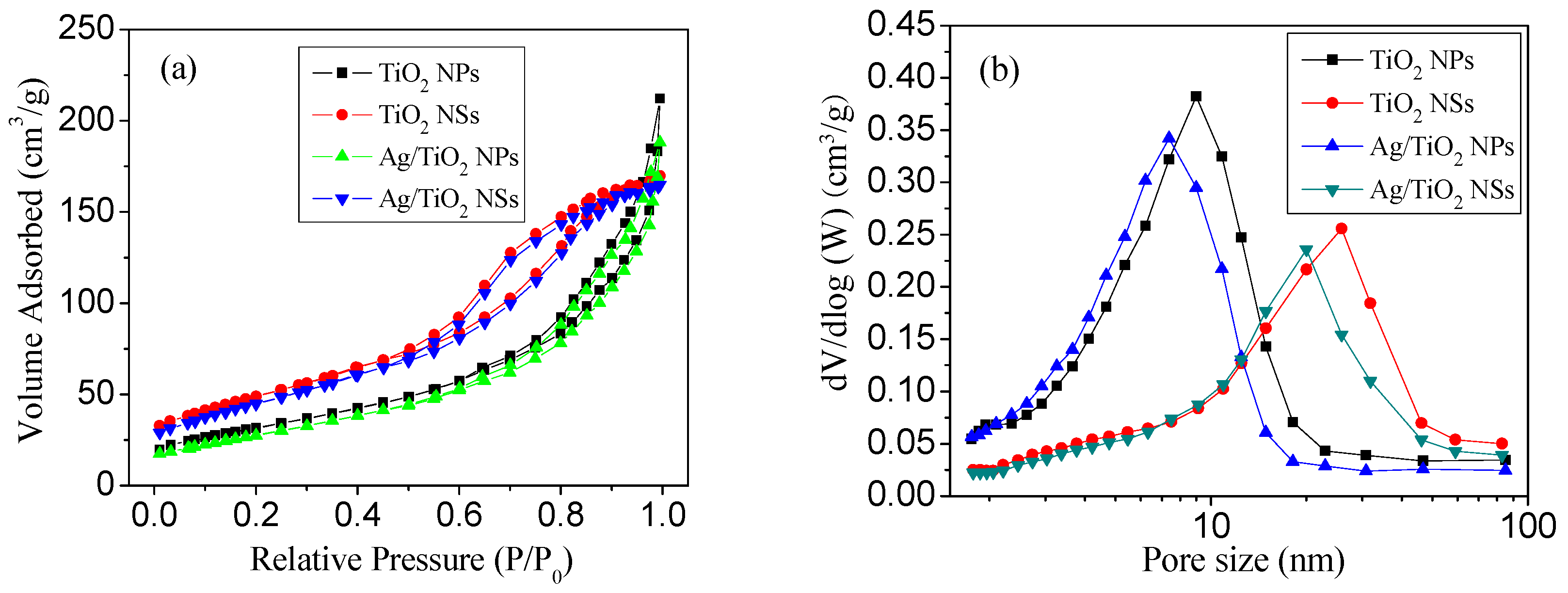
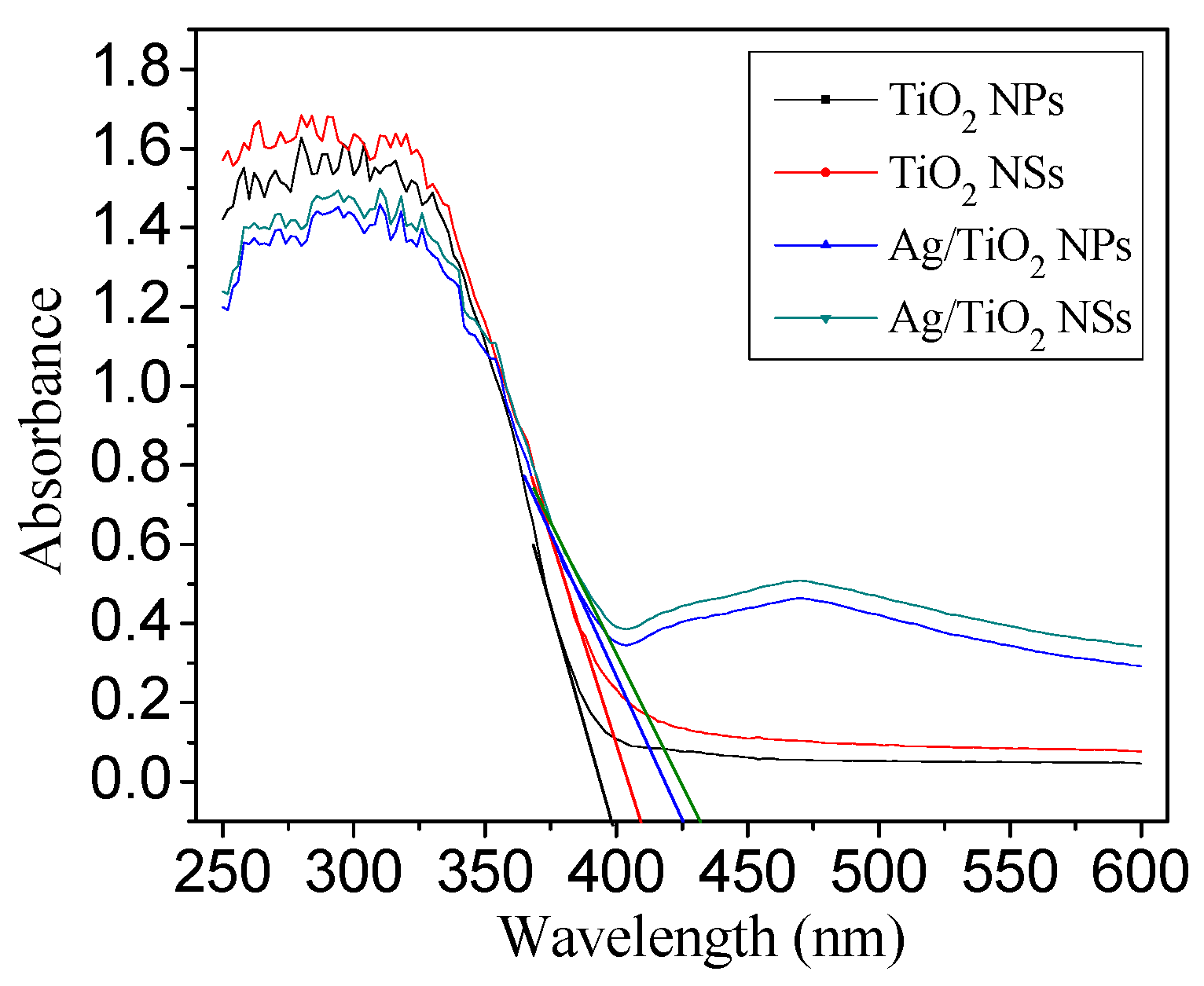
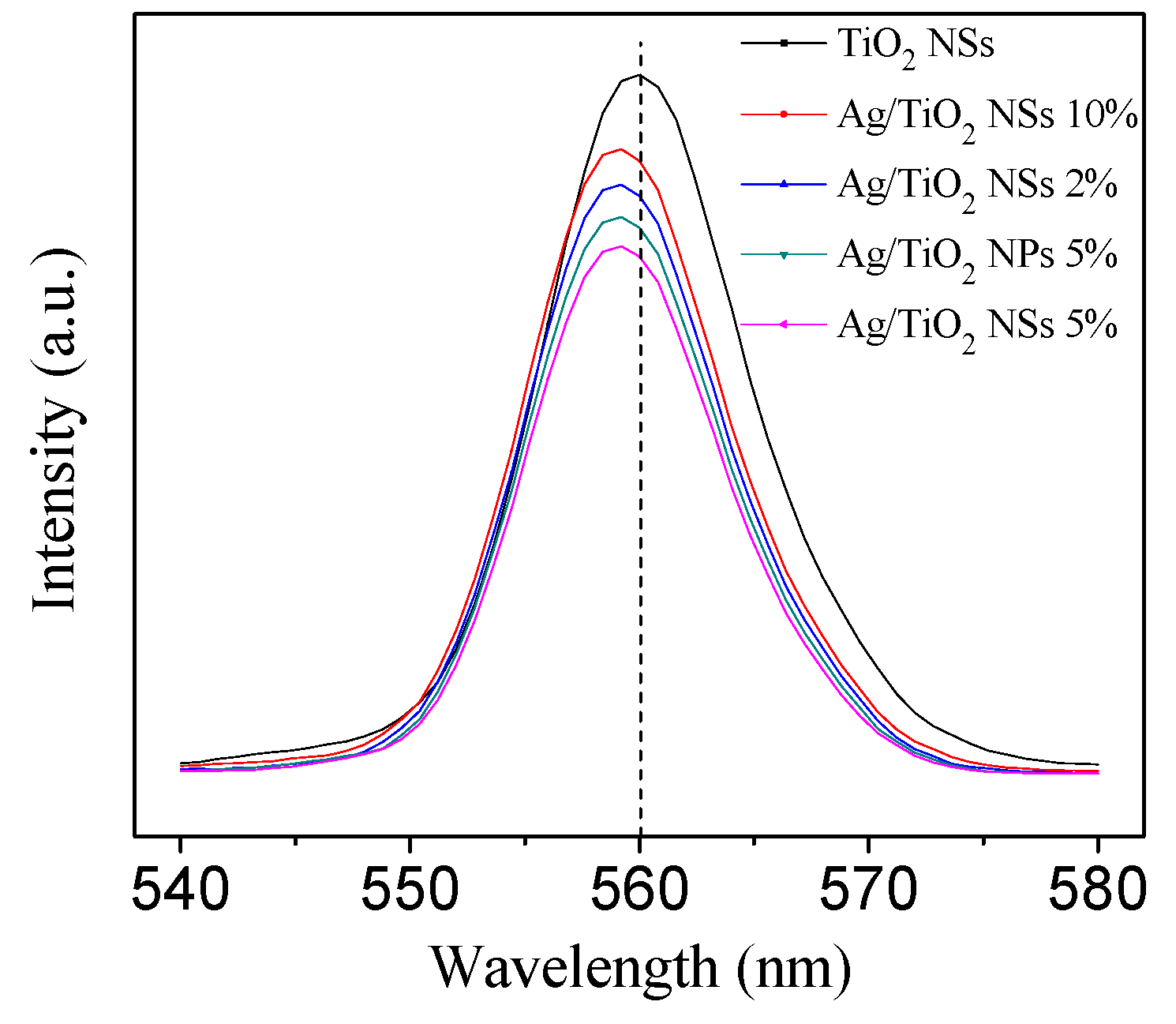
3.1. Structural Characteristics
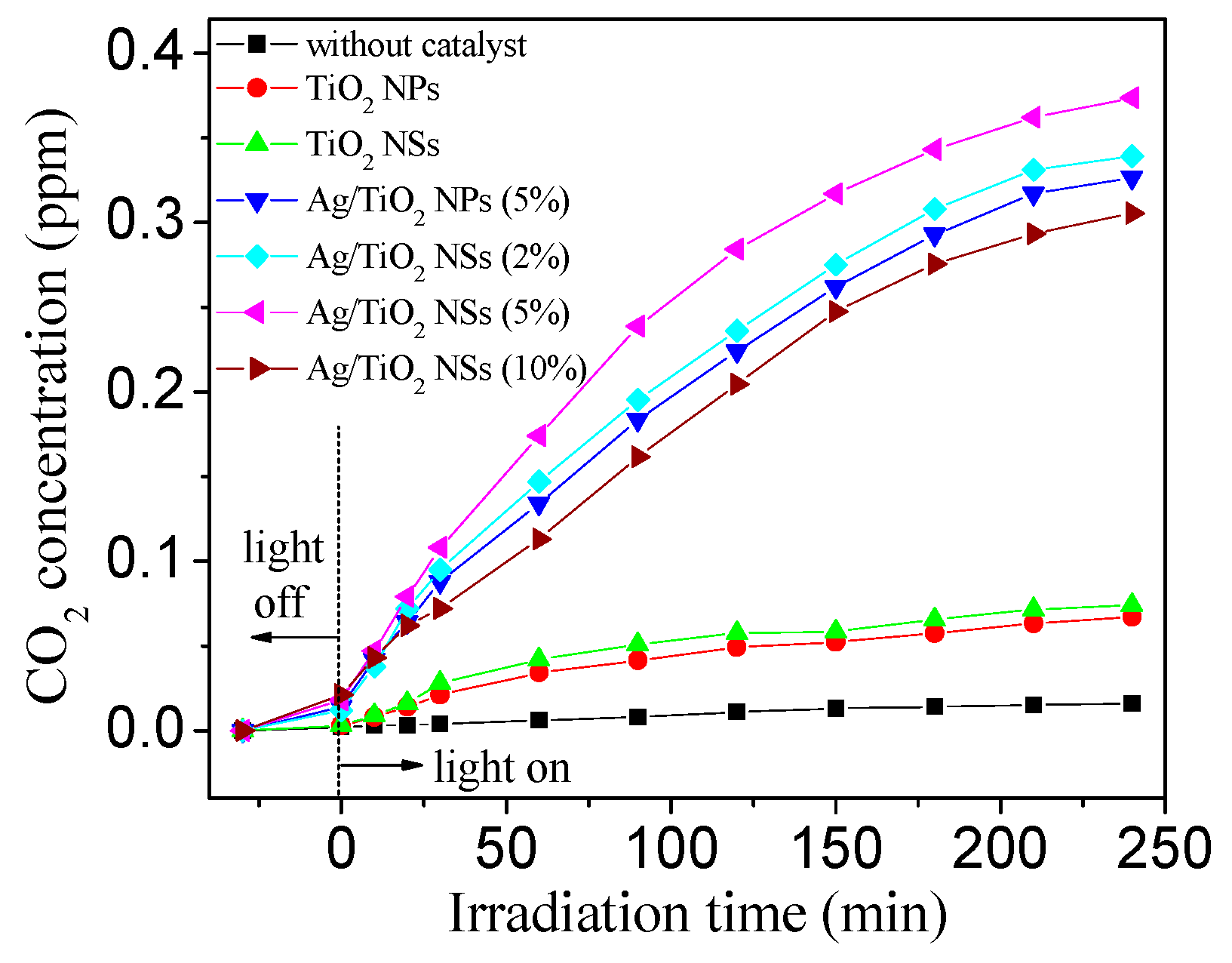
3.2. Photocatalytic Performance
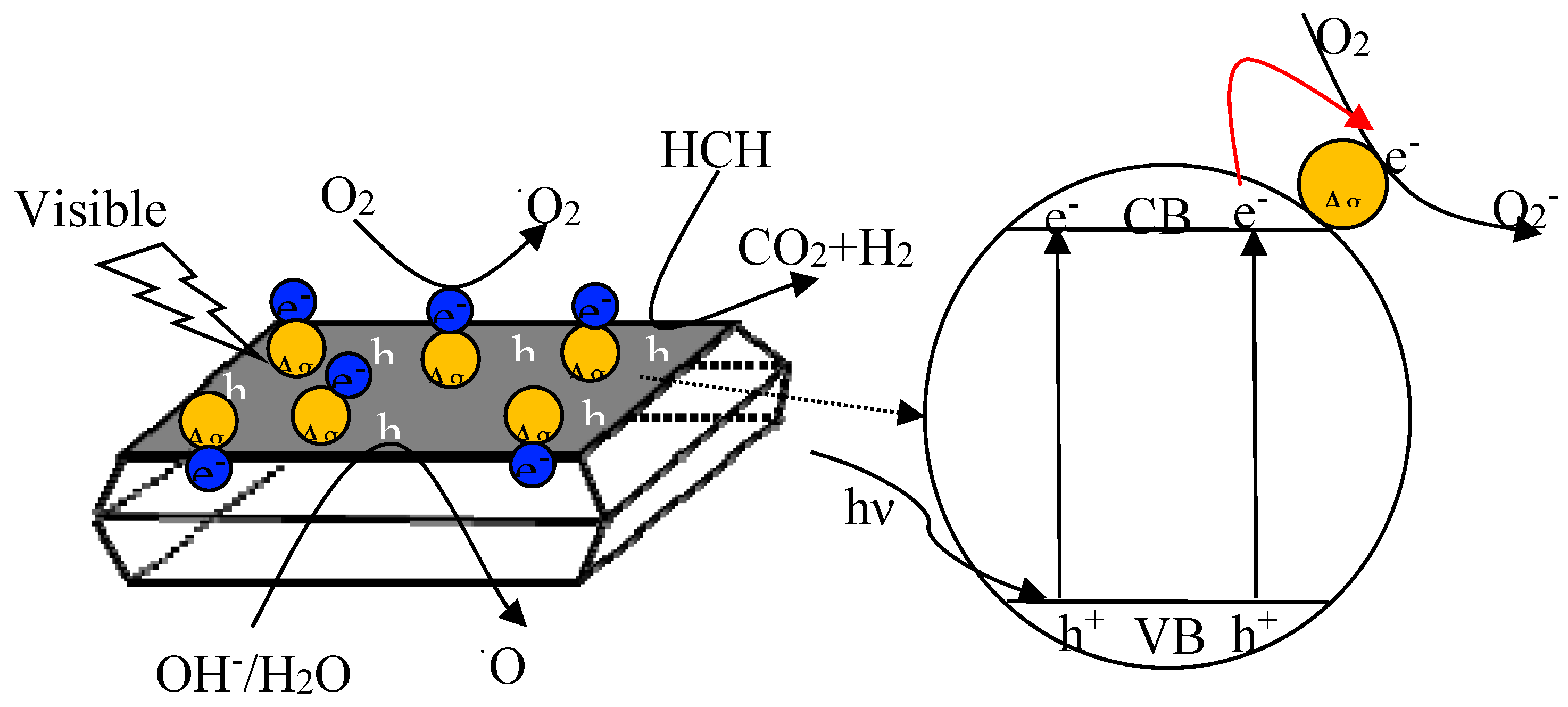
3.3. Mechanism of HCHO Photocatalytic Oxidation on Ag/TiO2 NSs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.C.; Fan, W.J.; Long, B.; Li, H.B.; Qiu, W.T.; Zhao, F.Y.; Tong, Y.X.; Ji, H.B. Alkali-modified non-precious metal 3D-NiCo2O4 nanosheets for efficient formaldehyde oxidation at low temperature. J. Mater. Chem. A 2016, 4, 3648–3654. [Google Scholar] [CrossRef]
- Yu, J.G.; Li, X.Y.; Xu, Z.H.; Xiao, W. NaOH-Modified Ceramic Honeycomb with Enhanced Formaldehyde Adsorption and Removal Performance. Environ. Sci. Technol. 2013, 47, 9928–9933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhu, X.F.; Yu, J.G.; Xiao, W. Effects of Adsorbed F, OH, and Cl Ions on Formaldehyde Adsorption Performance and Mechanism of Anatase TiO2 Nanosheets with Exposed {001} Facets. ACS Appl. Mater. Interfaces 2013, 5, 8165–8172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Zhang, C.B.; Ma, J.Z.; Chen, M.; Deng, H.; He, H. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation. Appl. Catal. B Environ. 2017, 217, 560–569. [Google Scholar] [CrossRef]
