Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sage Extract
2.3. Characterization of Sage Extract
2.3.1. Antioxidant Activity
2.3.2. Antimicrobial Activity
2.4. Preparation of Poly(ε-caprolactone) Based Films
2.4.1. Preparation of Solutions for Electrospinning
2.4.2. Characterization of the Solutions
2.4.3. Electro-Hydrodynamic Processing
2.5. Characterization of The PCL Based Films
2.5.1. Film Thickness
2.5.2. Morphology
2.5.3. Transparency
2.5.4. Water Contact Angle
2.5.5. Thermal Analysis
2.5.6. Mechanical Properties
2.5.7. Water Vapor Permeability (WVP)
2.5.8. D-Limonene Permeability (LP)
2.5.9. Antioxidant Activity
2.5.10. Antimicrobial Activity
2.5.11. Statistical Analysis
3. Results and Discussion
3.1. Sage Extract Characterization
3.1.1. Antioxidant Activity
3.1.2. Antimicrobial Activity
3.2. Solution Characterization
3.3. Film Characterization
3.3.1. Morphology
3.3.2. Optical Properties
3.3.3. Water Contact Angle
3.3.4. Thermogravimetric Analysis
3.3.5. Mechanical Properties
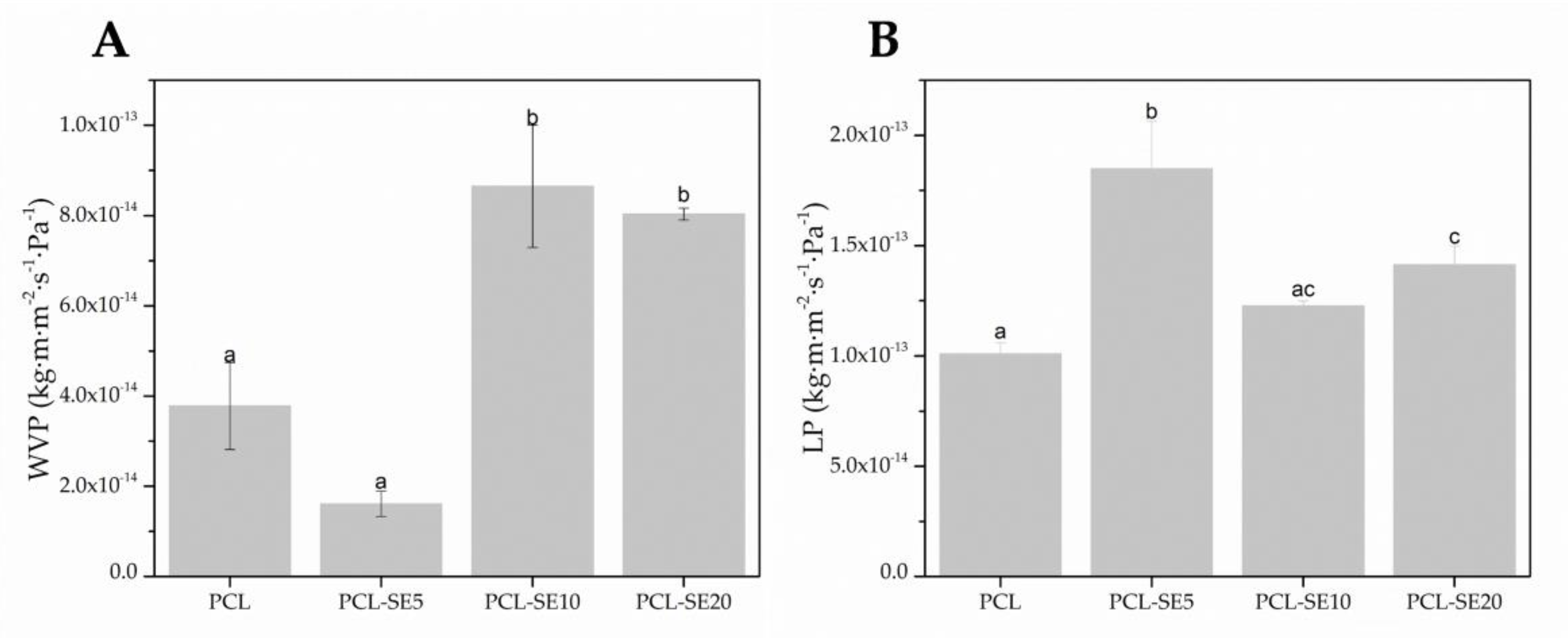
3.3.6. Water Vapor and D-Limonene Permeability
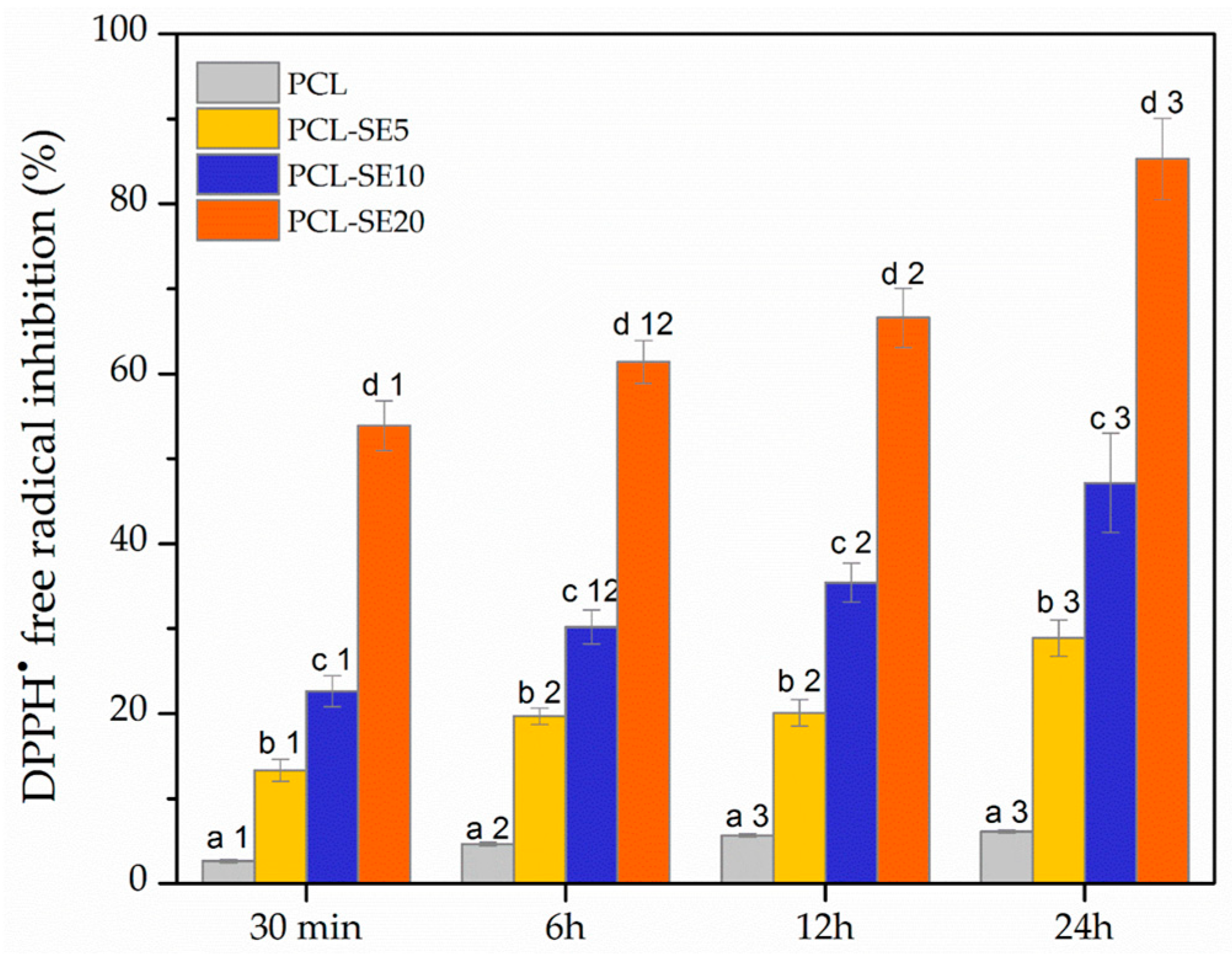
3.3.7. Antioxidant Activity
3.3.8. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crop. Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Đorđević, V.; Karlović, S.; Pavlović, V.; Komes, D.; Ježek, D.; Bugarski, B.; Nedović, V. Protein-reinforced and chitosan-pectin coated alginate microparticles for delivery of flavan-3-ol antioxidants and caffeine from green tea extract. Food Hydrocoll. 2015, 51, 361–374. