Oxygen-Scavenging Multilayered Biopapers Containing Palladium Nanoparticles Obtained by the Electrospinning Coating Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Films
2.3. Characterization of the Films
2.3.1. Film Thickness
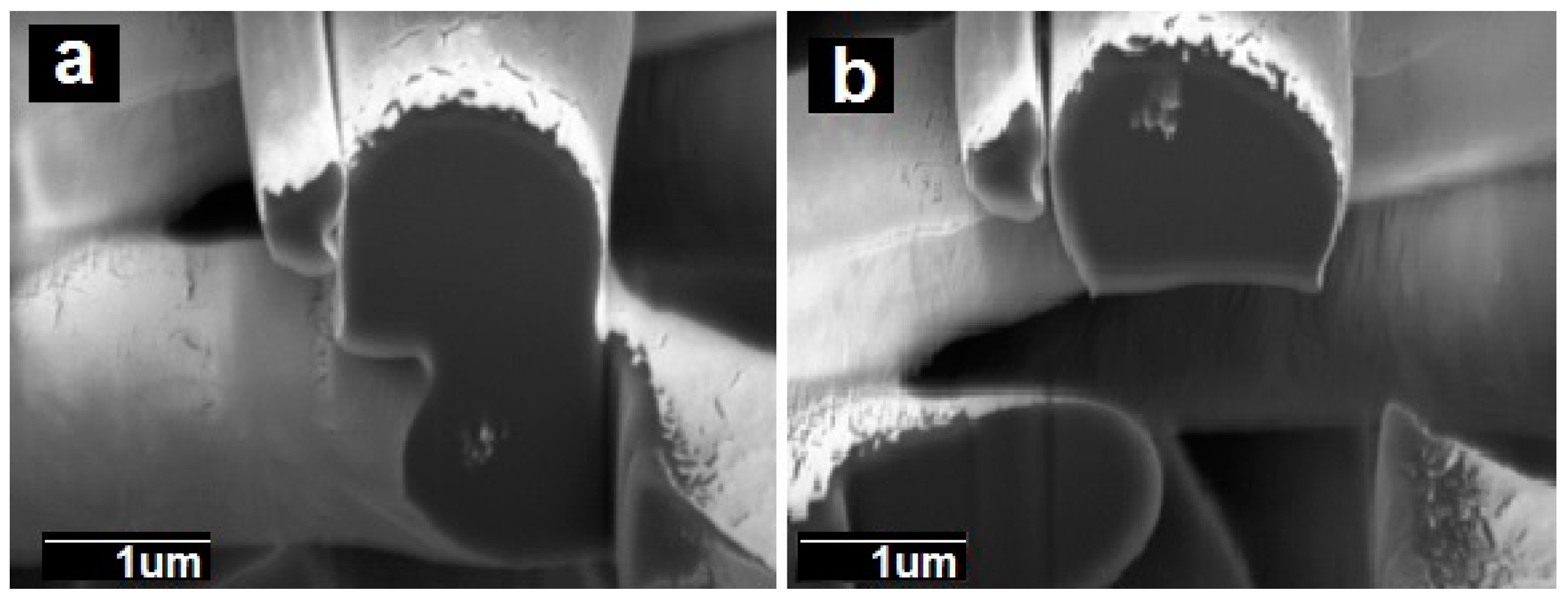
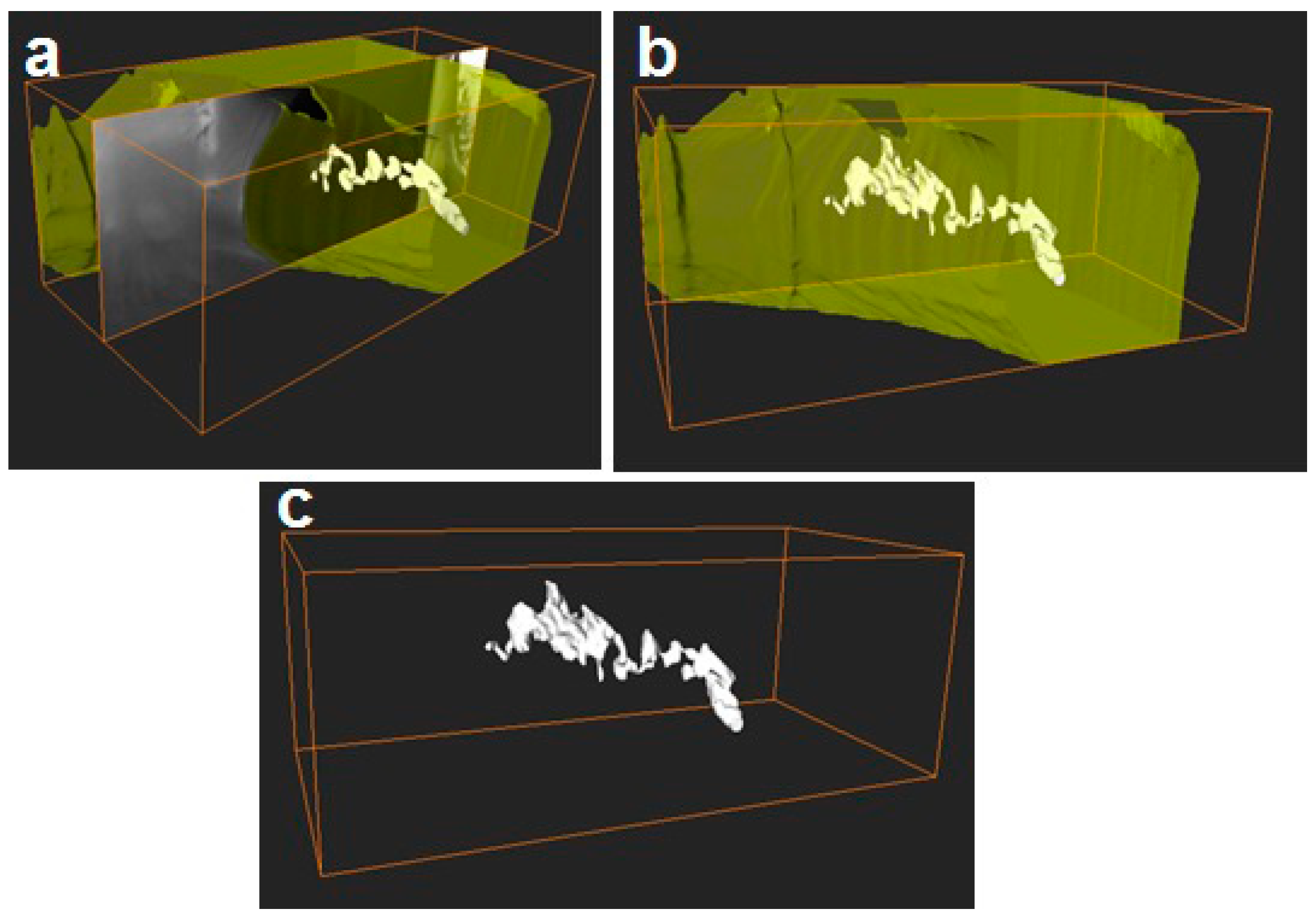
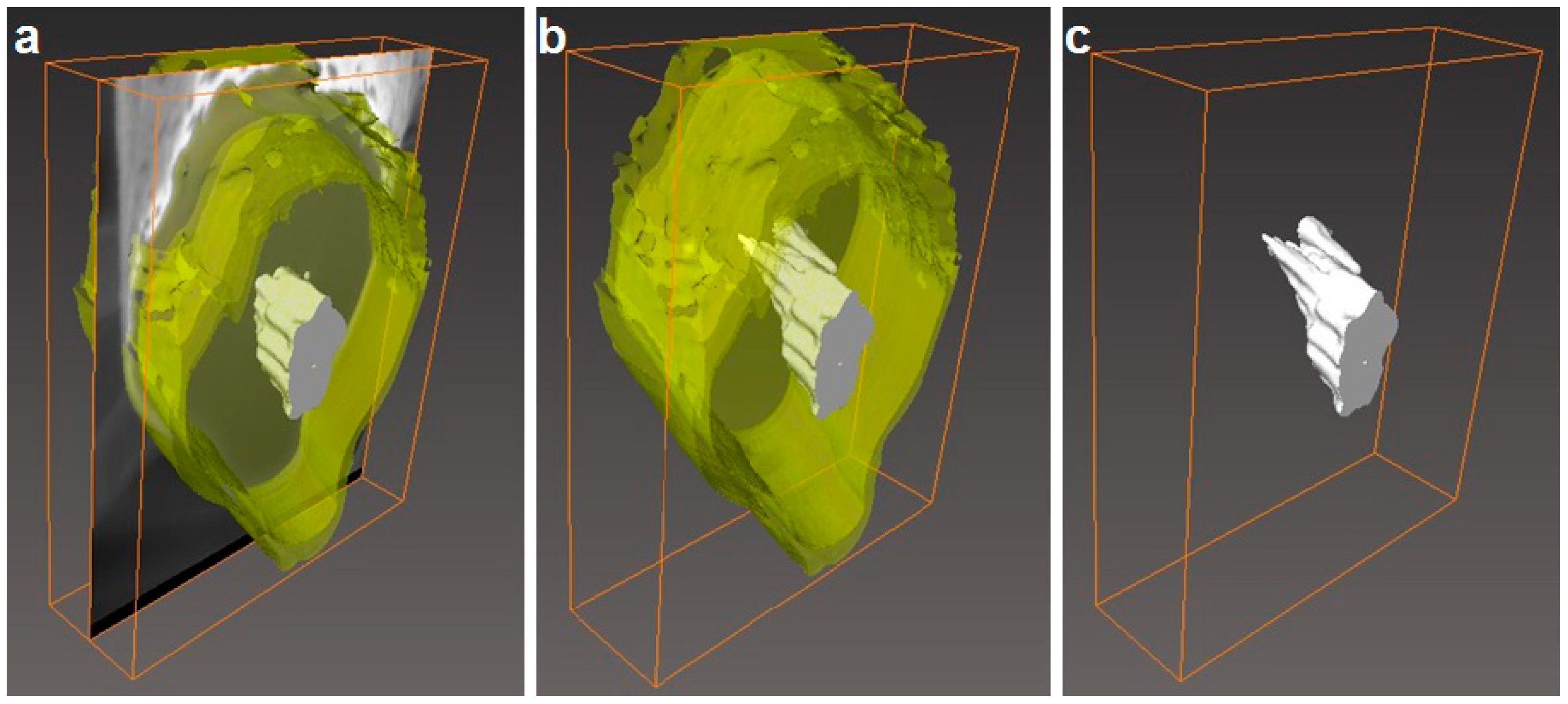
2.3.2. Focus Ion Beam Scanning Electron Microscopy (FIB-SEM)
2.3.3. Scanning Electron Microscopy
