3.1. Characterization of Fe3O(BDC)3 and MPC700
The MPC700, which was formed by the pyrolysis of Fe
3O(BDC)
3 at 700 °C, was chosen as a representative to analyze the characterization along with its precursor Fe
3O(BDC)
3. Initially, the Fe
3O(BDC)
3 and MPC700 were characterized by XRD and FT-IR spectra as shown in
Figure 2a,b. For the XRD profiles, the crystalline structure of Fe
3O(BDC)
3 showed the typical peaks at around 9.6° (101), 18.6° (002) and 28.1° (302), which matched well with previous papers [
30,
33]. Meanwhile, the diffraction profile for MPC700 indicated the presence of zero-valent iron (JCPDS No. 65–4899) at around 45° (110) [
6]. Moreover, the diffraction region at 20–30° indicates the π-stacking of the nano-graphitic platelets of the graphite sheets. The formation of zero-valent iron (ZVI) encapsulated on MPC700 can be explained through the physical pyrolysis and in situ chemical reduction (ISCR) [
34]. In fact, the calcination of Fe
3O(BDC)
3 at 700 °C allows to remove the volatile components e.g. H
2O, and gradually generate the hierarchical carbon multilayers by deconstructing the aromatic rings [
26]. The chemical reduction followed is expected to crack the Fe-O coordination bonds, and create the zero-valent Fe by ISCR process, proceeding in assembling the sort of carbon-encapsulated iron composite [
35].
The surface chemistry of adsorbents involving Fe
3O(BDC)
3 and MPC700 could be diagnosed using the FT-IR spectra profiles as illustrated in
Figure 2b. In general, the Fe
3O(BDC)
3 showed its typical functional groups including conjugated ketones C=O (1666 cm
−1), and C–O (1380 cm
−1) chemical bonds of carboxylate, which were consistent with footprints reported by previous publications [
21,
36]. Also, the existence of coordination bonds Fe–O (744 cm
−1) and Fe
3(μ
3–O) (540 cm
−1) could be confirmed clearly, revealing the formation of Fe (III) clusters with carboxylate groups of H
2BDC ligands [
21,
37,
38]. Meanwhile, although MPC700 repeated almost typical peaks as mentioned on Fe
3O(BDC)
3, the absence of important footprints of Fe (III)–O at the respectively low wavenumbers convinced that the ISCR process via reduction of Fe(III) (Fe
3O(BDC)
3) to zero-valent Fe (MPC700) is highly likely to be proceeded [
22]. Therefore, this observation was again commensurate with the XRD profile evidence mentioned of the existence of zero-valent Fe on the MPC700.
The Raman spectra and pH of point zero charge curves can be used to identify more properties of materials and their profiles are shown in
Figure 2c,d. The Raman spectra in
Figure 2c reveals the bonds of aromatic C–H (870 cm
−1), C–C (1160 cm
−1), COO– (1450 cm
−1), and C=C vibrations (1610 cm
−1) on the Fe
3O(BDC)
3, which are familiar with the previous publications [
39]. In the meantime, the graphitic nature of MPC700 structure can be exposed by the presence of typical D- (1330 cm
−1) and G- (1600 cm
−1) bands, suggesting the structurally hierarchical, amorphous, and disordered phase of materials (I
D/I
G = 1.61). Meanwhile, according to
Figure 2d, the pH
pzc values were found to be 4.0 and 6.4 for Fe
3O(BDC)
3 and MPC700. Note that the surface of materials tends to be more negative if the pH solution surpassed the pH of point zero charge and more positive at pH < pH
pzc. These values are vital to explain the adsorption mechanisms [
31].
Figure 2e,f plotted the profiles of N
2 adsorption/desorption isotherm and pore distribution curves of Fe
3O(BDC)
3 and MPC700. In
Figure 2e, the shape of isotherm plot for MPC700 is relatively corresponding to Type IV (IUPAC) with the presence of a hysteresis loop at high P/P
o ratio, indicating that MPC700 possessed the dominance of mesoporous structure. Meanwhile, the figure for Fe
3O(BDC)
3 seems to show the similarity to Type II (IUPAC), demonstrating the non-porous or macroporous (>50 nm in diameter) structure.
