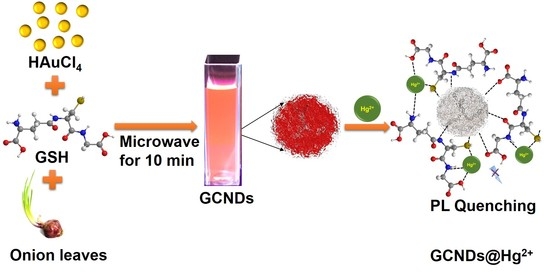
Biosynthesized Highly Stable Au/C Nanodots: Ideal Probes for the Selective and Sensitive Detection of Hg2+ Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
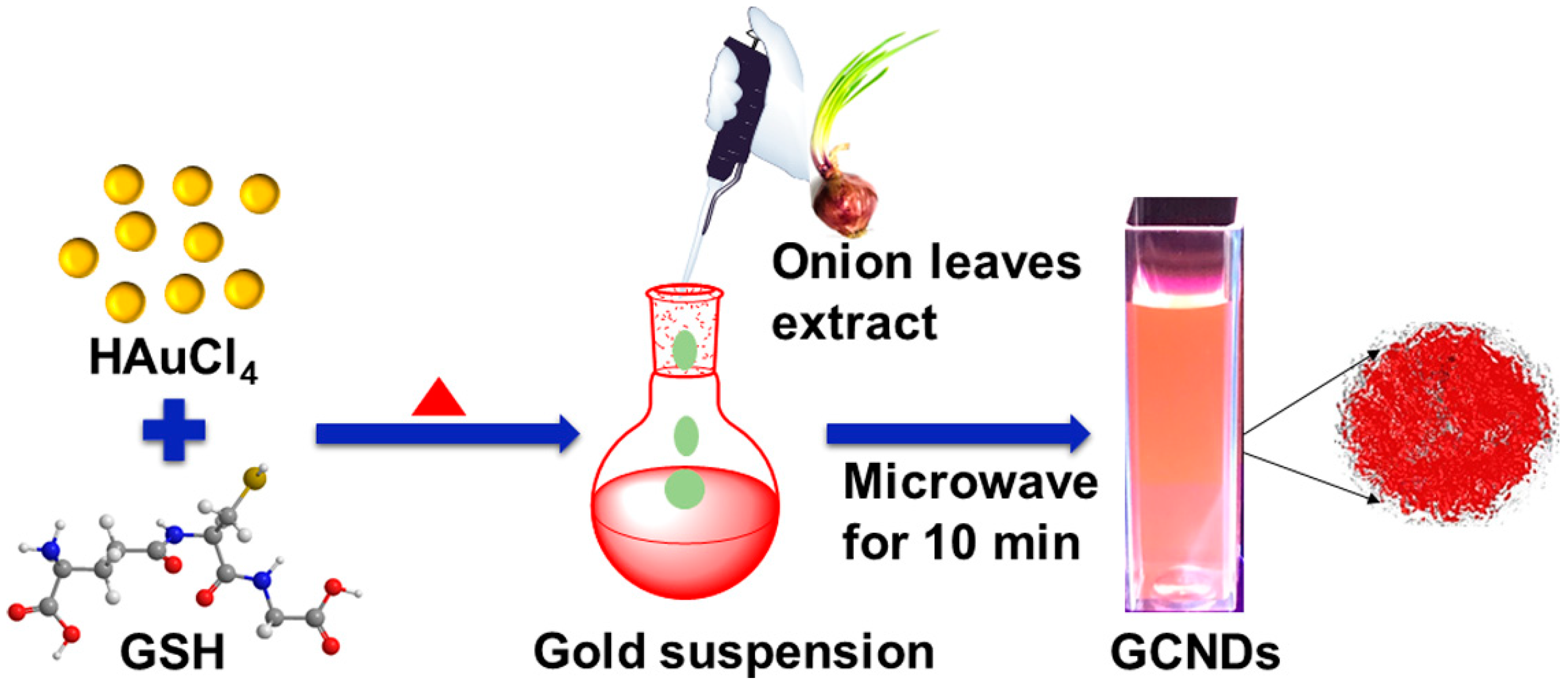
2.2. Synthesis of GCNDs
2.3. Characterization
2.4. Detection of Hg2+ Ions Using Fluorescent GCNDs Probe
3. Results and Discussion
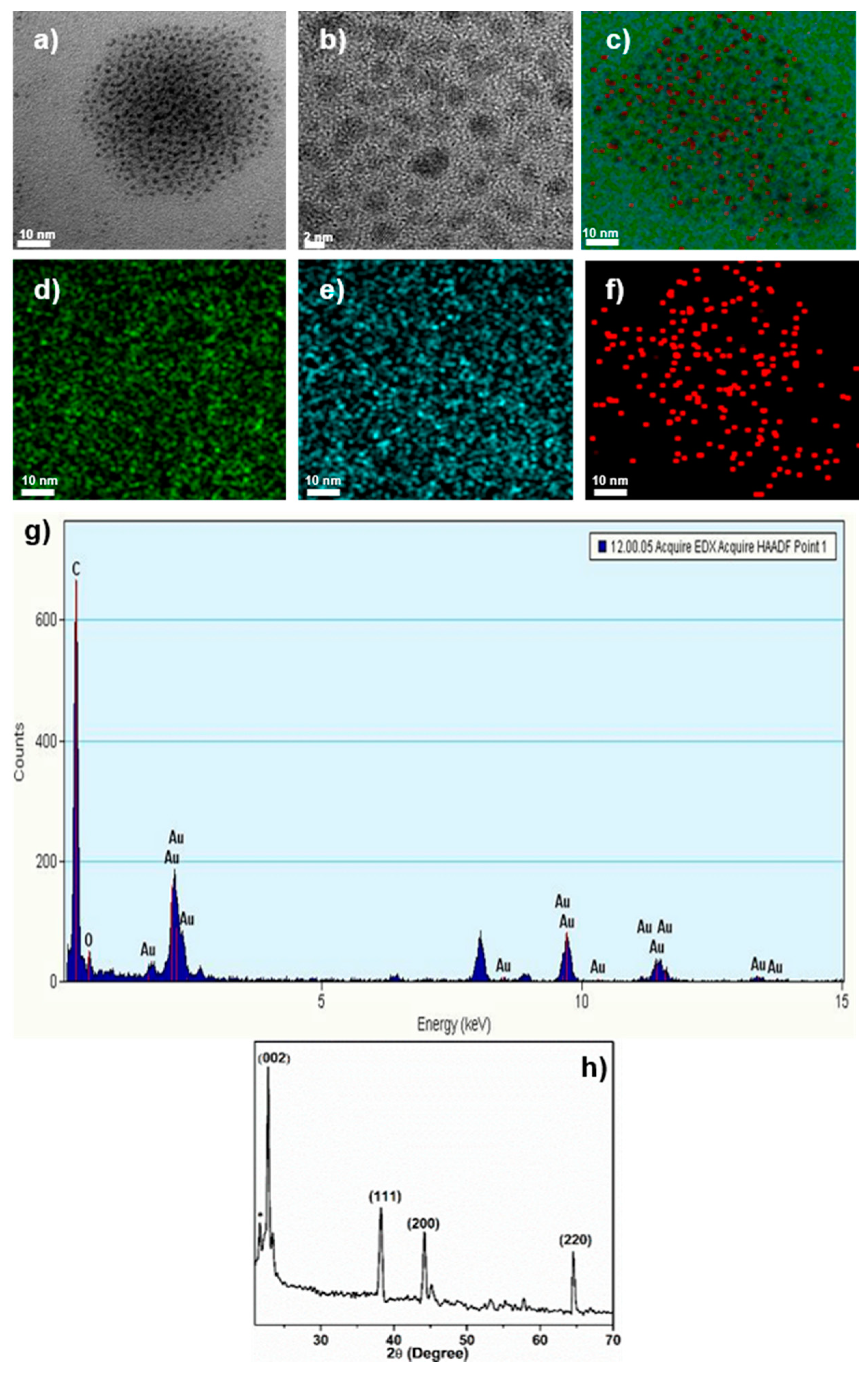
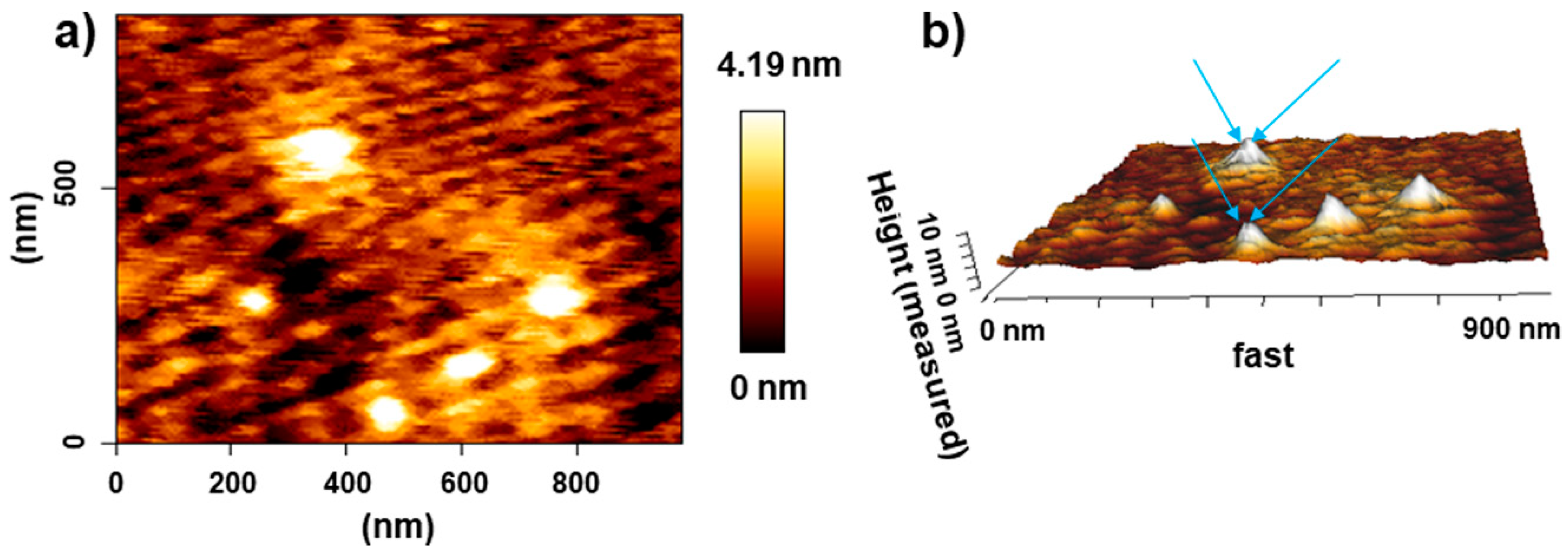
3.1. Material Characterization
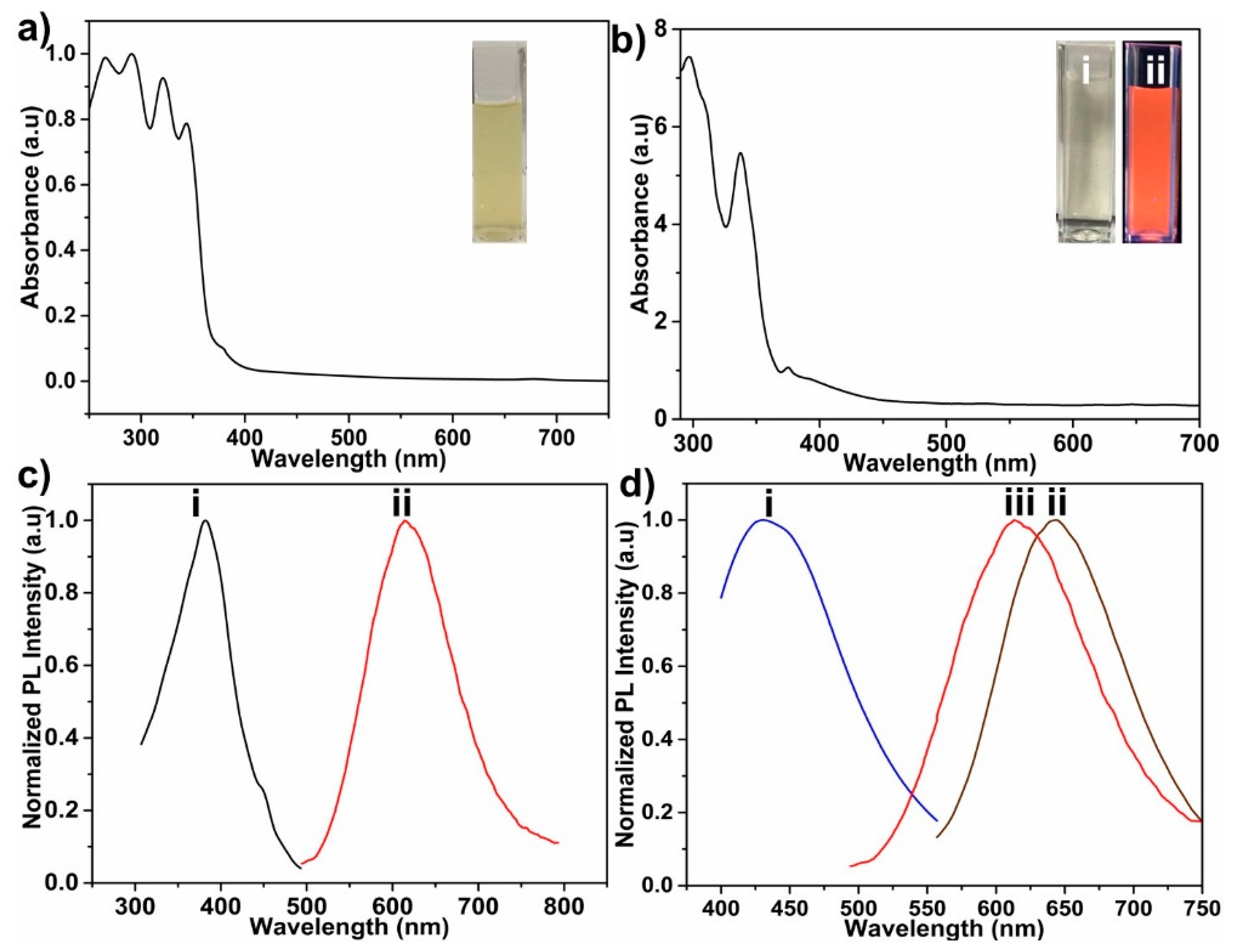
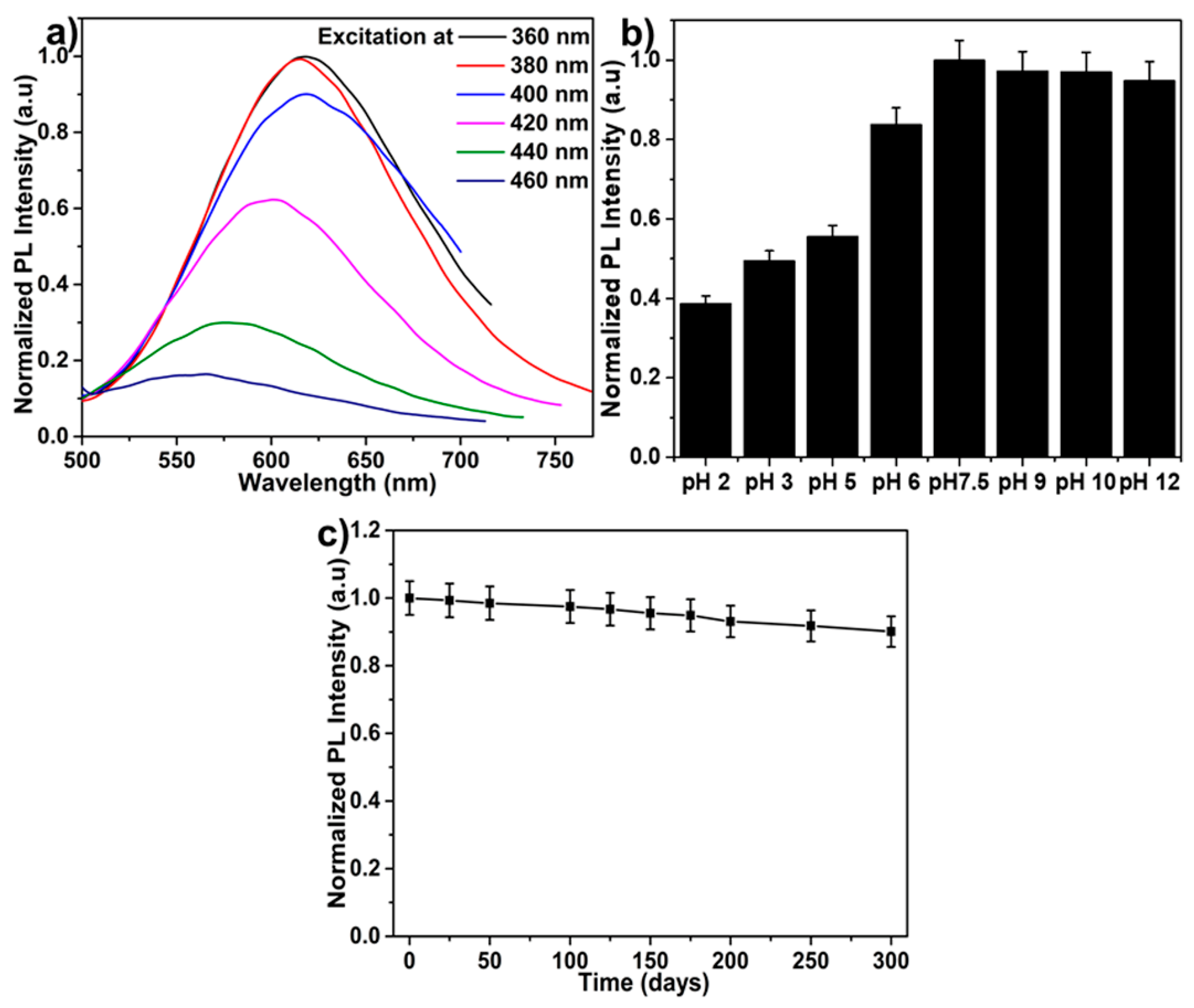
3.2. Optical Properties of GCNDs
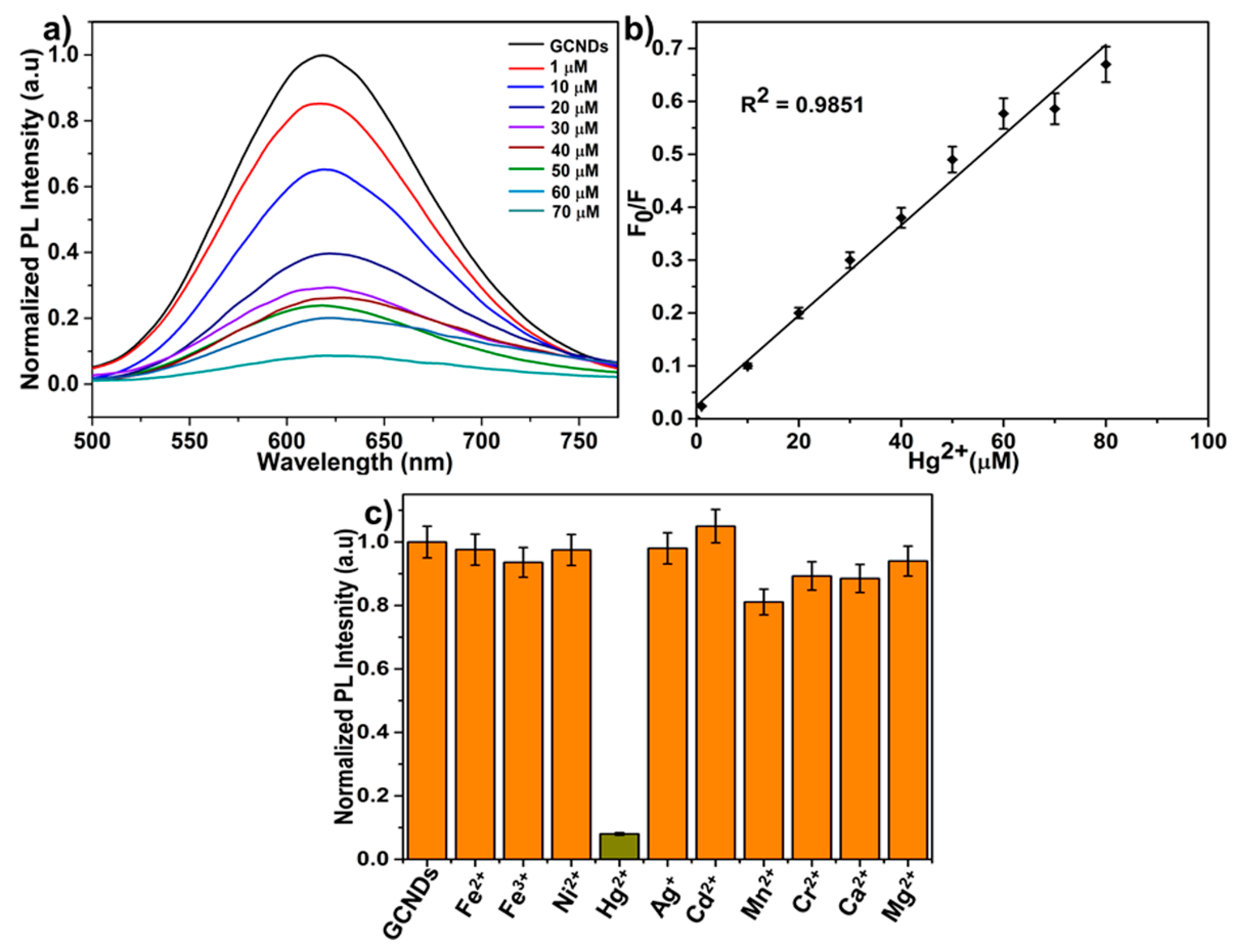
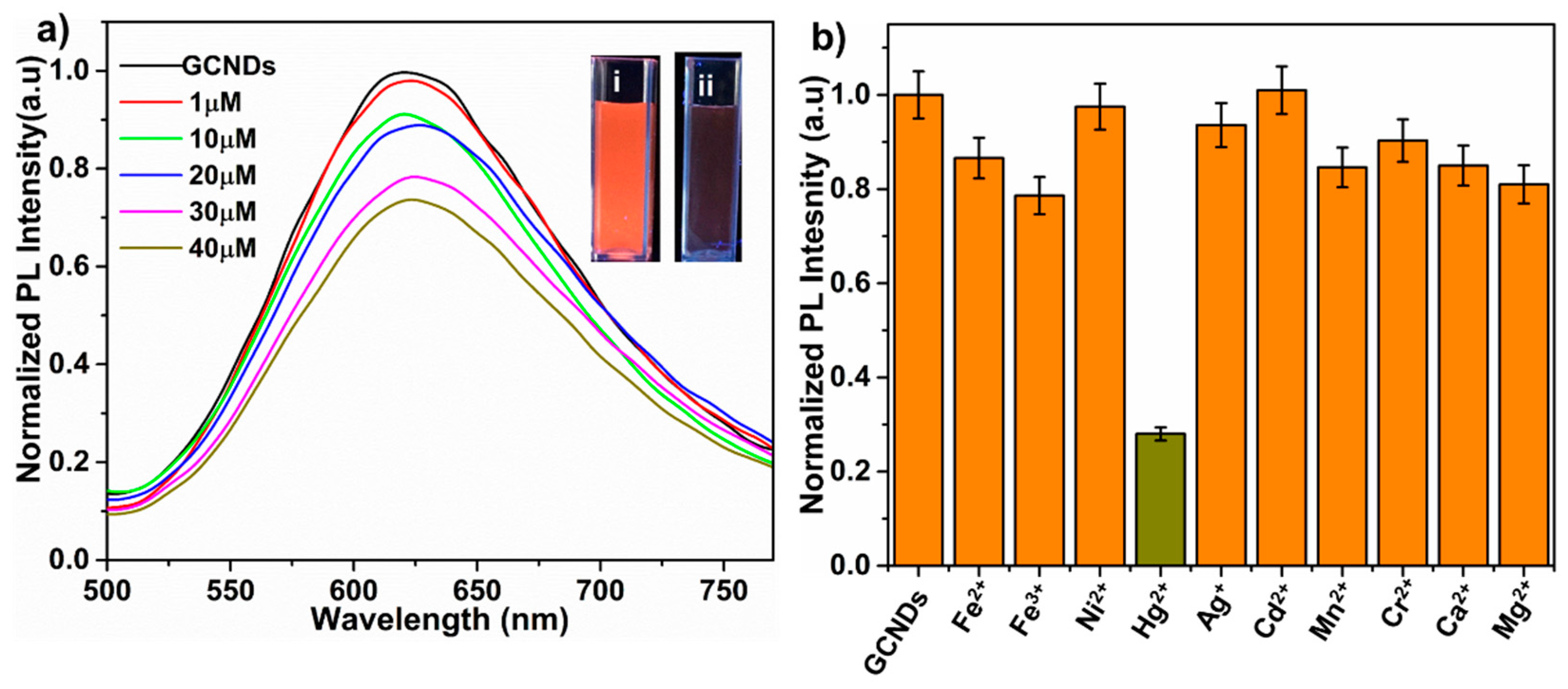
3.3. Sensitivity and Selectivity of GCNDs Towards Hg2+ Ions
3.4. Reproducibility Study
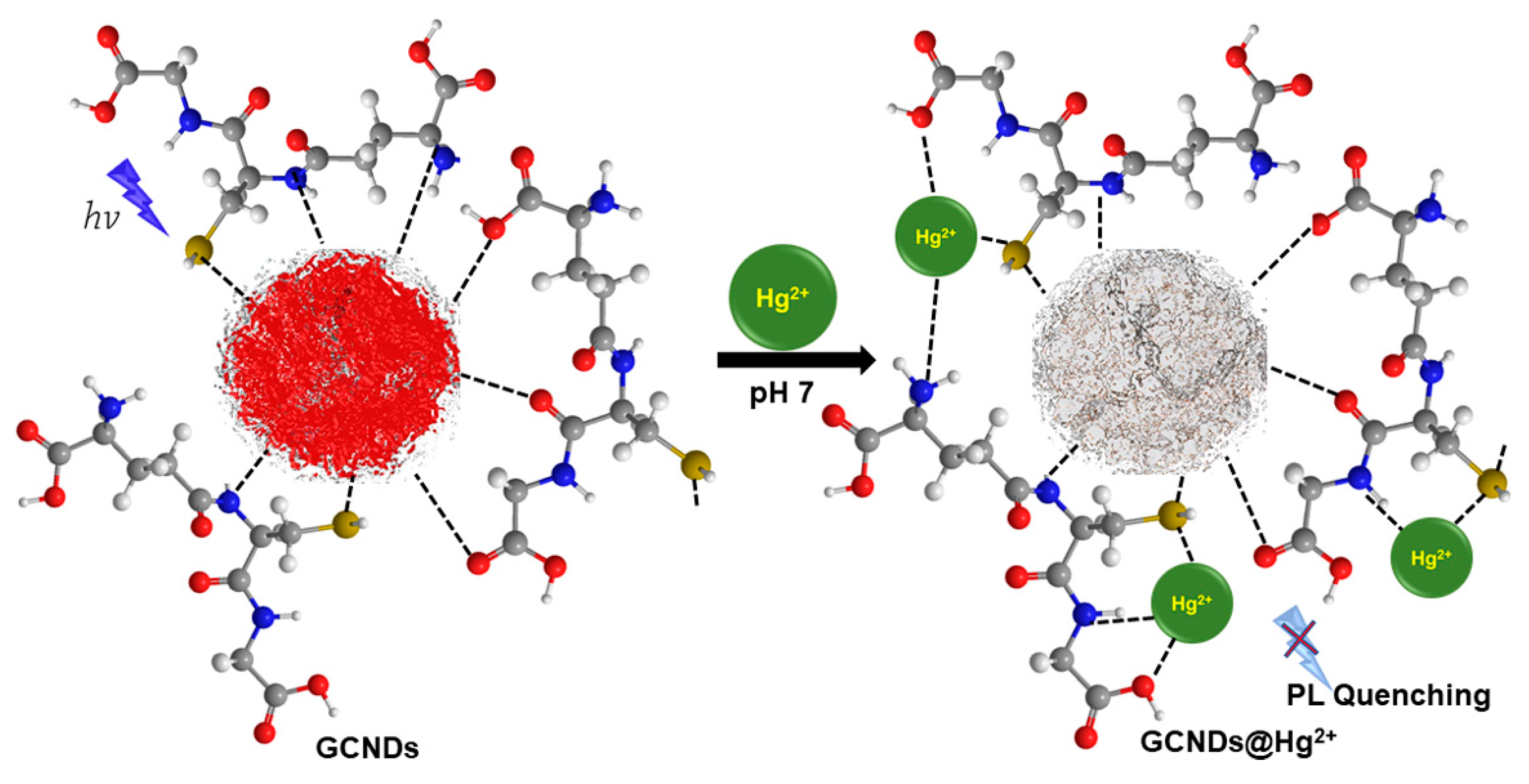
3.5. Quenching Mechanism of GCNDs by Hg2+ Ions
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Govindaraju, S.; Puthiaraj, P.; Lee, M.H.; Yun, K. Photoluminescent AuNCs@ Uio-66 for Ultrasensitive Detection of Mercury in Water Samples. ACS Omega 2018, 3, 12052–12059. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Panda, A.; Kim, E.; Yoon, M. Biopolymer-Coated Magnetite Nanoparticles and Metal–Organic Framework Ternary Composites for Cooperative Pb (II) Adsorption. ACS Appl. Nano Mater. 2018, 1, 4198–4210. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of Contaminants of Emerging Concern by Membranes in Water and Wastewater: A Review. Chem. Eng. J. 2017, 335, 896–914. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Lee, D.; Yoon, M. Bioinspired 2D-Carbon Flakes and Fe3O4 Nanoparticles Composite for Arsenite Removal. ACS Appl. Mater. Interfaces 2016, 8, 23876–23885. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Mazumder, M.A.J. A New Resin Embedded With Chelating Motifs of Biogenic Methionine for the Removal of Hg (II) at ppb Levels. J. Hazard. Mater. 