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.; Fabra, M.; Cabedo, L.; Lagaron, J. On the Use of the Electrospinning Coating Technique to Produce Antimicrobial Polyhydroxyalkanoate Materials Containing In Situ-Stabilized Silver Nanoparticles. Nanomaterials 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Salmieri, S.; Lacroix, M. Physicochemical properties of alginate/polycaprolactone-based films containing essential oils. J. Agric. Food Chem. 2006, 54, 10205–10214. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19. [Google Scholar] [CrossRef]
- Ozdemir, M.; Floros, J.D. Active food packaging technologies. Crit. Rev. Food Sci. Nutr. 2004, 44, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crop. Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2014, 170, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. Jaocsj. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Food for Human Consumption, Part 182—Substances Generally Recognized as Safe. Fed. Regist. 2016, 81, 54960–55055. [Google Scholar]
- Cherpinski, A.; Gozutok, M.; Sasmazel, H.; Torres-Giner, S.; Lagaron, J.; Cherpinski, A.; Gozutok, M.; Sasmazel, H.T.; Torres-Giner, S.; Lagaron, J.M. Electrospun Oxygen Scavenging Films of Poly(3-hydroxybutyrate) Containing Palladium Nanoparticles for Active Packaging Applications. Nanomaterials 2018, 8, 469. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abad, A.; Sánchez, G.; Fuster, V.; Lagaron, J.M.; Ocio, M.J. Antibacterial performance of solvent cast polycaprolactone (PCL) films containing essential oils. Food Control 2013, 34, 214–220. [Google Scholar] [CrossRef]