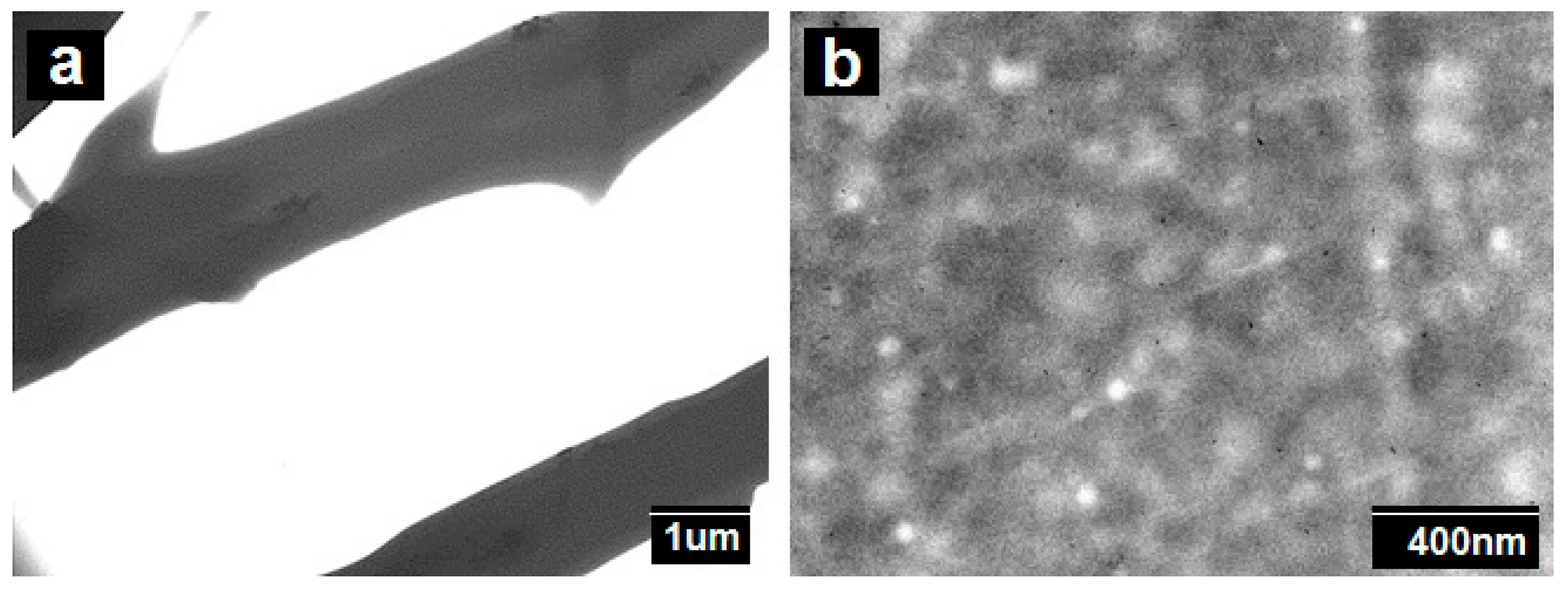
2.3.4. Transmission Electronic Microscopy
2.3.5. Differential Scanning Calorimetry (DSC)
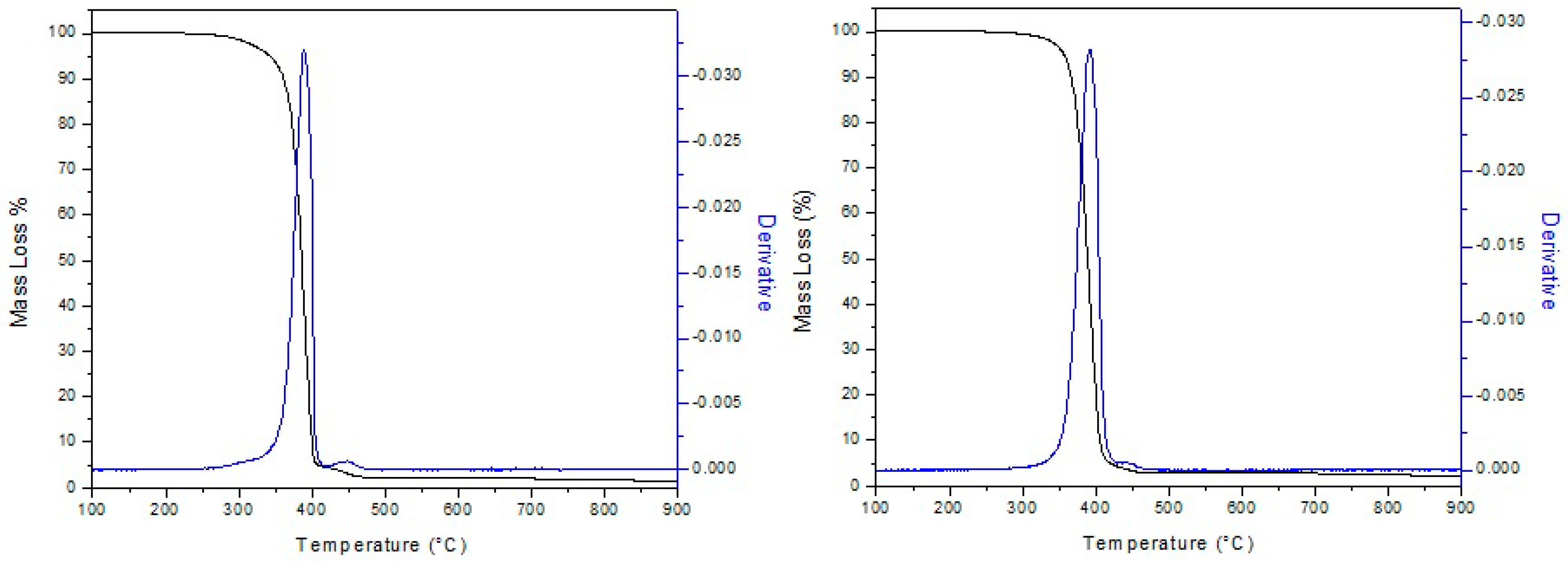
2.3.6. Thermogravimetric Analysis (TGA)
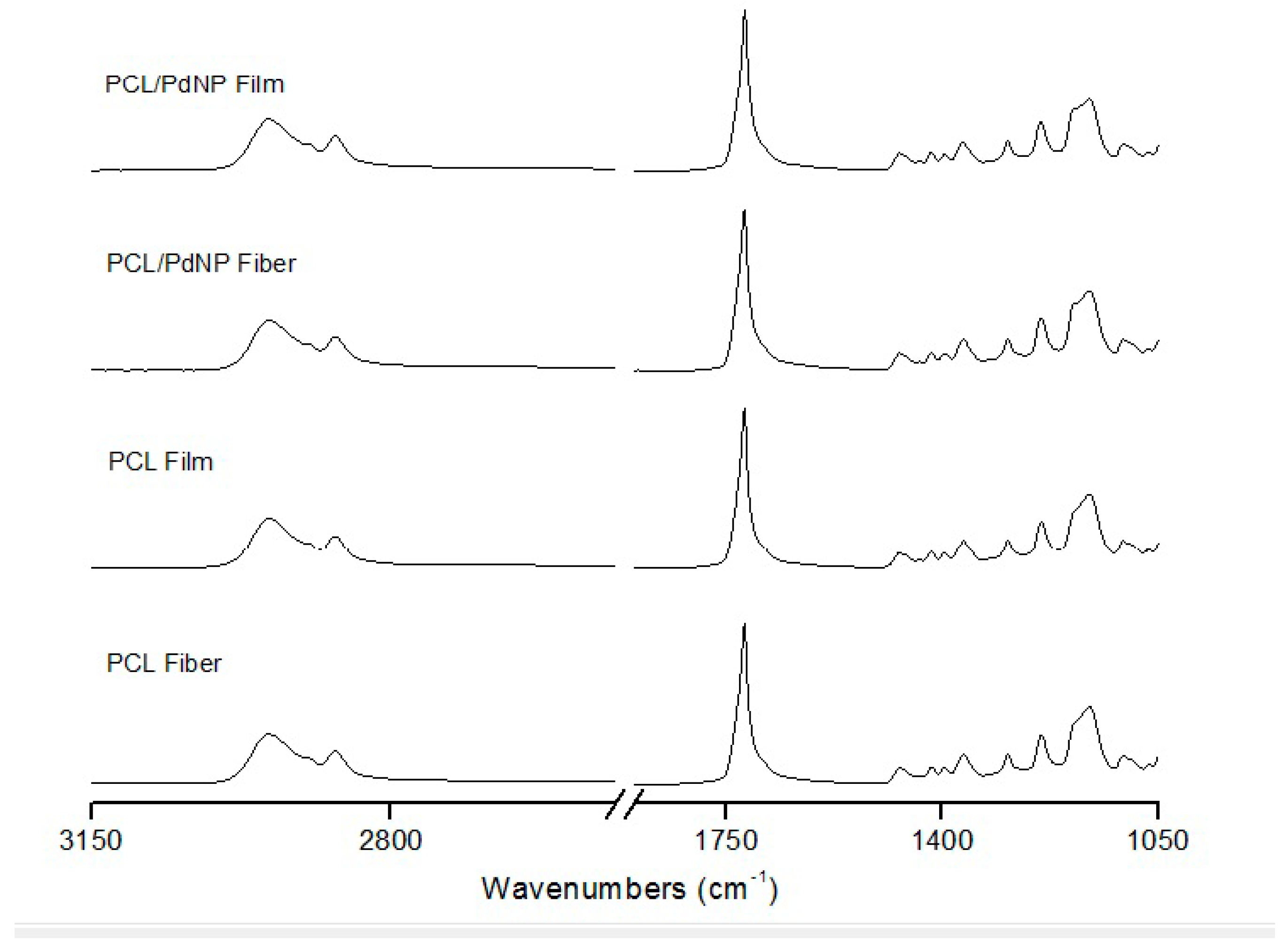
2.3.7. Infrared Spectroscopy
2.3.8. Water Vapor Permeance
2.3.9. Measurement of Oxygen Scavenging Activity
2.4. Statistical Analysis
3. Results and Discussion
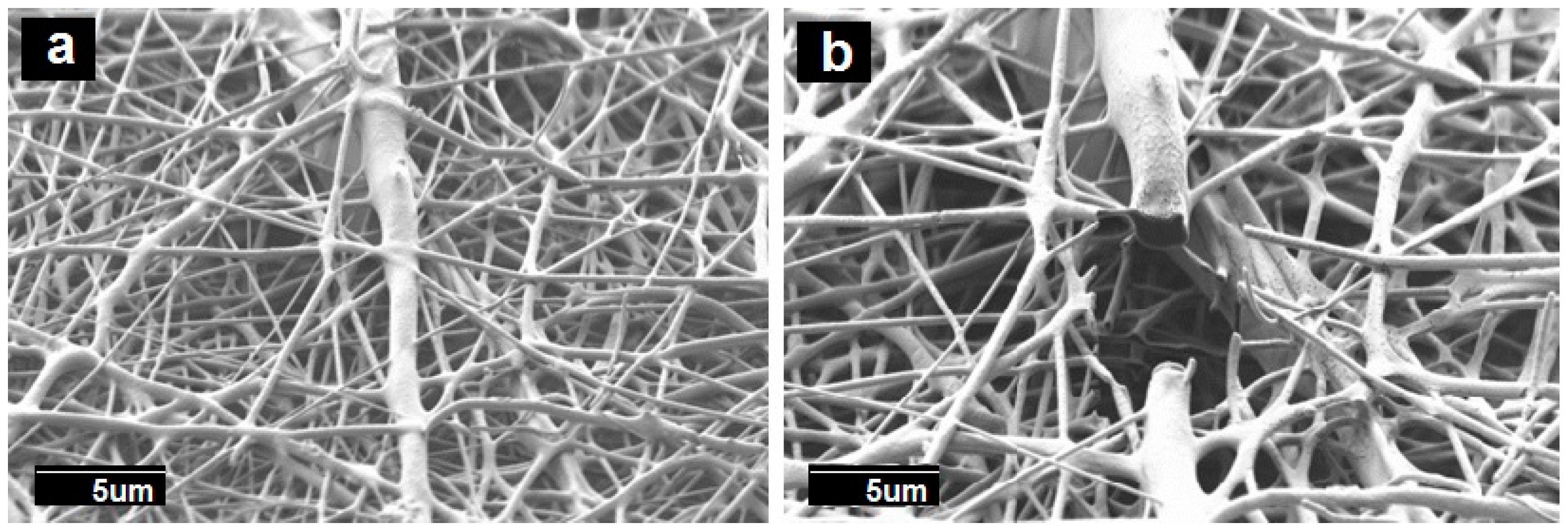
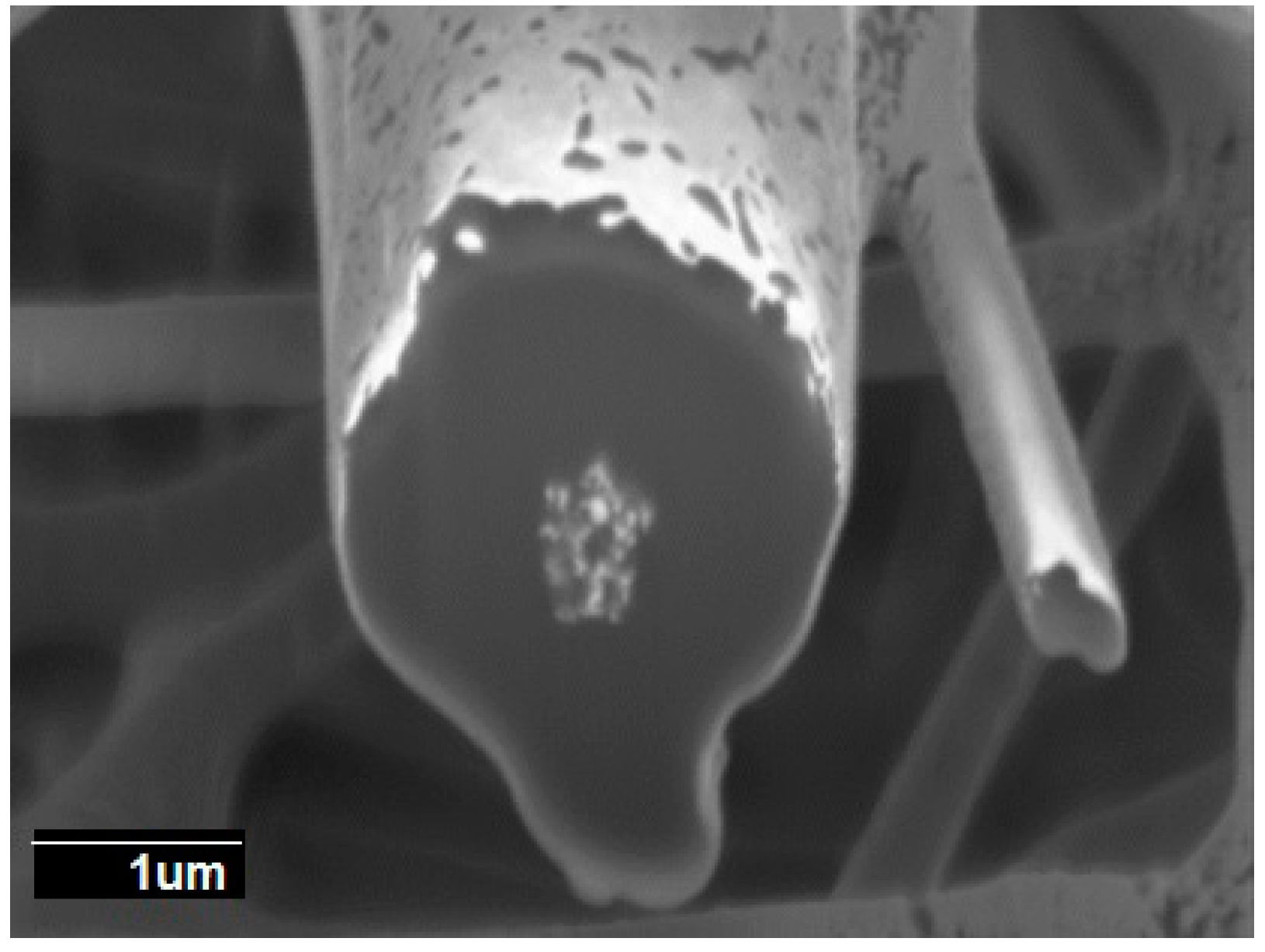