2018, 350, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.F.O.; Hott, R.C.; Ladeira, P.C.C.; Batista, B.L.; Andrade, T.G.; Santos, M.S.; Faria, M.C.S.; Oliveira, L.C.A.; Monteiro, D.S.; Pereira, M.C.; et al. Simple Synthesis and Characterization of l-Cystine Functionalized δ-FeOOH for Highly Efficient Hg (II) Removal From Contamined Water and Mining Waste. Chemosphere 2019, 215, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Ma, S.; Zhang, S.; Liu, H.; Gong, B.; Zhang, J.; Zheng, C. Emission Controls of Mercury and Other Trace Elements During Coal Combustion in China: A Review. Int. Geol. Rev. 2018, 60, 638–670. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.H. Nanomaterials for the Adsorptive Treatment of Hg (II) Ions from Water. Chem. Eng. J. 2019, 358, 264–282. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M. Surfactant-free Green Synthesis of Fe3O4 Nanoparticles Capped with 3, 4-Dihydroxyphenethylcarbamodithioate: Stable Recyclable Magnetic Nanoparticles for the Rapid and Efficient Removal of Hg (II) Ions from Water. Dalton Trans. 2015, 44, 18427–18437. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Zhou, Y.; Chang, L.; Zhang, J. Removal of Gaseous Elemental Mercury by Modified Diatomite. Sci. Total Environ. 2019, 652, 651–659. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Wang, L.; Zhang, J.; Song, S.; Fan, C.; Wang, S. Optical Detection of Mercury (II) in Aqueous Solutions by Using Conjugated Polymers and Label-free Oligonucleotides. Adv. Mater. 2007, 19, 1471–1474. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhao, M.; He, Y.; Cheng, F.; Chen, S. Fabrication of ZnO/rGO/PPy Heterostructure for Electrochemical Detection of Mercury ion. J. Electroanal. Chem. 2018, 826, 90–95. [Google Scholar] [CrossRef]
- Rout, K.; Manna, A.K.; Sahu, M.; Patra, G.K. A guanidine Based bis Schiff Base Chemosensor for Colorimetric Detection of Hg (II) and Fluorescent Detection of Zn (II) ions. Inorganica Chim. Acta 2019, 486, 733–741. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M. Fungus-derived Photoluminescent Carbon Nanodots for Ultrasensitive Detection of Hg2+ Ions and Photoinduced Bactericidal Activity. Sens. Actuator B Chem. 2017, 258, 172–183. [Google Scholar] [CrossRef]