- Zhu, X.B.; Gao, X.; Qin, R.; Zeng, Y.X.; Qu, R.Y.; Zheng, C.H.; Tu, X. Plasma-catalytic removal of formaldehyde over Cu–Ce catalysts in a dielectric barrier discharge reactor. Appl. Catal. B Environ. 2015, 170, 293–300. [Google Scholar] [CrossRef]
- Kibanova, D.; Sleiman, M.; Cervini-Silva, J.; Destaillats, H. Adsorption and photocatalytic oxidation of formaldehyde on a clay-TiO2 composite. J. Hazard. Mater. 2012, 211, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Long, B.; Tang, M.N.; Rui, Z.B.; Balogun, M.S.; Tong, Y.X.; Ji, H.B. Bifunctional catalytic material: An ultrastable and high-performance surface defect CeO2 nanosheets for formaldehyde thermal oxidation and photocatalytic oxidation. Appl. Catal. B Environ. 2016, 181, 779–787. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhang, P.Y.; Li, J.G.; Jiang, C.J.; Yunus, R.; Kim, J. Room-Temperature Oxidation of Formaldehyde by Layered Manganese Oxide: Effect of Water. Environ. Sci. Technol. 2015, 49, 12372–12379. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Jansson, I.; Suárez, S.; Villarroel, M.; Sánchez, B.; Avila, P. Natural silicate-TiO2 hybrids for photocatalytic oxidation of formaldehyde in gas phase. Chem. Eng. J. 2017, 310, 560–570. [Google Scholar] [CrossRef]