- Erbay, E.A.; Dağtekin, B.B.; Türe, M.; Yeşilsu, A.F.; Torres-Giner, S. Quality improvement of rainbow trout fillets by whey protein isolate coatings containing electrospun poly(ε-caprolactone) nanofibers with Urtica dioica L. extract during storage. LWT 2017, 78, 340–351. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Ghorani, B.; Tucker, N. Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Fabra, M.J.; López-Rubio, A.; Sentandreu, E.; Lagaron, J.M. Development of multilayer corn starch-based food packaging structures containing β-carotene by means of the electro-hydrodynamic processing. Starch/Staerke 2016, 68, 603–610. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.; Castro-Mayorga, J.; Andrade-Mahecha, M.; Cabedo, L.; Lagaron, J. Antibacterial and Barrier Properties of Gelatin Coated by Electrospun Polycaprolactone Ultrathin Fibers Containing Black Pepper Oleoresin of Interest in Active Food Biopackaging Applications. Nanomaterials 2018, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Akcan, T.; Estévez, M.; Serdaroğlu, M. Antioxidant protection of cooked meatballs during frozen storage by whey protein edible films with phytochemicals from Laurus nobilis L. and Salvia officinalis. LWT-Food Sci. Technol. 2017, 77, 323–331. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocoll. 2018, 74, 324–332. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- ASTM D638-10. Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM E96-95. Standard Test Methods for Water Vapour Transmission of Materials; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- Japanese Industrial Standard Committee. JIS Z 2801:2000 Antimicrobial Products—Test for Antimicrobial Activity and Efficacy; Japanese Standards Association: Tokyo, Japan, 2000. [Google Scholar]