- Shahat, A.; Elsalam, S.A.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F.; Ramis-Ramos, G. Optical Recognition and Removal of Hg (II) Using a New Self-Chemosensor Based on a Modified Amino-Functionalized Al-MOF. Sens. Actuator B Chem. 2017, 253, 164–172. [Google Scholar] [CrossRef]
- Borthakur, P.; Darabdhara, G.; Das, M.R.; Boukherroub, R.; Szunerits, S. Solvothermal Synthesis of CoS/Reduced Porous Graphene Oxide Nanocomposite for Selective Colorimetric Detection of Hg (II) Ion in Aqueous Medium. Sens. Actuator B Chem. 2017, 244, 684–692. [Google Scholar] [CrossRef]
- Sareen, D.; Kaur, P.; Singh, K. Strategies in Detection of Metal Ions Using Dyes. Coordin. Chem. Rev. 2014, 265, 125–154. [Google Scholar] [CrossRef]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Selective Nanomolar Detection of Mercury Using Coumarin Based Fluorescent Hg (II)-Ion Imprinted Polymer. Sens. Actuator B Chem. 2017, 246, 597–605. [Google Scholar] [CrossRef]
- Rao, L.; Tang, Y.; Lu, H.; Yu, S.; Ding, X.; Xu, K.; Li, Z.; Zhang, J.Z. Highly Photoluminescent and Stable N-Doped Carbon Dots as Nanoprobes for Hg2+ Detection. Nanomaterials 2018, 8, 900. [Google Scholar] [CrossRef]
- Baig, M.M.F.; Chen, Y.C. Bright Carbon dots as Fluorescence Sensing Agents for Bacteria and Curcumin. J. Colloid Interface Sci. 2017, 501, 341–349. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Mukherjee, S.; Punugupati, N.; Vasudevan, D.; Patra, C.R.; Narayan, R.; Kothapalli, R.V. Facile Synthesis of Carbon Dot and Residual Carbon Nanobeads: Implications for Ion Sensing, Medicinal and Biological Applications. Mater. Sci. Eng. C 2017, 73, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Shokri, R.; Hallaj, T. Interaction of Glucose-Derived Carbon Quantum Dots with Silver and Gold Nanoparticles and Its Application for the Fluorescence Detection