Figure 3f showing the pore distribution curves of Fe
3O(BDC)
3 and MPC700 also supported these observations. Herein, the Brunauer-Emmett-Teller (BET) surface area values made a great difference, which was found to be 224.7 m
2/g (MPC700) compared with 7.6 m
2/g (Fe
3O(BDC)
3). A very low value of specific surface area of Fe
3O(BDC)
3 may be attributable to anhydrous form of Fe
3O(BDC)
3 exhibits closed pores with almost no accessible porosity to N
2 at 77 K [
21]. Finally,
Table 2 summarized the important properties of MPC700 and Fe
3O(BDC)
3 including BET surface area, pore diameter, and saturation magnetization (Ms) values. With dominant favorability of surface area, porous structure, pore size, and functional groups, MPC700 was expected to show more efficient adsorption and favorable separation than Fe
3O(BDC)
3.
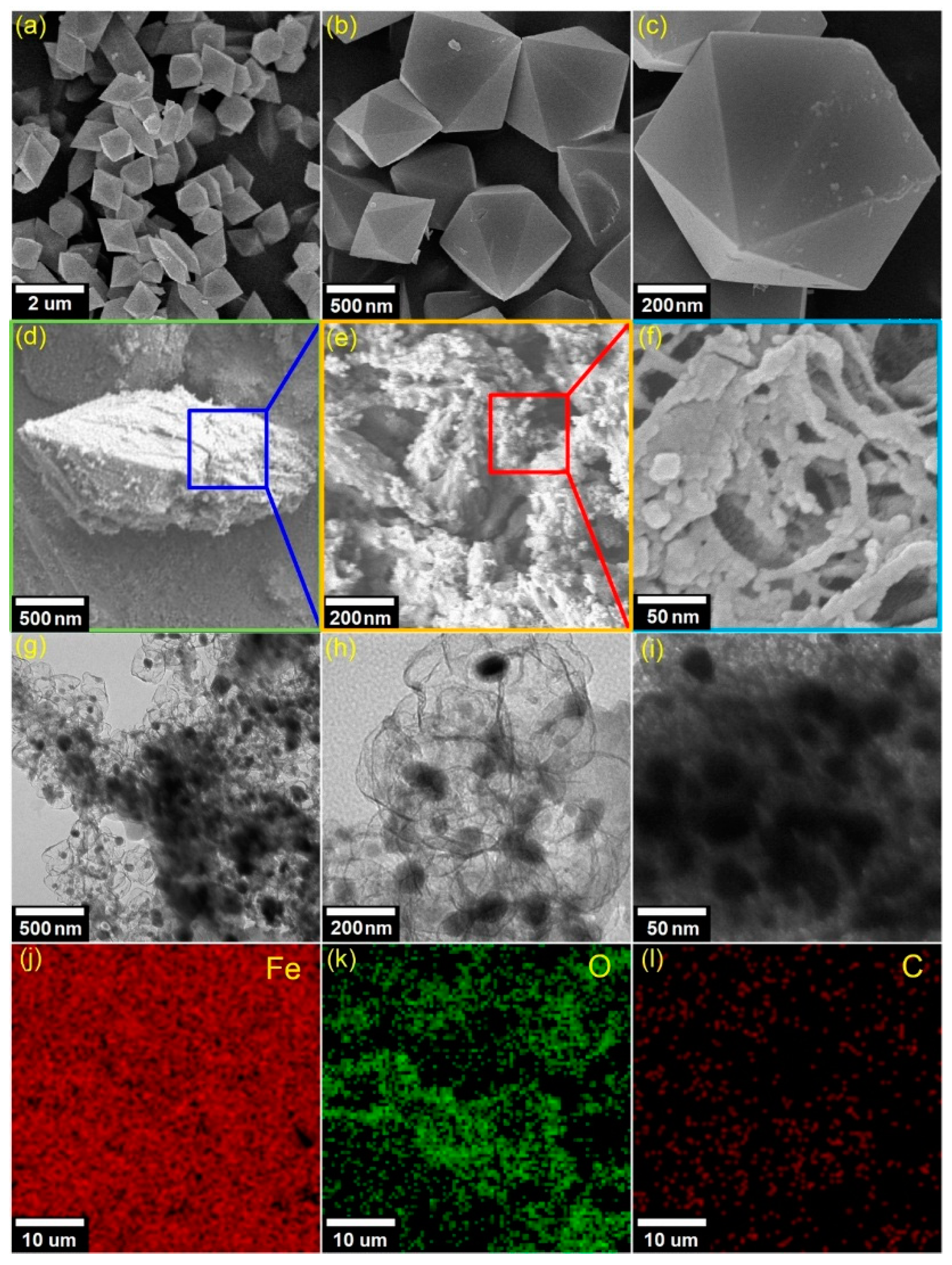
The morphological properties of the Fe
3O(BDC)
3 and MPC700 can be analyzed by the SEM, and TEM as shown in
Figure 3. Overall, there was a morphologically noticeable difference between Fe
3O(BDC)
3 precursor and MPC700. In detail, the Fe
3O(BDC)
3 crystals in
Figure 3a–c are likely to adhere to a perfect hexagonal structure at scale 500 nm, while the MPC700 obtained the relatively defective, amorphous, and heterogeneous structure as seen from
Figure 3d–f. To better understand of structure of MPC700, TEM images in