- Chen, H.Y.; Lin, Y.C.; Hsieh, C.L. Evaluation of antioxidant activity of aqueous extract of some selected nutraceutical herbs. Food Chem. 2007, 104, 1418–1424. [Google Scholar] [CrossRef]
- Farhat Ben, M.; Landoulsi, A.; Chaouch-hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crop. Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant activities of polyphenols from sage (Salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Gorjanović, S.; Komes, D.; Pastor, F.T.; Belščak-Cvitanović, A.; Pezo, L.; Hečimović, I.; Suźnjević, D.S. Antioxidant Capacity of Teas and Herbal Infusions: Polarographic Assessment. J. Agric. Food Chem. 2012, 60, 9573–9580. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial efficacy of plant phenolic compounds against Salmonella and Escherichia Coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Tampau, A.; Gonzalez-Martinez, C.; Chiralt, A. Carvacrol encapsulation in starch or PCL based matrices by electrospinning. J. Food Eng. 2017, 214, 245–256. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocoll. 2018, 79, 158–169. [Google Scholar] [CrossRef]
- Radisavljevic, A.; Stojanovic, D.B.; Perisic, S.; Djokic, V.; Radojevic, V.; Rajilic-Stojanovic, M.; Uskokovic, P.S. Cefazolin-loaded polycaprolactone fibers produced via different electrospinning methods: Characterization, drug release and antibacterial effect. Eur. J. Pharm. Sci. 2018, 124, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Three-Layer Films Based on Wheat Gluten and Electrospun PHA. Food Bioprocess Technol. 2015, 8, 2330–2340. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669–680. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Sci. 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Krochta, J.M. Wetting properites and water vapor permeability of whey-protein-coated paper. Trans. Asae 1999, 42, 1375–1382. [Google Scholar] [CrossRef]