of 6-Thioguanine. Luminescence 2017, 32, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Zhang, J.; Lin, S.; Huang, Y.; Yuan, R.; Liang, X.; Xiang, W. Intense Enhancement of Yellow Luminescent Carbon Dots Coupled with Gold Nanoparticles Toward White LED. Dyes Pigm. 2017, 140, 122–130. [Google Scholar] [CrossRef]
- Chen, T.; Sheng, A.; Hu, Y.; Mao, D.; Ning, L.; Zhang, J. Modularization of Three-Dimensional Gold Nanoparticles/Ferrocene/Liposome Cluster for Electrochemical Biosensor. Biosens. Bioelectron. 2019, 124, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; New, S.Y.; Xie, J.; Su, X.; Tan, Y.N. Protein-Based Fluorescent Metal Nanoclusters for Small Molecular Drug Screening. Chem. Commun. 2014, 50, 13805–13808. [Google Scholar] [CrossRef] [PubMed]
- Kraszkiewicz, P.; Mista, W. Thermally Stable SBA-15 Supported sub-2 nm Gold Clusters, Highly Active in Room Temperature CO Oxidation: Effect of Thermal Pretreatment. Catal. Commun. 2018, 110, 14–17. [Google Scholar] [CrossRef]
- Govindaraju, S.; Ankireddy, S.R.; Viswanath, B.; Kim, J.; Yun, K. Fluorescent Gold Nanoclusters for Selective Detection of Dopamine in Cerebrospinal Fluid. Sci. Rep. 2017, 7, 40298. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Chou, S.S.; De, M.; Luo, J.; Rotello, V.M.; Huang, J.; Dravid, V.P. Nanoscale Graphene Oxide (nGO) as Artificial Receptors: Implications for Biomolecular Interactions and Sensing. J. Am. Chem. Soc. 2012, 134, 16725–16733. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Wang, Q.M. Homo and Heterometallic Gold (I) Clusters with Hypercoordinated Carbon. Coordin. Chem. Rev. 2019, 378, 382–394. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, H.; Wang, Y.; Liu, X.; Luo, Z.; Yao, J. Enhanced Stability and Fluorescence of Mixed-Proteins-Protected Gold/Silver Clusters Used for Mercury Ions Detection. Sens. Actuator B Chem. 2017, 251, 773–780. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Lou, T.; Chen, Z. Iodide-Responsive Cu@ Au Nanoparticle-Based Colorimetric Assay for Sensitive Mercury (II) Detection. Sens. Actuator B Chem. 2017, 252, 663–670. [Google Scholar] [CrossRef]