Figure 3g–i reveal a great dispersion of dark sites, which may be attributable to the aggregation of magnetic Fe particles and encapsulated by opaque regions (mesoporous carbon). In addition, the EDS elemental mapping in
Figure 3j–l implies that structure of MPC700 was constructed by iron, carbon and oxygen elements [
40].
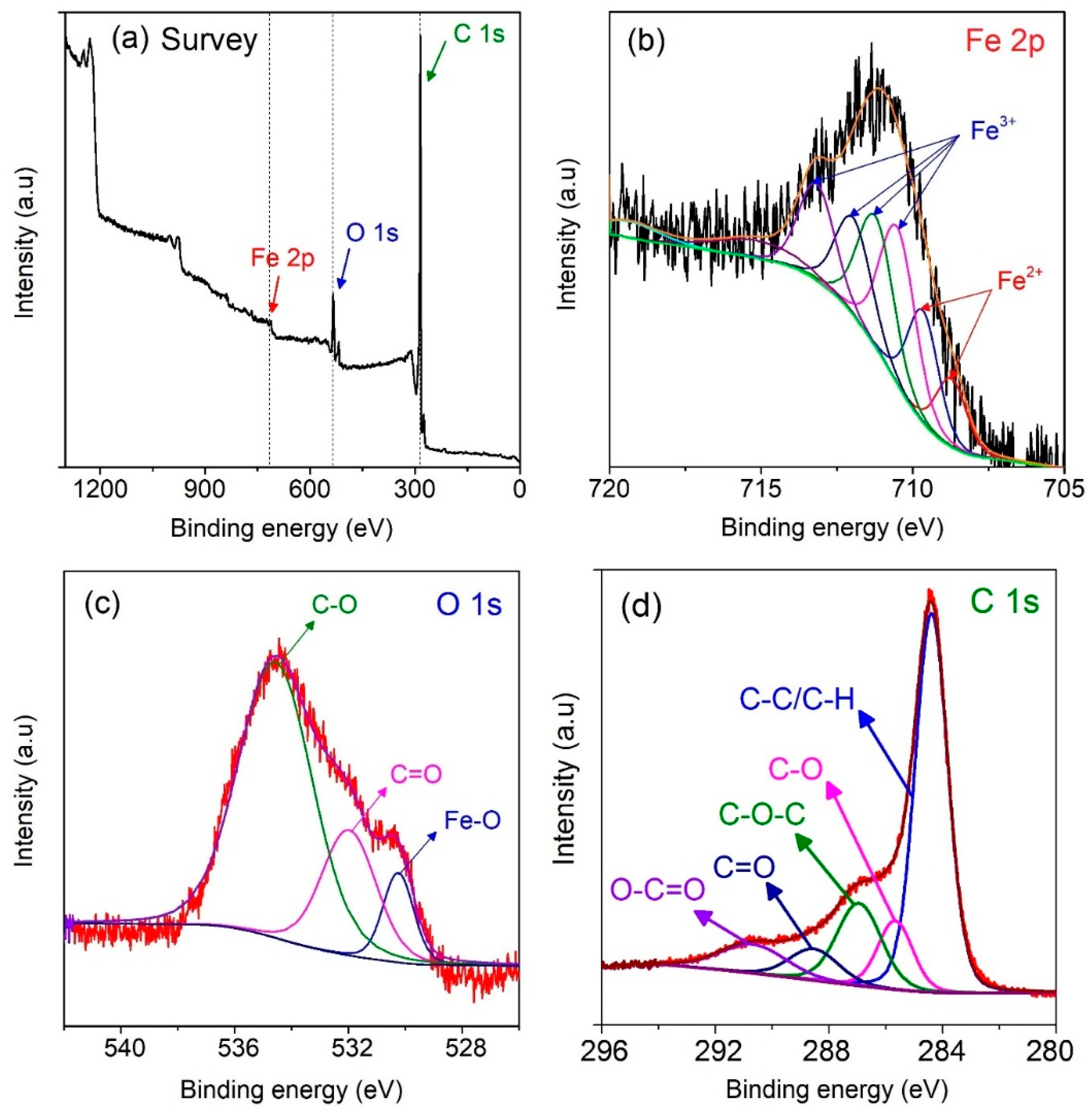
To gain the insight into the existence of chemical bonds in MPC700 nanocomposite, the XPS technique was further studied, and depicted in
Figure 4. As observed from
Figure 4a, the preliminary survey results indicate that MPC700 was solely constituted of three elements: iron (Fe 2p), carbon (C 1s) and oxygen (O 1s). For details, the
Figure 4b shows the broad photoelectronic peak of Fe 2p
3/2 sub level at around 710.8 eV. This level can be converted into various signals of Fe
3+ species at 710.6, 711.3, 712.1, 713.3 eV, and Fe
2+ species at 709.7, 708.8 eV, suggesting that the composite may contain both Fe
2+ and Fe
3+ species (FeO, Fe
3O
4, Fe
2O
3, FeOOH) without detecting any trace of ZVI [
41,
42,
43]. However, according to the XRD and FT-IR spectra, there was the only diagnostic peak of ZVI found in the structure of MPC700. Hence, it is possible that Fe
2+ and Fe
3+ species encapsulate the outside shell of ZVI particles, leading to no detection of ZVI signals in the XPS spectrum of MPC700 composite because of its sensibility at limited range of depth (<10 nm) [