- Wang, J.G.; Rao, P.H.; An, W.; Xu, J.L.; Men, Y. Boosting photocatalytic activity of Pd decorated TiO2 nanocrystal with exposed (001) facets for selective alcohol oxidations. Appl. Catal. B Environ. 2016, 195, 141–148. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, H.; Zhang, P.Y.; Xu, T.Z. Pt deposited TiO2 films with exposed {001} facets for photocatalytic degradation of a pharmaceutical pollutant. Appl. Catal. A Gen. 2016, 521, 75–82. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.H.; Liu, D.Y.; Mubeen, S.; Chuong, T.T.; Moskovits, M.; Stucky, G.D. Anisotropic Growth of TiO2 onto Gold Nanorods for Plasmon-Enhanced Hydrogen Production from Water Reduction. J. Am. Chem. Soc. 2016, 138, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, F.; Antonio D’Archivio, A.; Fanelli, M.; Santucci, S. Photocatalytic degradation of linuron in aqueous suspensions of TiO2. RSC Adv. 2011, 1, 611–618. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.Z.; Xu, J.H. Photodegradation of formaldehyde by photocatalyst TiO2: Effects on the presences of NO, SO2 and VOCs. Appl. Catal. B Environ. 2004, 54, 41–50. [Google Scholar] [CrossRef]