- Verma, S.; Rao, B.T.; Srivastava, A.K.; Patel, H.S.; Satapathy, S.; Joshi, M.P.; Sahu, V.K.; Kukreja, L.M. Studies on Interdependent Optical Properties of Rhodamine 6G Dye and Gold Nanoparticles at Different Dilutions of Aqueous Solutions. J. Lumin. 2014, 155, 156–164. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Wang, Y.; Li, J.; Shen, D.; Kang, Q.; Chen, L. Ratiometric Fluorescence Sensor Based on Dithiothreitol Modified Carbon Dots-Gold Nanoclusters for the Sensitive Detection of Mercury Ions in Water Samples. Sens. Actuator B Chem. 2018, 262, 810–817. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Talib, A.; Wu, H.F. One Pot Synthesis of Gold–Carbon Dots Nanocomposite and Its Application for Cytosensing of Metals for Cancer Cells. Talanta 2017, 166, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Kumar, D.; Rizvi, S.I. Red Onion Extract (Allium cepa L.) Supplementation Improves Redox Balance in Oxidatively Stressed Rats. Food Sci. Hum. Well. 2013, 2, 99–104. [Google Scholar] [CrossRef]
- Pérez-Gregorio, R.M.; García-Falcón, M.S.; Simal-Gándara, J.; Rodrigues, A.S.; Almeida, D.P. Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 2010, 23, 592–598. [Google Scholar] [CrossRef]
- Gitîn, L.; Dinicâ, R.; Neagu, C.; Dumitrascu, L. Sulfur Compounds Identification and Quantification from Allium spp. Fresh Leaves. J. Food Drug Anal. 2014, 22, 425–430. [Google Scholar] [CrossRef]
- Zhang, S.L.; Deng, P.; Xu, Y.C.; Lu, S.W.; Wang, J.J. Quantification and Analysis of Anthocyanin and Flavonoids Compositions, and Antioxidant Activities in Onions with Three Different Colors. J. Integr. Agric. 2016, 15, 2175–2181. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Borzoo, M. Green Synthesis of Silver Nanoparticles Using Onion Extract and Their Application for the Preparation of a Modified Electrode for Determination of Ascorbic acid. J. Food Compos. Anal. 2016, 24, 796–803. [Google Scholar] [CrossRef]