44]. This observation also supported again the fact of the partial reduction of Fe-O bonds by carbon during the pyrolysis. Moreover, The O 1s XPS spectrum in
Figure 3c exhibits three peaks at binding energies 534.6, 533.0, 530.0 eV, corresponding to chemisorbed O, C-O/C=O, and iron oxides Fe-O, while the C 1s XPS spectrum in
Figure 3d indicates the presence of chemical bonds consisting of O-C=O (290.6 eV), C=O (288.5 eV), C-O-C (286.9 eV), C-O (285.7 eV), C-C/C-H (284.4 eV) [
45]. These chemical bonds along with respective energies from the fitting of C 1s is very much in line with recent work [
46].
Finally,
Table 3 shows the quantity of functional groups on MPC700 including carboxylic (1.1 mmol/g), lactonic (0.5 mmol/g), phenolic (0.7 mol/g), and total basic (0.85 mmol/g) groups via Boehm titration. These functional groups may play a crucial role in the adsorption of CAP in aqueous solutions.
3.3. Thermodynamic and Recyclability Studies
The thermodynamic study shows the effect of temperature on the adsorption of CAP, and give the prediction about whether the adsorption is a spontaneous process or not. Their standard parameters could be represented as follows (Equation (7)):
where,
KC and
T (K) are the adsorption equilibrium constant and temperature, respectively.