- Pardo-Figuerez, M.; López-Córdoba, A.; Torres-Giner, S.; Lagaron, J. Superhydrophobic Bio-Coating Made by Co-Continuous Electrospinning and Electrospraying on Polyethylene Terephthalate Films Proposed as Easy Emptying Transparent Food Packaging. Coatings 2018, 8, 364. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Torres-Giner, S.; Pardo-Figuerez, M.; Álvarez-Láinez, M.; Lagaron, J.M. Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings 2018, 8, 173. [Google Scholar] [CrossRef]
- Jaramillo, C.M.; González Seligra, P.; Goyanes, S.; Bernal, C.; Famá, L. Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. Starch/Staerke 2015, 67, 780–789. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur. Polym. J. 2016, 79, 140–149. [Google Scholar] [CrossRef]
- Yang, X.; Cui, C.; Tong, Z.; Sabanayagam, C.R.; Jia, X. Poly(ε-caprolactone)-based copolymers bearing pendant cyclic ketals and reactive acrylates for the fabrication of photocrosslinked elastomers. Acta Biomater. 2013, 9, 8232–8244. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Lee, S.M.; Lee, J.S.; Lee, Y.S. Development of antimicrobial polyolefin films containing lauroyl arginate and their use in the packaging of strawberries. J. Food Meas. Charact. 2017, 11, 1706–1716. [Google Scholar] [CrossRef]
- Péroval, C.; Debeaufort, F.; Despré, D.; Voilley, A. Edible arabinoxylan-based films. 1. Effects of lipid type on water vapor permeability, film structure, and other physical characteristics. J. Agric. Food Chem. 2002, 50, 3977–3983. [Google Scholar] [CrossRef]