- Liu, D.; Wu, F. Biosynthesis of Pd Nanoparticle Using Onion Extract for Electrochemical Determination of Carbendazim. Int. J. Electrochem. Sci. 2017, 12, 2125–2134. [Google Scholar] [CrossRef]
- Yu, J.; Gao, L.Z.; Li, X.L.; Wu, C.; Gao, L.L.; Li, C.M. Porous Carbons Produced by the Pyrolysisof Green Onion Leaves and Their Capacitive Behavior. New Carbon Mater. 2016, 31, 475–484. [Google Scholar] [CrossRef]
- Donner, H.; Gao, L.; Mazza, G. Separation and Characterization of Simple and Malonylated Anthocyanins in Red Onions, Allium cepa L. Food Res. Int. 1997, 30, 637–643. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M. Core–Shell Ferromagnetic Nanorod Based on Amine Polymer Composite (Fe3O4@DAPF) for Fast Removal of Pb (II) from Aqueous Solutions. ACS Appl. Mater. Interfaces 2015, 7, 25362–25372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Ma, Y.L.; Wang, C.H.; Wang, H.; Ren, Y.F.; Zhang, J.G.; Thakur, K.; Wei, Z.J. Insights into Physicochemical and Functional Properties of Polysaccharides Sequentially Extracted from Onion (Allium cepa L.). Int. J. Biol. Macromol. 2017, 105, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Demir, T.; Gunesli, Z.; Sonmez, F.; Bilen, C.; Yavuz, E.; Gencer, N. Inhibition of Carbonic Anhydrase I and II with Total Anthocyanins Extracted from Sweet Cherry Cultivars. Environ. Eng. Manag. J. 2015, 14, 935–941. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Tatode, A.; Bhoyar, B. Development and Validation of UV Spectrophotometric Method for the Estimation of Kaempferol in Kaempferol: Hydrogenated Soy Phosphatidylcholine (HSPC) complex. Pharm. Meth. 2014, 5, 34–38. [Google Scholar] [CrossRef]
- Sonmezoglu, S.; Akyurek, C.; Akin, S. High-Efficiency Dye-Sensitized Solar Cells Using Ferrocene-Based Electrolytes and Natural Photosensitizers. J. Phys. D Appl. Phys. 2012, 45, 425101. [Google Scholar] [CrossRef]