KC can be calculated through the ratio of equilibrium concentration of adsorbent between liquid and solid phase and determined as follows (Equation (8)):
where,
CA (mg/g) and
Ce (mg/L) are the equilibrium CAP concentrations in solid phase and solution phase, respectively [
50]. Standard enthalpy (
ΔH) and entropy (
ΔS) can be calculated by van’t Hoff isotherm equation as follows (Equation (9)):
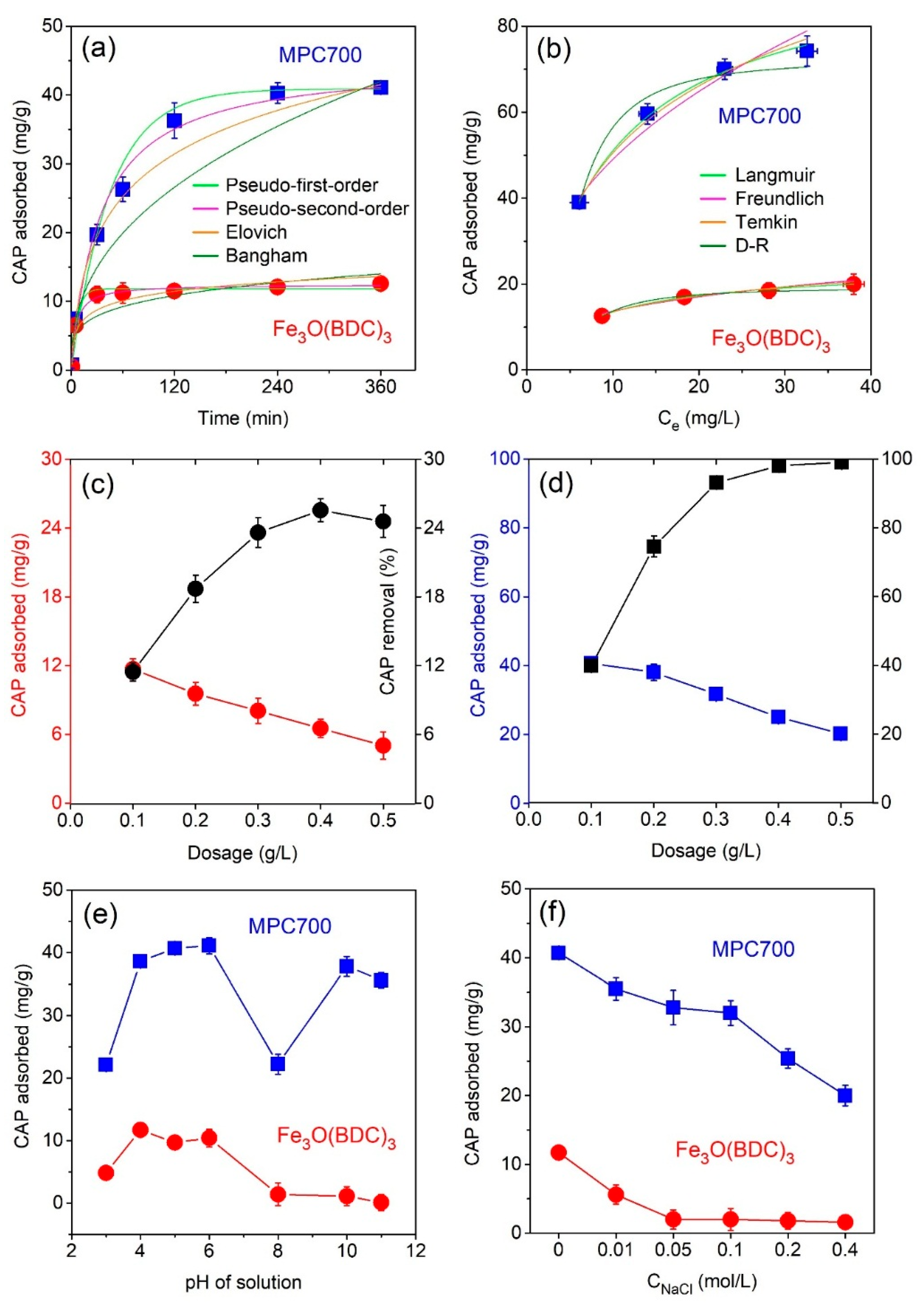
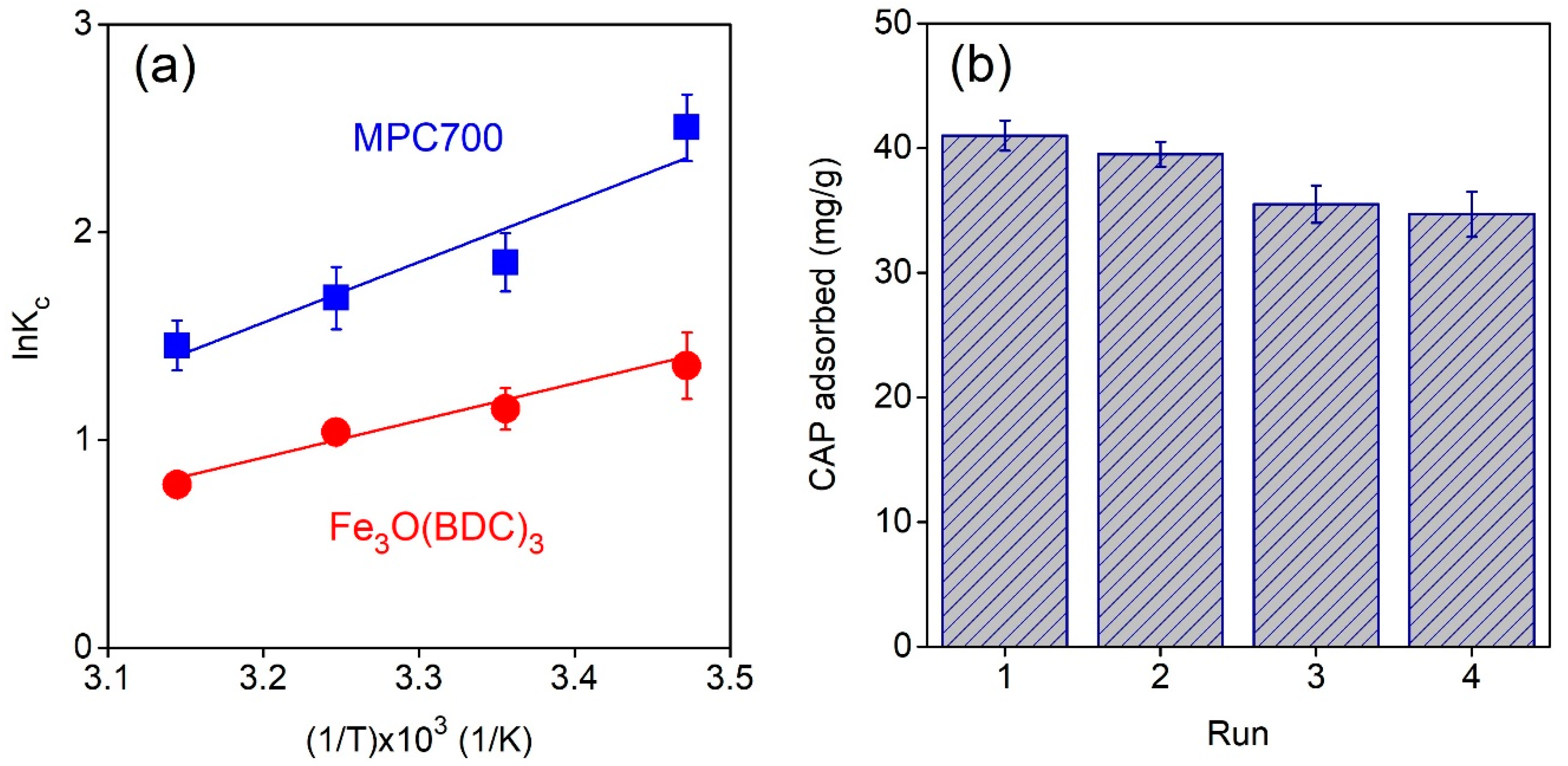
Figure 7a plots the impact of temperature (288–318 K) on CAP adsorption onto MPC700 and Fe
3O(BDC)
3. In addition, thermodynamic constants involving enthalpy (
∆H), entropy (
∆S) and Gibbs free energy (
∆G) were shown in
Table 7. The negative
∆H values indicate the adsorption of CAP over MPC700 and Fe
3O(BDC)
3 was an exothermic process, which totally agreed with a recent work [
51]. Meanwhile, negative values of
∆S show a decline in disorder occurring in heterogeneous phase because of migration between solvent and CAP molecules during sorption [
45]. Finally, the negative values of Gibbs free energy indicate that the adsorption of CAP onto MPC700 and Fe
3O(BDC)
3 was a spontaneous process.