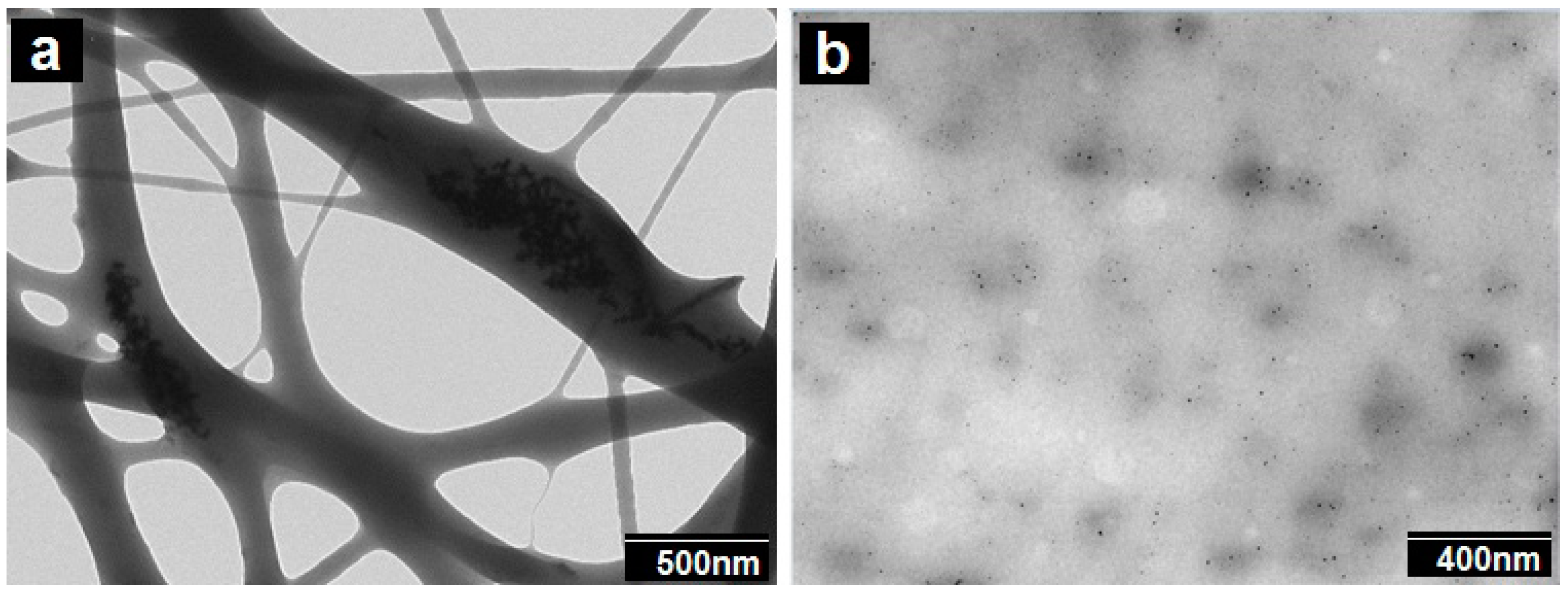
3.1. Morphology of the Electrospun PCL Fibers and Films
3.2. FTIR Analysis of the PCL Electrospun Fibers and Films
3.3. Thermal Properties of the PCL Electrospun Fibers
3.4. PHB Electrospun Fiber Morphology
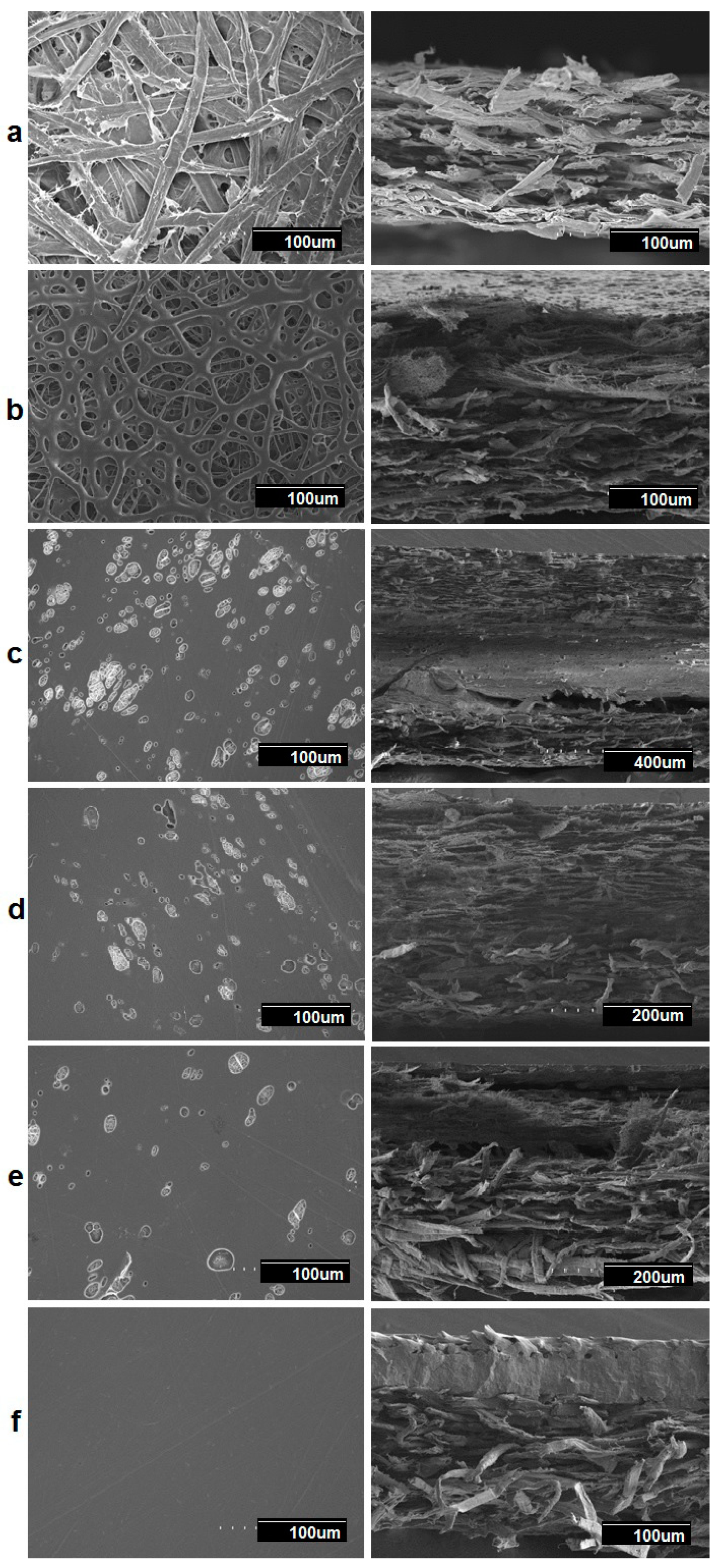
3.5. Morphology of the Multilayers
3.6. Passive and Active Barrier Properties of the Multilayers
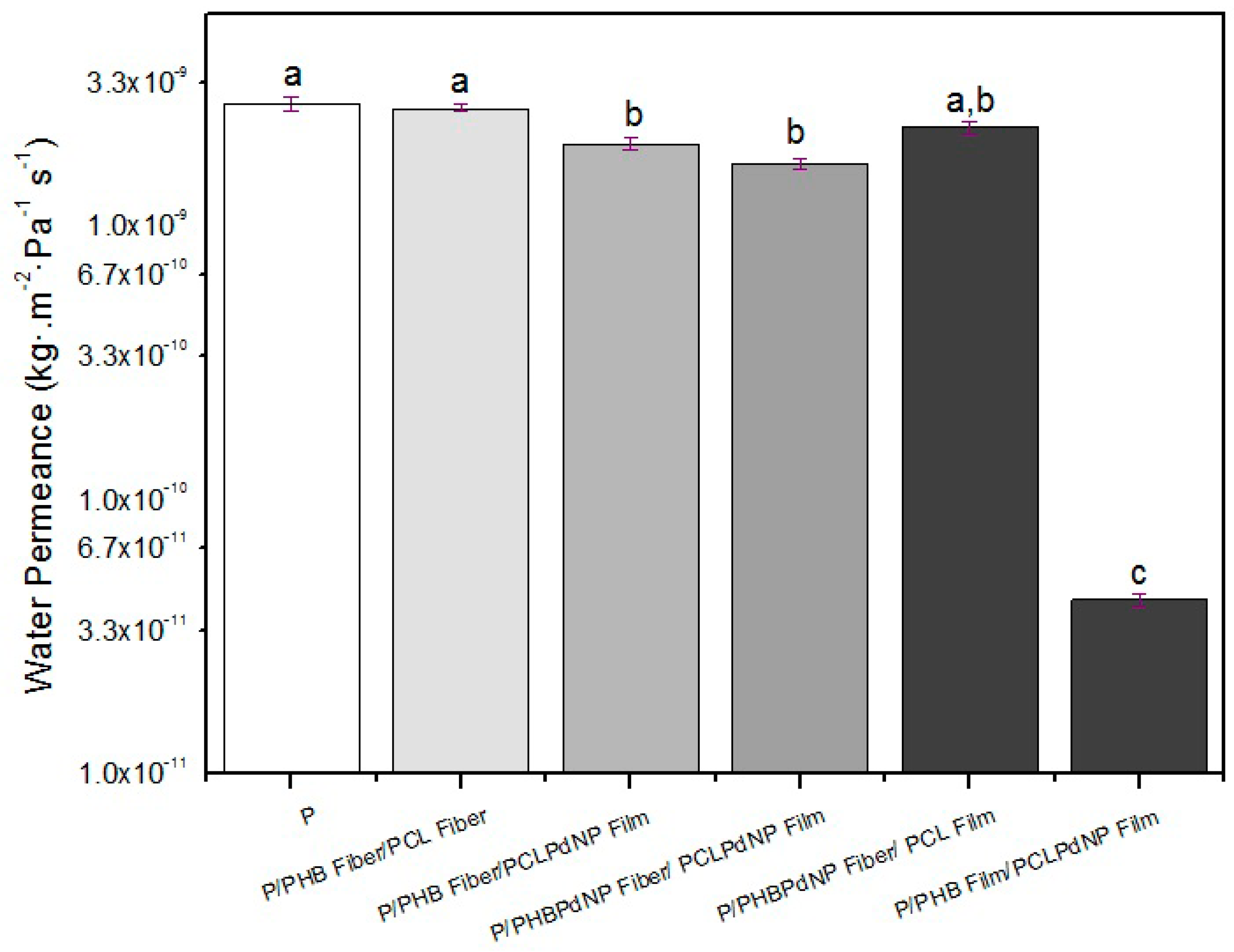