- He, Y.S.; Pan, C.G.; Cao, H.X.; Yue, M.Z.; Wang, L.; Liang, G.X. Highly Sensitive and Selective Dual-Emission Ratiometric Fluorescence Detection of Dopamine Based on Carbon Dots-Gold Nanoclusters Hybrid. Sens. Actuator B Chem. 2018, 265, 371–377. [Google Scholar] [CrossRef]
- Tabaraki, R.; Sadeghinejad, N. Microwave Assisted Synthesis of Doped Carbon Dots and Their Application as Green and Simple Turn Off–on Fluorescent Sensor for Mercury (II) and Iodide in Environmental Samples. Ecotox. Environ. Safe 2018, 153, 101–106. [Google Scholar] [CrossRef]
- Anandababu, A.; Anandan, S.; Ashokkumar, M. A Simple Discriminating p-tert-Butylcalix[4]arene Thiospirolactam Rhodamine B Based Colorimetric and Fluorescence Sensor for Mercury Ion and Live Cell Imaging Applications. Chem. Sel. 2018, 3, 4413–4420. [Google Scholar] [CrossRef]
- Devi, N.R.; Sasidharan, M.; Sundramoorthy, A.K. Gold Nanoparticles-Thiol-Functionalized Reduced Graphene Oxide Coated Electrochemical Sensor System for Selective Detection of Mercury Ion. J. Electrochem. Soc. 2018, 165, B3046–B3053. [Google Scholar] [CrossRef]
- Tabaraki, R.; Abdi, O. Green and Simple Turn off/on Fluorescence Sensor for Mercury (II), Cysteine and Histidine. J. Mol. Liq. 2018, 251, 77–82. [Google Scholar] [CrossRef]
- Liu, Y.; Ouyang, Q.; Li, H.; Chen, M.; Zhang, Z.Z.; Chen, Q. A Turn-on Fluoresence Sensor for Hg2+ in Food Based on FRET between Aptamers-Functionalized Upconversion Nanoparticles and Gold Nanoparticles. J. Agric. Food Chem. 2018, 66, 6188–6195. [Google Scholar] [CrossRef] [PubMed]
- Ju, E.; Liu, Z.; Du, Y.; Tao, Y.; Ren, J.; Qu, X. Heterogeneous Assembled Nanocomplexes for Ratiometric Detection of Highly Reactive Oxygen Species in Vitro and in Vivo. ACS Nano 2014, 8, 6014–6023. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Yuan, J.; Wang, E. Oligonucleotide-stabilized Ag Nanoclusters as Novel Fluorescence Probes for the Highly Selective and Sensitive Detection of the Hg2+ Ion. Chem. Commun. 2009, 23, 3395–3397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Wu, F.Y.; Wu, Y.M.; Zhan, X.S. Fluorescent Method for the Determination of Sulfide Anionwith ZnS: Mn Quantum Dots. J. Fluoresc. 2010, 20, 243–250. [Google Scholar] [CrossRef] [PubMed]