- Zhou, W.; Li, T.; Wang, J.Q.; Qu, Y.; Pan, K.; Xie, Y.; Tian, G.H.; Wang, L.; Ren, Z.Y.; Jiang, B.J.; et al. Composites of small Ag clusters confined in the channels of well-ordered mesoporous anatase TiO2 and their excellent solar-light-driven photocatalytic performance. Nano Res. 2014, 7, 731–742. [Google Scholar] [CrossRef]
- Lee, J.E.; Bera, S.; Choi, Y.S.; Lee, W.I. Size-dependent plasmonic effects of M and M@SiO2 (M = Au or Ag) deposited on TiO2 in photocatalytic oxidation reactions. Appl. Catal. B Environ. 2017, 214, 15–22. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. TiO2/SrTiO3 and SrTiO3 microspheres decorated with Rh, Ru or Pt nanoparticles: Highly UV–vis responsible photoactivity and mechanism. J. Catal. 2017, 350, 159–173. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.W.; Nikolla, E.; Medlin, J.W. Directing Reaction Pathways through Controlled Reactant Binding at Pd–TiO2 Interfaces. Angew. Chem. Int. Ed. 2017, 56, 6594–6598. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, S.; Aït-Mansour, K.; Harbich, W.; Brune, H. Reaction-Induced Cluster Ripening and Initial Size-Dependent Reaction Rates for CO Oxidation on Ptn/TiO2(110)-(1×1). J. Am. Chem. Soc. 2014, 136, 8702–8707. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.L.; Kumar, S.; Krishnan, V.; Sathish, M.; Shankar, M.V. Multifunctional Cu/Ag quantum dots on TiO2 nanotubes as highly efficient photocatalysts for enhanced solar hydrogen evolution. J. Catal. 2017, 350, 226–239. [Google Scholar] [CrossRef]