3.6.1. Water Vapor Passive Permeance
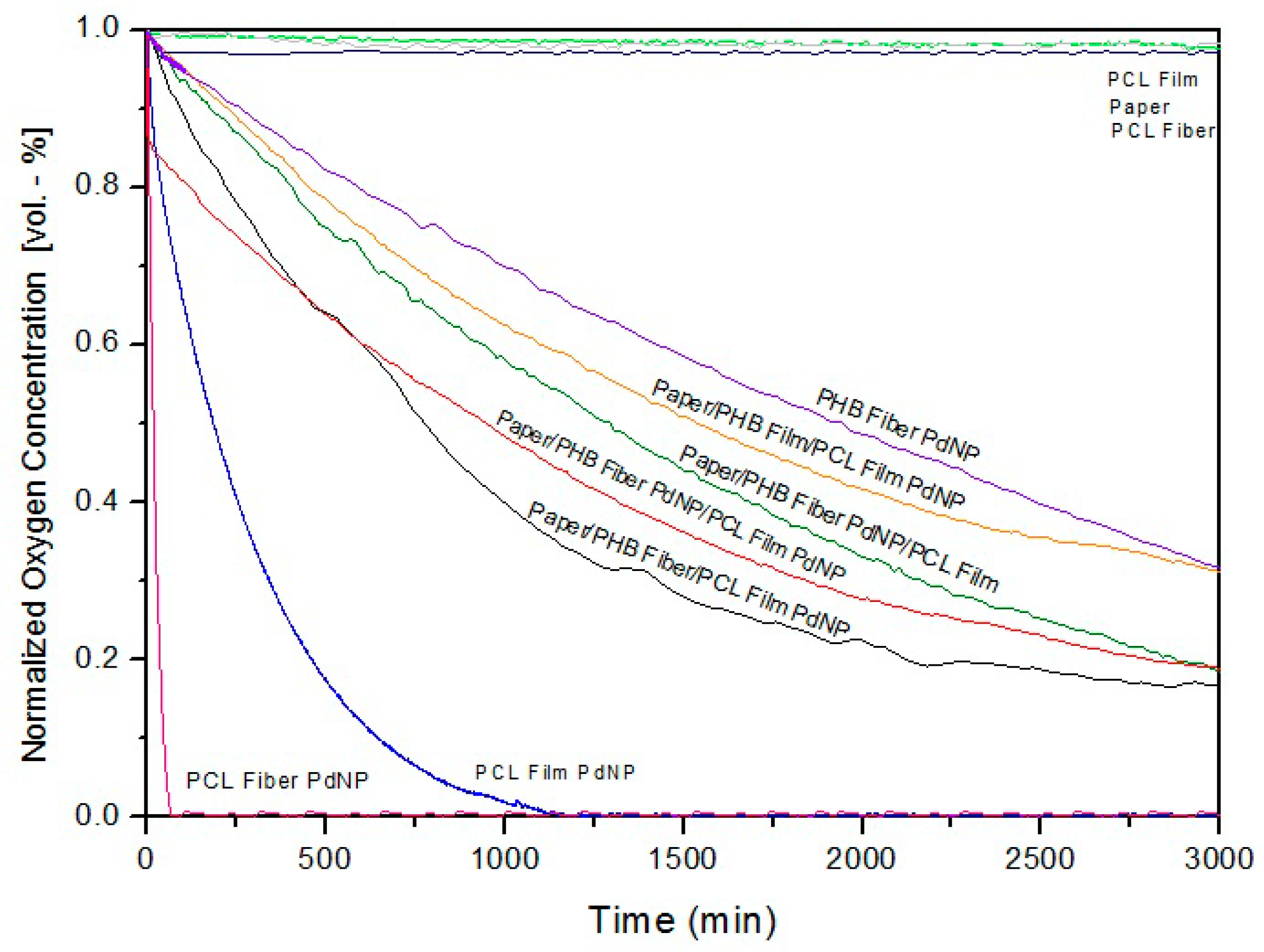
3.6.2. Active Oxygen Scavenging Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kerry, J.P.; O’Grady, M.N.; Hogan, S.A. Past, Current and Potential Utilisation of Active and Intelligent Packaging Systems for Meat and Muscle-Based Products: A Review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Wong, D.E.; Andler, S.M.; Lincoln, C.; Goddard, J.M.; Talbert, J.N. Oxygen Scavenging Polymer Coating Prepared by Hydrophobic Modification of Glucose Oxidase. J. Coat. Technol. Res. 2017, 14, 489–495. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Rüegg, N.; Lohwasser, W. Development of Palladium-Based Oxygen Scavenger: Optimization of Substrate and Palladium Layer Thickness. Packag. Technol. Sci. 2015, 28, 710–718. [Google Scholar] [CrossRef]
- Brody, A.L.; Strupinsky, E.P.; Kline, L.R. Active Packaging for Food Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Dobrucka, R.; Cierpiszewski, R. Active and Intelligent Packaging Food-Research and Development—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 7–15. [Google Scholar] [CrossRef]
- Kaufman, J.; Lacoste, A.; Schulok, J.; Shehady, E.; Yam, K. An Overview of Oxygen Scavenging Packaging and Applications. 2000. Available online: https://www.bakeryonline.com/doc/an-overview-of-oxygen-scavenging-packaging-an-0002 (accessed on 8 October 2017).