| Nanomaterial | Technique | Limit of Detection (LOD) | Reference |
|---|---|---|---|
| Carbon dots | FRET (fluorescence resonance energy transfer) | 62 nM | 50 |
| p-tert-Butylcalixarene Thiospirolactam Rhodamine B | FRET | 9.65 nM | 51 |
| Dithiothreitol modified carbon dots-gold nanoclusters | Ratiometric fluorescence sensor | 8.7 nM | 34 |
| rGO-SH/Au-NPs | Electrochemical sensor | 200 nM | 52 |
| Nitrogen and sulfur doped carbon dots | Fluorescence sensor | 8 nM | 53 |
| Aptamers-Functionalized Upconversion Nanoparticles and Gold Nanoparticles | FRET | 60 nM | 54 |
| Au/C Nanodots | Fluorescence sensor | 1.3 nM | Current work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkateswarlu, S.; Govindaraju, S.; Sangubotla, R.; Kim, J.; Lee, M.-H.; Yun, K. Biosynthesized Highly Stable Au/C Nanodots: Ideal Probes for the Selective and Sensitive Detection of Hg2+ Ions. Nanomaterials 2019, 9, 245. https://doi.org/10.3390/nano9020245
Venkateswarlu S, Govindaraju S, Sangubotla R, Kim J, Lee M-H, Yun K. Biosynthesized Highly Stable Au/C Nanodots: Ideal Probes for the Selective and Sensitive Detection of Hg2+ Ions. Nanomaterials. 2019; 9(2):245. https://doi.org/10.3390/nano9020245
Chicago/Turabian StyleVenkateswarlu, Sada, Saravanan Govindaraju, Roopkumar Sangubotla, Jongsung Kim, Min-Ho Lee, and Kyusik Yun. 2019. "Biosynthesized Highly Stable Au/C Nanodots: Ideal Probes for the Selective and Sensitive Detection of Hg2+ Ions" Nanomaterials 9, no. 2: 245. https://doi.org/10.3390/nano9020245
APA StyleVenkateswarlu, S., Govindaraju, S., Sangubotla, R., Kim, J., Lee, M.-H., & Yun, K. (2019). Biosynthesized Highly Stable Au/C Nanodots: Ideal Probes for the Selective and Sensitive Detection of Hg2+ Ions. Nanomaterials, 9(2), 245. https://doi.org/10.3390/nano9020245