- He, J.X.; Yang, P.J.; Sato, H.; Umemura, Y.; Yamagishi, A. Effects of Ag-photodeposition on photocurrent of an ITO electrode modified by a hybrid film of TiO2 nanosheets. J. Electroanal. Chem. 2004, 566, 227–233. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Z.H.; Yang, H.; Shen, K.B.; Huang, Y.; Shen, S. Preparation of TiO2 loaded with crystalline nano Ag by a one-step low-temperature hydrothermal method. J. Mater. Chem. 2012, 22, 16306–16311. [Google Scholar] [CrossRef]
- Gao, F.Q.; Yang, Y.; Wang, T.H. Preparation of porous TiO2/Ag heterostructure films with enhanced photocatalytic activity. Chem. Eng. J. 2015, 270, 418–427. [Google Scholar] [CrossRef]
- Jiang, Z.F.; Lv, X.M.; Jiang, D.L.; Xie, J.M.; Mao, D.J. Natural leaves-assisted synthesis of nitrogen-doped, carbon-rich nanodots-sensitized, Ag-loaded anatase TiO2 square nanosheets with dominant {001} facets and their enhanced catalytic applications. J. Mater. Chem. A 2013, 1, 14963–14972. [Google Scholar] [CrossRef]
- Li, Y.X.; Jiang, Y.; Peng, S.Q.; Jiang, F.Y. Nitrogen-doped TiO2 modified with NH4F for efficient photocatalytic degradation of formaldehyde under blue light-emitting diodes. J. Hazard. Mater. 2010, 182, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, J.Y.; Peng, H.G.; Xu, X.L.; Liu, W.M.; Wang, Z.; Zhang, N.; Wang, X. Ag supported on meso-structured SiO2 with different morphologies for CO oxidation: On the inherent factors influencing the activity of Ag catalysts. Microporous Mesoporous Mater. 2017, 242, 90–98. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Fang, J.L.; Li, D.Z.; Chen, J.; Zhang, X.Y.; Shao, Y.; He, Y.H. A facile synthesis of CdSe quantum dots-decorated anatase TiO2 with exposed {001} facets and its superior photocatalytic activity. Appl. Catal. B Environ. 2016, 181, 838–847. [Google Scholar] [CrossRef]
- Li, X.R.; Wang, J.G.; Men, Y.; Bian, Z.F. TiO2 mesocrystal with exposed (001) facets and CdS quantum dots as an active visible photocatalyst for selective oxidation reactions. Appl. Catal. B Environ. 2016, 187, 115–121. [Google Scholar] [CrossRef]
- Yu, D.H.; Yu, X.D.; Wang, C.H.; Liu, X.C.; Xing, Y. Synthesis of Natural Cellulose-Templated TiO2/Ag Nanosponge Composites and Photocatalytic Properties. ACS Appl. Mater. Interfaces 2012, 4, 2781–2787. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.Q.; Zhu, B.; Li, X.S.; Liu, J.L.; Zhu, X.B.; Zhu, A.M. Visible-light photocatalytic oxidation of CO over plasmonic Au/TiO2: Unusual features of oxygen plasma activation. Appl. Catal. B Environ. 2016, 188, 48–55. [Google Scholar] [CrossRef]
- Liu, R.R.; Wang, J.; Zhang, J.J.; Xie, S.; Wang, X.Y.; Ji, Z.J. Honeycomb-like micro-mesoporous structure TiO2/sepiolite composite for combined chemisorption and photocatalytic elimination of formaldehyde. Microporous Mesoporous Mater. 2017, 248, 234–245. [Google Scholar] [CrossRef]
- Zhang, G.X.; Sun, Z.M.; Duan, Y.W.; Ma, R.X.; Zheng, S.L. Synthesis of nano-TiO2/diatomite composite and its photocatalytic degradation of gaseous formaldehyde. Appl. Surf. Sci. 2017, 412, 105–112. [Google Scholar] [CrossRef]
- Qi, L.F.; Ho, W.K.; Wang, J.L.; Zhang, P.Y.; Yu, J.G. Enhanced catalytic activity of hierarchically macro-/mesoporous Pt/TiO2 toward room-temperature decomposition of formaldehyde. Catal. Sci. Technol. 2015, 5, 2366–2377. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Novel adsorption and photocatalytic oxidation for removal of gaseous toluene by V-doped TiO2/PU under visible light. J. Hazard. Mater. 2015, 300, 493–503. [Google Scholar] [CrossRef] [PubMed]






| Sample | C0 (mg/L) | k (min−1) | R2 | r0 (mg/(L·min)) |