- Ozdemir, M.; Floros, J.D. Active Food Packaging Technologies. Crit. Rev. Food Sci. Nutr. 2004, 44, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Solovyov, S.E. Oxygen Scavengers. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley and Sons: New York, NY, USA, 2000; pp. 1–31. [Google Scholar]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef]
- Hutter, S.; Rüegg, N.; Yildirim, S. Use of Palladium Based Oxygen Scavenger to Prevent Discoloration of Ham. Food Packag. Shelf Life 2016, 8, 56–62. [Google Scholar] [CrossRef]
- Li, M.; Posevins, D.; Gustafson, K.P.J.; Tai, C.; Shchukarev, A.; Qiu, Y.; Bäckvall, J. Diastereoselective Cyclobutenol Synthesis: A Heterogeneous Palladium-Catalyzed Oxidative Carbocyclization-Borylation of Enallenols. Chem. Eur. J. 2019, 25, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Inge, A.K.; Posevins, D.; Gustafson, K.P.J.; Qiu, Y.; Backvall, J. Chemodivergent and Diastereoselective Synthesis of Γ-Lactones and Γ-Lactams: A Heterogeneous Palladium-Catalyzed Oxidative Tandem Process. J. Am. Chem. Soc. 2018, 140, 14604–14608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, B.; Backvall, J. Highly Selective Cascade C–C Bond Formation Via Palladium-Catalyzed Oxidative Carbonylation–Carbocyclization–Carbonylation–Alkynylation of Enallenes. J. Am. Chem. Soc. 2015, 137, 11868–11871. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, B.; Jiang, T.; Bäckvall, J. Olefin-Directed Palladium-Catalyzed Regio-and Stereoselective Oxidative Arylation of Allenes. Angew. Chem. Int. Ed. 2015, 54, 9066–9069. [Google Scholar] [CrossRef] [PubMed]
- Mahieu, A.; Terrié, C.; Youssef, B. Thermoplastic Starch Films and Thermoplastic Starch/Polycaprolactone Blends with Oxygen-Scavenging Properties: Influence of Water Content. Ind. Crop Prod. 2015, 72, 192–199. [Google Scholar] [CrossRef]
- Rhim, J.; Park, H.; Ha, C. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Farmahini-Farahani, M.; O’Hearn, P.; Xiao, H.; Ocampo, H. An Overview of Bio-Based Polymers for Packaging Materials. J. Bioresour. Bioprod. 2016, 1, 106–113. [Google Scholar]
- Castro-Mayorga, J.L.; Martínez-Abad, A.; Fabra, M.J.; Olivera, C.; Reis, M.; Lagarón, J.M. Stabilization of Antimicrobial Silver Nanoparticles by a Polyhydroxyalkanoate Obtained from Mixed Bacterial Culture. Int. J. Biol. Macromol. 2014, 71, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Cherpinski, A.; Gozutok, M.; Sasmazel, H.; Torres-Giner, S.; Lagaron, J. Electrospun Oxygen Scavenging Films of Poly(3-Hydroxybutyrate) Containing Palladium Nanoparticles for Active Packaging Applications. Nanomaterials 2018, 8, 469. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Lagaron, J.M. Post-Processing Optimization of Electrospun Sub-Micron Poly(3-Hydroxybutyrate) Fibers to Obtain Continuous Films of Interest in Food Packaging Applications. Food Addit. Contam. Part A 2017, 34, 1817–1830. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Cabedo, L.; Méndez, J.A.; Lagaron, J.M. Multilayer Structures Based on Annealed Electrospun Biopolymer Coatings of Interest in Water and Aroma Barrier Fiber-Based Food Packaging Applications. J. Appl. Polym. Sci. 2018, 135, 45501. [Google Scholar] [CrossRef]
- Hu, M.; Li, C.; Li, X.; Zhou, M.; Sun, J.; Sheng, F.; Shi, S.; Lu, L. Zinc Oxide/Silver Bimetallic Nanoencapsulated in Pvp/Pcl Nanofibres for Improved Antibacterial Activity. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1248–1257. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.E.A.; Tan, N.C.B. The Effect of Processing Variables on the Morphology of Electrospun Nanofibers and Textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Theron, S.A.; Zussman, E.; Yarin, A.L. Experimental Investigation of the Governing Parameters in the Electrospinning of Polymer Solutions. Polymer 2004, 45, 2017–2030. [Google Scholar] [CrossRef]
- Zander, N.E. Hierarchically Structured Electrospun Fibers. Polymers 2013, 5, 19–44. [Google Scholar] [CrossRef]
- Tarrés, Q.; Delgado-Aguilar, M.; Pèlach, M.A.; González, I.; Boufi, S.; Mutjé, P. Remarkable Increase of Paper Strength by Combining Enzymatic Cellulose Nanofibers in Bulk and Tempo-Oxidized Nanofibers as Coating. Cellulose 2016, 23, 3939–3950. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Castro-Mayorga, J.L.; Andrade-Mahecha, M.M.; Cabedo, L.; Lagaron, J.M. Antibacterial and Barrier Properties of Gelatin Coated by Electrospun Polycaprolactone Ultrathin Fibers Containing Black Pepper Oleoresin of Interest in Active Food Biopackaging Applications. Nanomaterials 2018, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Lasprilla-Botero, J.; STorres-Giner, E.; Pardo-Figuerez, M.; Álvarez-Láinez, M.; Lagaron, J.M. Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly (Ε-Caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings 2018, 8, 173. [Google Scholar] [CrossRef]
- Stachewicz, U.; Modaresifar, F.; Bailey, R.J.; Peijs, T.; Barber, A.H. Manufacture of Void-Free Electrospun Polymer Nanofiber Composites with Optimized Mechanical Properties. ACS Appl. Mater. Interfaces 2012, 4, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Stachewicz, U.; Qiao, T.; Rawlinson, S.C.F.; Almeida, F.V.; Li, W.; Cattell, M.; Barber, A.H. 3d Imaging of Cell Interactions with Electrospun Plga Nanofiber Membranes for Bone Regeneration. Acta Biomater. 2015, 27, 88–100. [Google Scholar] [CrossRef]
- Bailey, R.J.; Geurts, R.; Stokes, D.J.; de Jong, F.; Barber, A.H. Evaluating Focused Ion Beam Induced Damage in Soft Materials. Micron 2013, 50, 51–56. [Google Scholar] [CrossRef]
- Stachewicz, U.; Bailey, R.J.; Zhang, H.; Stone, C.A.; Willis, C.R.; Barber, A.H. Wetting Hierarchy in Oleophobic 3d Electrospun Nanofiber Networks. ACS Appl. Mater. Interfaces 2015, 7, 16645–16652. [Google Scholar] [CrossRef]