- Cava, D.; Giménez, E.; Gavara, R.; Lagaron, J.M. Comparative performance and barrier properties of biodegradable thermoplastics and nanobiocomposites versus PET for food packaging applications. J. Plast. Film Sheeting 2006, 22, 265–274. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Novel PET nanocomposites of interest in food packaging applications and comparative barrier performance with biopolyester nanocomposites. J. Plast. Film Sheeting 2007, 23, 133–148. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Tanner, C.; Cayot, P.; Karbowiak, T.; Debeaufort, F. Impact of functional properties and release kinetics on antioxidant activity of biopolymer active films and coatings. Food Chem. 2018, 242, 369–377. [Google Scholar] [CrossRef]
- Hromiš, N.M.; Lazić, V.L.; Markov, S.L.; Vaštag, Ž.G.; Popović, S.Z.; Šuput, D.Z.; Džinić, N.R.; Velićanski, A.S.; Popović, L.M. Optimization of chitosan biofilm properties by addition of caraway essential oil and beeswax. J. Food Eng. 2015, 158, 86–93. [Google Scholar] [CrossRef]
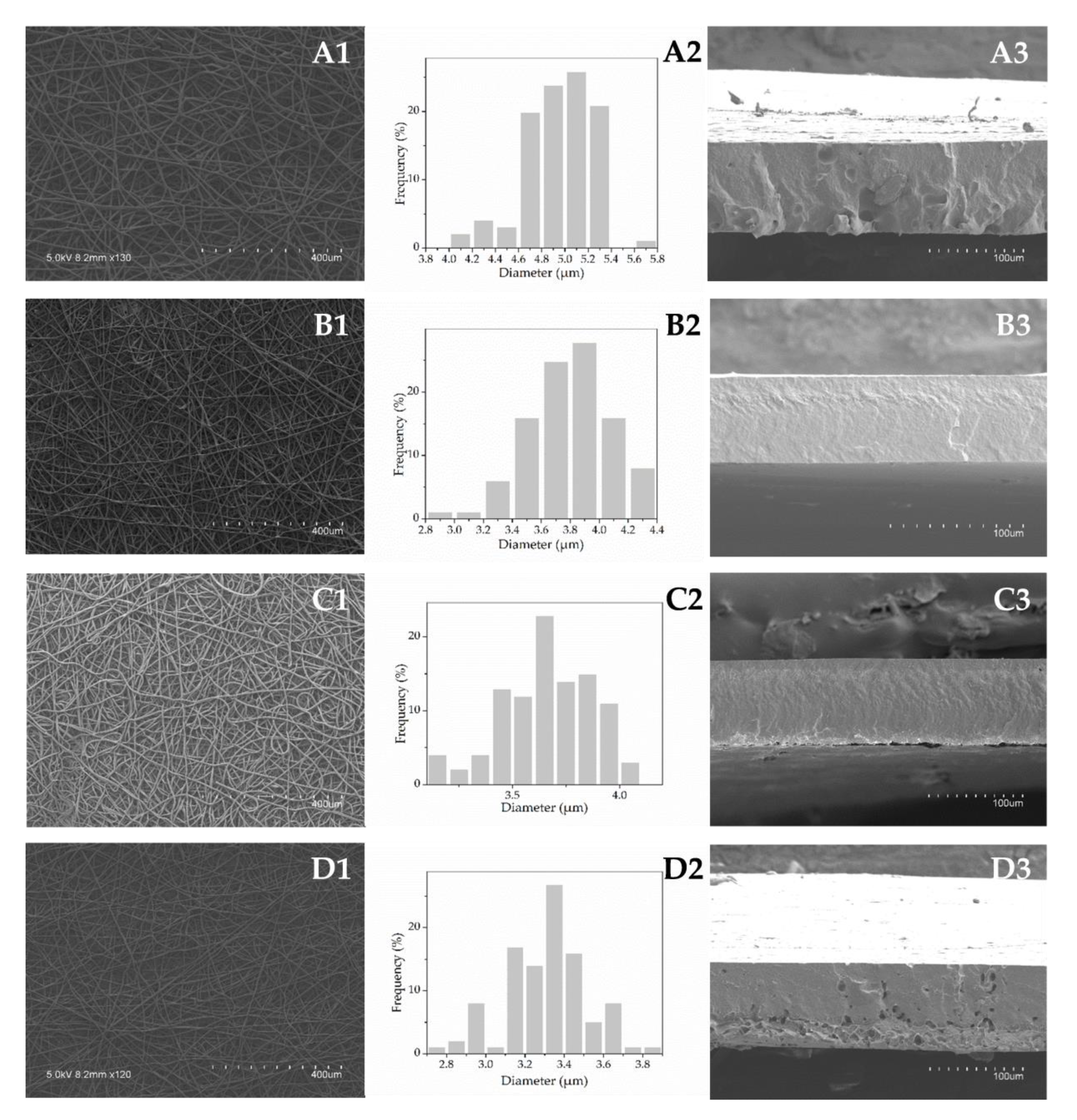

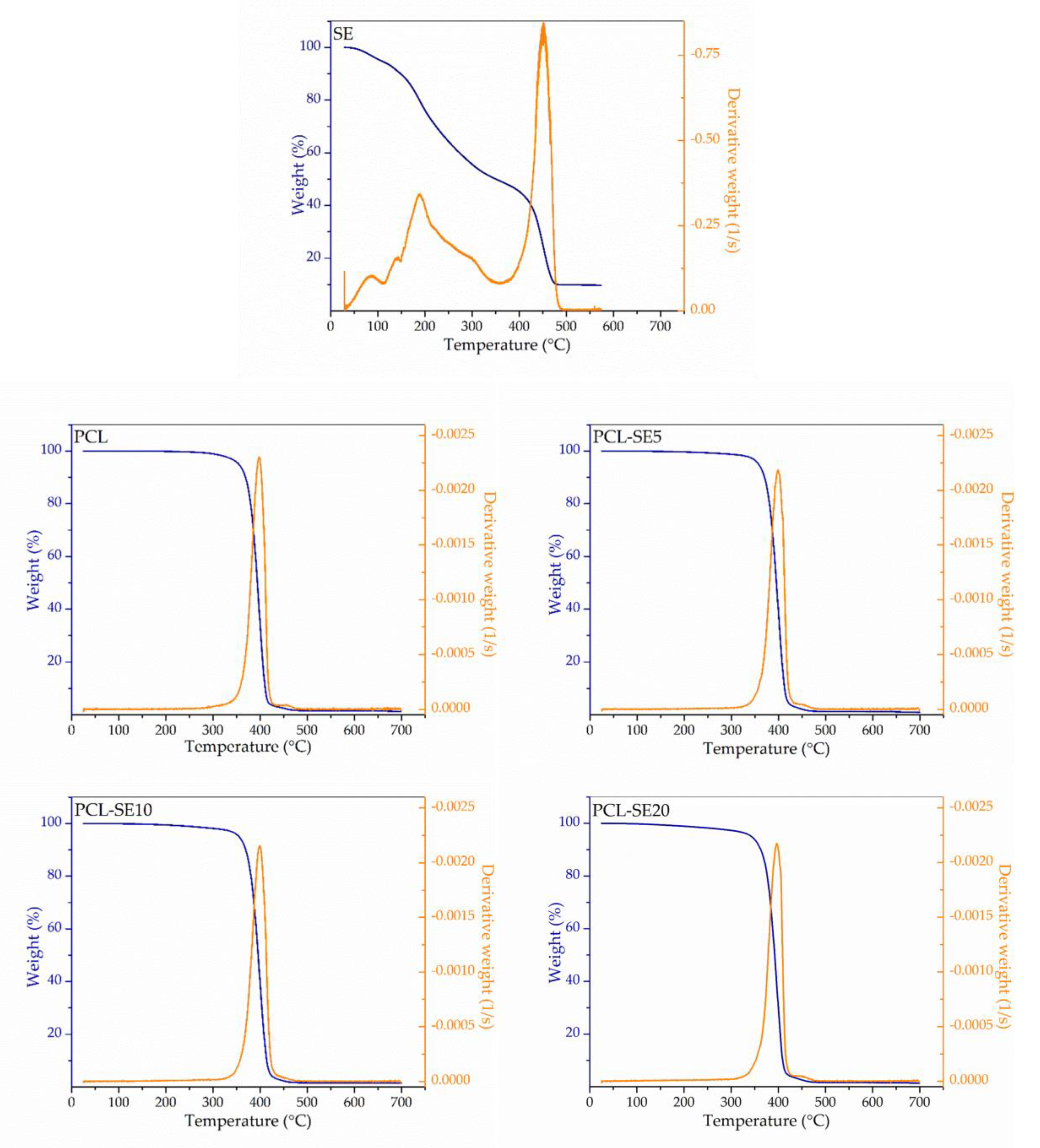
| Formulation | Viscosity (cP) | Conductivity (µS/cm) | Surface Tension (mN/m) | Fiber Diameter (µm) | Film Thickness (mm) |
|---|---|---|---|---|---|
| PCL | 1908.1 ± 41.2 a | 0.02 ± 0.00 a | 27.2 ± 0.2 a | 4.95 ± 0.29 | 0.09 ± 0.01 a |
| PCL-SE5 | 1695.6 ± 36.6 b | 0.02 ± 0.00 a | 27.4 ± 0.1 a | 3.80 ± 0.28 | 0.10 ± 0.01 a |
| PCL-SE10 | 1579.2 ± 33.7 b,c | 0.10 ± 0.00 b | 27.5 ± 0.1 a | 3.65 ± 0.21 | 0.08 ± 0.01 b |
| PCL-SE20 | 1565.9 ± 35.6 c | 0.09 ± 0.00 b | 24.7 ± 0.1 b | 3.31 ± 0.21 | 0.08 ± 0.01 b |