|---|---|---|---|---|
| blank | 0.488 | 0.00022 | 0.9883 | 0.00011 |
| TiO2 NPs | 0.447 | 0.00040 | 0.9917 | 0.00018 |
| TiO2 NSs | 0.464 | 0.00073 | 0.9639 | 0.00034 |
| Ag/TiO2 NPs (5%) | 0.446 | 0.00856 | 0.9945 | 0.00382 |
| Ag/TiO2 NSs (2%) | 0.452 | 0.00743 | 0.9964 | 0.00335 |
| Ag/TiO2 NSs (5%) | 0.454 | 0.01022 | 0.9884 | 0.00464 |
| Ag/TiO2 NSs (10%) | 0.456 | 0.00548 | 0.9943 | 0.00250 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Xu, W.; Yu, L. Photocatalytic Decomposition of Gaseous HCHO over Ag Modified TiO2 Nanosheets at Ambient Temperature. Nanomaterials 2019, 9, 338. https://doi.org/10.3390/nano9030338
Jiang X, Xu W, Yu L. Photocatalytic Decomposition of Gaseous HCHO over Ag Modified TiO2 Nanosheets at Ambient Temperature. Nanomaterials. 2019; 9(3):338. https://doi.org/10.3390/nano9030338
Chicago/Turabian StyleJiang, Xueding, Weicheng Xu, and Lian Yu. 2019. "Photocatalytic Decomposition of Gaseous HCHO over Ag Modified TiO2 Nanosheets at Ambient Temperature" Nanomaterials 9, no. 3: 338. https://doi.org/10.3390/nano9030338
APA StyleJiang, X., Xu, W., & Yu, L. (2019). Photocatalytic Decomposition of Gaseous HCHO over Ag Modified TiO2 Nanosheets at Ambient Temperature. Nanomaterials, 9(3), 338. https://doi.org/10.3390/nano9030338