- Garg, K.; Bowlin, G.L. Electrospinning Jets and Nanofibrous Structures. Biomicrofluidics 2011, 5, 013403. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lan, C.; Wang, S.; Fang, P.; Sun, Y. Influence of Zinc Oxide Nanoparticles on the Crystallization Behavior of Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanofibers. Polymer 2010, 51, 2403–2409. [Google Scholar] [CrossRef]
- Kane, M. Investigation and Characterization of the Dispersion of Nanoparticles in a Polymer Matrix by Scattering Techniques. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2007. [Google Scholar]
- Powers, K.W.; Palazuelos, M.; Moudgil, B.M.; Roberts, S.M. Characterization of the Size, Shape, and State of Dispersion of Nanoparticles for Toxicological Studies. Nanotoxicology 2007, 1, 42–51. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Hezma, A.M.; El-khodary, A.; Elzayat, A.M. Spectroscopic Studies and Thermal Properties of Pcl/Pmma Biopolymer Blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Liverani, L.; Lacina, J.; Roether, J.A.; Boccardi, E.; Killian, M.S.; Schmuki, P.; Schubert, D.W.; Boccaccini, A.R. Incorporation of Bioactive Glass Nanoparticles in Electrospun Pcl/Chitosan Fibers by Using Benign Solvents. Bioact. Mater. 2018, 3, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Finkenstadt, V.L.; Gordon, S.H.; Biresaw, G.; Palmquist, D.E.; Rayas-Duarte, P. Thermal Properties of Pcl/Gluten Bioblends Characterized by Tga, Dsc, Sem, and Infrared-Pas. J. Appl. Polym. Sci. 2008, 110, 3256–3266. [Google Scholar] [CrossRef]
- Aliah, N.N.; Ansari, M.N.M. Thermal Analysis on Characterization of Polycaprolactone (Pcl) E Chitosan Scaffold for Tissue Engineering. Int. J. Sci. Res. Eng. Technol. 2017, 6, 2278–2882. [Google Scholar]
- Bajsić, E.G.; Bulatović, V.O.; Slouf, M.; Šitum, A. Characterization of Biodegradable Polycaprolactone Containing Titanium Dioxide Micro and Nanoparticles. Int. J. Chem. Nucl. Met. Mater. Eng. 2014, 8, 572–576. [Google Scholar]
- Wang, G.; Yang, S.; Wei, Z.; Dong, X.; Wang, H.; Qi, M. Facile Preparation of Poly(Ε-Caprolactone)/Fe3o4@Graphene Oxide Superparamagnetic Nanocomposites. Polym. Bull. 2013, 70, 2359–2371. [Google Scholar] [CrossRef]
- Hong, R.Y.; Qian, J.Z.; Cao, J.X. Synthesis and Characterization of Pmma Grafted Zno Nanoparticles. Powder Technol. 2006, 163, 160–168. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Lagaron, J.M. Stabilized Nanosilver Based Antimicrobial Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanocomposites of Interest in Active Food Packaging. Innov. Food Sci. Emerg. Technol. 2016, 33, 524–533. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly (3-Hydroxybutyrate)/Zno Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Ionita, E.R.; Nicolae, C.; Gabor, A.R.; Ionita, M.D.; Trusca, R.; Lixandru, B.; Codita, I.; Dinescu, G. Poly (3-Hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications. Polymers 2018, 10, 1249. [Google Scholar] [CrossRef]
- Cherpinski, A.; Torres-Giner, S.; Vartiainen, J.; Peresin, M.S.; Lahtinen, P.; Lagaron, J.M. Improving the Water Resistance of Nanocellulose-Based Films with Polyhydroxyalkanoates Processed by the Electrospinning Coating Technique. Cellulose 2018, 25, 1291–1307. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012. [Google Scholar] [CrossRef]
- Zeman, S.; Kubík, L. Permeability of Polymeric Packaging Materials. Tech. Sci. 2007, 10, 26–34. [Google Scholar] [CrossRef]
- Cavalcante, M.; Rodrigues, E.; Tavares, M.I. Avaliação Da Cristalinidade De Blendas De Polihidróxibutirato E Policaprolactona. In Proceedings of the 13th Congresso Brasileiro de Polímeros, Natal-RN, Brazil, 18 October 2015. [Google Scholar]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and Barrier Properties of Nanobiocomposites of Poly (3-Hydroxybutyrate) and Layered Silicates. J. Appl. Polym. Sci. 2008, 108, 2787–2801. [Google Scholar] [CrossRef]
- Shogren, R. Water Vapor Permeability of Biodegradable Polymers. J. Environ. Polym. Degrad. 1997, 5, 91–95. [Google Scholar] [CrossRef]
- Gibis, D.; Rieblinger, K. Oxygen Scavenging Films for Food Application. Procedia Food Sci. 2011, 1, 229–234. [Google Scholar] [CrossRef]

| Sample | Tm (°C) | ΔHm (J/g) | Tc (J/g) | ΔHc (J/g) |
|---|---|---|---|---|
| PCL Fibers | 59.7 ± 1.2 a | 33.8 ± 2.0 b | 32.6 ± 0.9 b | 41.4 ± 2.1 b |
| PCL/PdNP Fibers | 64.7 ± 0.7 b | 27.8 ± 1.5 a | 31.2 ± 1.2 a | 38.2 ± 0.9 a |
| Sample | T5% (°C) | Td1 (°C) | Td2 (°C) | R900 (%) |
|---|---|---|---|---|
| PCL | 342.5 ± 4.1 | 388.0 ± 5.3 | 449.3 ± 4.2 | 1.3 ± 0.05 |
| PCL-PdNP | 355.1 ± 4.5 | 391.7 ± 3.6 | 447.8 ± 5.3 | 2.1 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherpinski, A.; Szewczyk, P.K.; Gruszczyński, A.; Stachewicz, U.; Lagaron, J.M. Oxygen-Scavenging Multilayered Biopapers Containing Palladium Nanoparticles Obtained by the Electrospinning Coating Technique. Nanomaterials 2019, 9, 262. https://doi.org/10.3390/nano9020262
Cherpinski A, Szewczyk PK, Gruszczyński A, Stachewicz U, Lagaron JM. Oxygen-Scavenging Multilayered Biopapers Containing Palladium Nanoparticles Obtained by the Electrospinning Coating Technique. Nanomaterials. 2019; 9(2):262. https://doi.org/10.3390/nano9020262
Chicago/Turabian StyleCherpinski, Adriane, Piotr K. Szewczyk, Adam Gruszczyński, Urszula Stachewicz, and Jose M. Lagaron. 2019. "Oxygen-Scavenging Multilayered Biopapers Containing Palladium Nanoparticles Obtained by the Electrospinning Coating Technique" Nanomaterials 9, no. 2: 262. https://doi.org/10.3390/nano9020262
APA StyleCherpinski, A., Szewczyk, P. K., Gruszczyński, A., Stachewicz, U., & Lagaron, J. M. (2019). Oxygen-Scavenging Multilayered Biopapers Containing Palladium Nanoparticles Obtained by the Electrospinning Coating Technique. Nanomaterials, 9(2), 262. https://doi.org/10.3390/nano9020262