| Formulation | E (MPa) | σb (MPa) | εb (%) | T (mJ/m3) |
|---|---|---|---|---|
| PCL | 420.77 ± 61.45 a | 24.32 ± 3.79 a | 4.60 ± 0.34 a | 1.41 ± 0.19 a |
| PCL-SE5 | 425.40 ± 40.85 a | 26.11 ± 2.76 a | 4.90 ± 0.48 a | 1.51 ± 0.09 a |
| PCL-SE10 | 410.78 ± 24.70 a | 31.98 ± 4.42 a | 5.44 ± 0.78 a | 1.62 ± 0.02 a |
| PCL-SE20 | 359.25 ± 63.43 a | 26.43 ± 5.19 a | 5.28 ± 0.57 a | 1.52 ± 0.15 a |
| Formulation | S. aureus | E. coli | ||
|---|---|---|---|---|
| Bacterial Counts log CFU/mL | Surface Reduction R | Bacterial Counts log CFU/mL | Surface Reduction R | |
| Control | 6.10 ± 0.09 | - | 7.09 ± 0.12 | - |
| PCL | 5.77 ± 0.15 | 0.33 | 6.89 ± 0.09 | 0.20 |
| PCL-SE5 | no viable counts | biocidal effect | 6.36 ± 0.13 | 0.73 |
| PCL-SE10 | no viable counts | biocidal effect | 6.18 ± 0.16 | 0.91 |
| PCL-SE20 | no viable counts | biocidal effect | 2.40 ± 0.52 | 4.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salević, A.; Prieto, C.; Cabedo, L.; Nedović, V.; Lagaron, J.M. Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials 2019, 9, 270. https://doi.org/10.3390/nano9020270
Salević A, Prieto C, Cabedo L, Nedović V, Lagaron JM. Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials. 2019; 9(2):270. https://doi.org/10.3390/nano9020270
Chicago/Turabian StyleSalević, Ana, Cristina Prieto, Luis Cabedo, Viktor Nedović, and Jose Maria Lagaron. 2019. "Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract" Nanomaterials 9, no. 2: 270. https://doi.org/10.3390/nano9020270
APA StyleSalević, A., Prieto, C., Cabedo, L., Nedović, V., & Lagaron, J. M. (2019). Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials, 9(2), 270. https://doi.org/10.3390/nano9020270