One of the most important properties of adsorbent relating to cost-effectiveness and practical applicability is their stability and recyclability [
52]. In this study, adsorbent MPC700 can be regenerated by using a green eluent mixture of methanol/acetic acid (9:1) [
53]. The regeneration procedure could be described as follows: The CAP@MPC700 was rapidly separated using a magnetic field, washing with a mixture of eluents (3 × 10 mL), drying and reactivating under 110 °C for 4 h. As shown in
Figure 7b, after for cycles, there was a slight decline in the CAP adsorption capacity from 41.0 mg/g to 34.7 mg/g, suggesting good stability and regeneration performance of MPC.
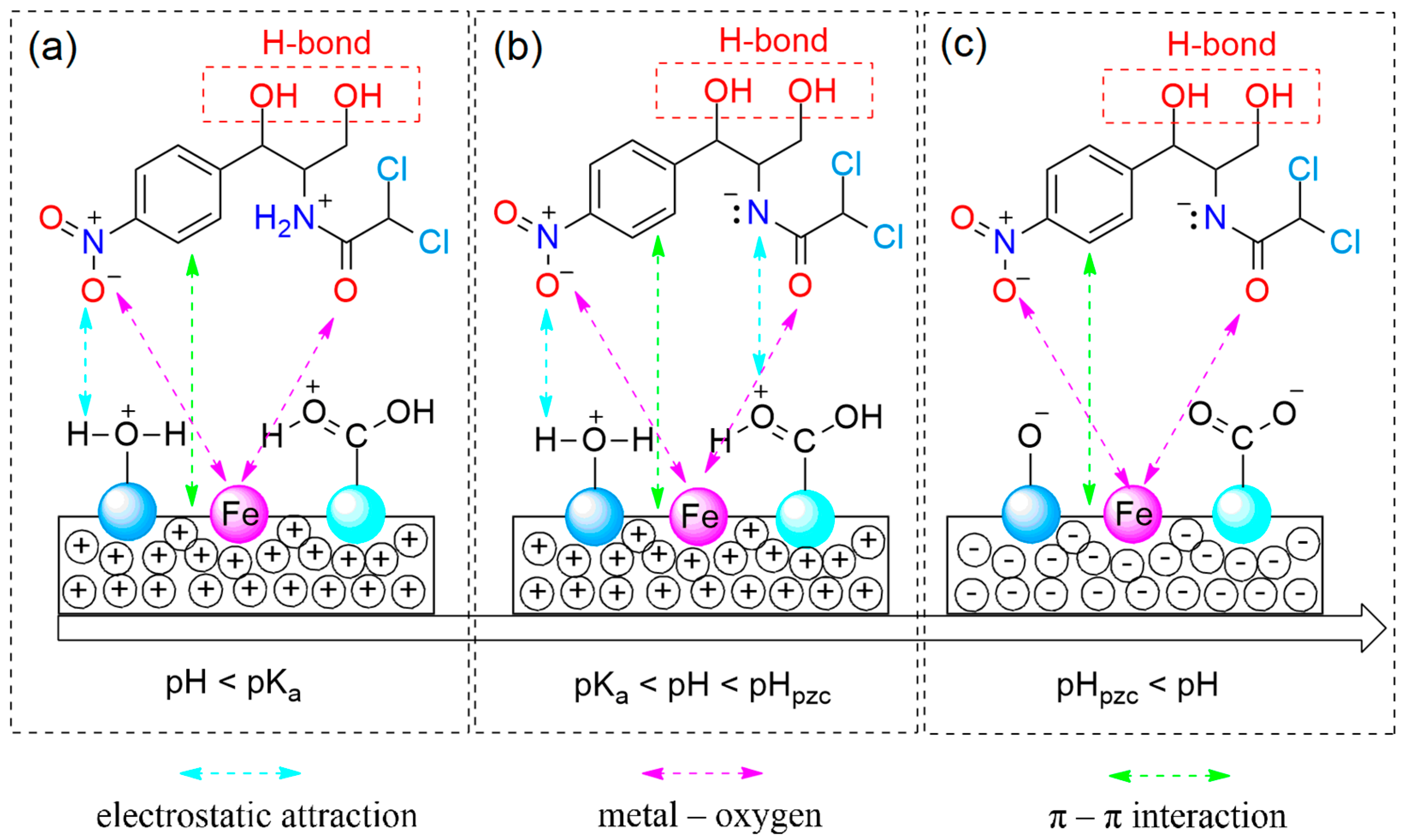
3.4. Proposed Mechanism
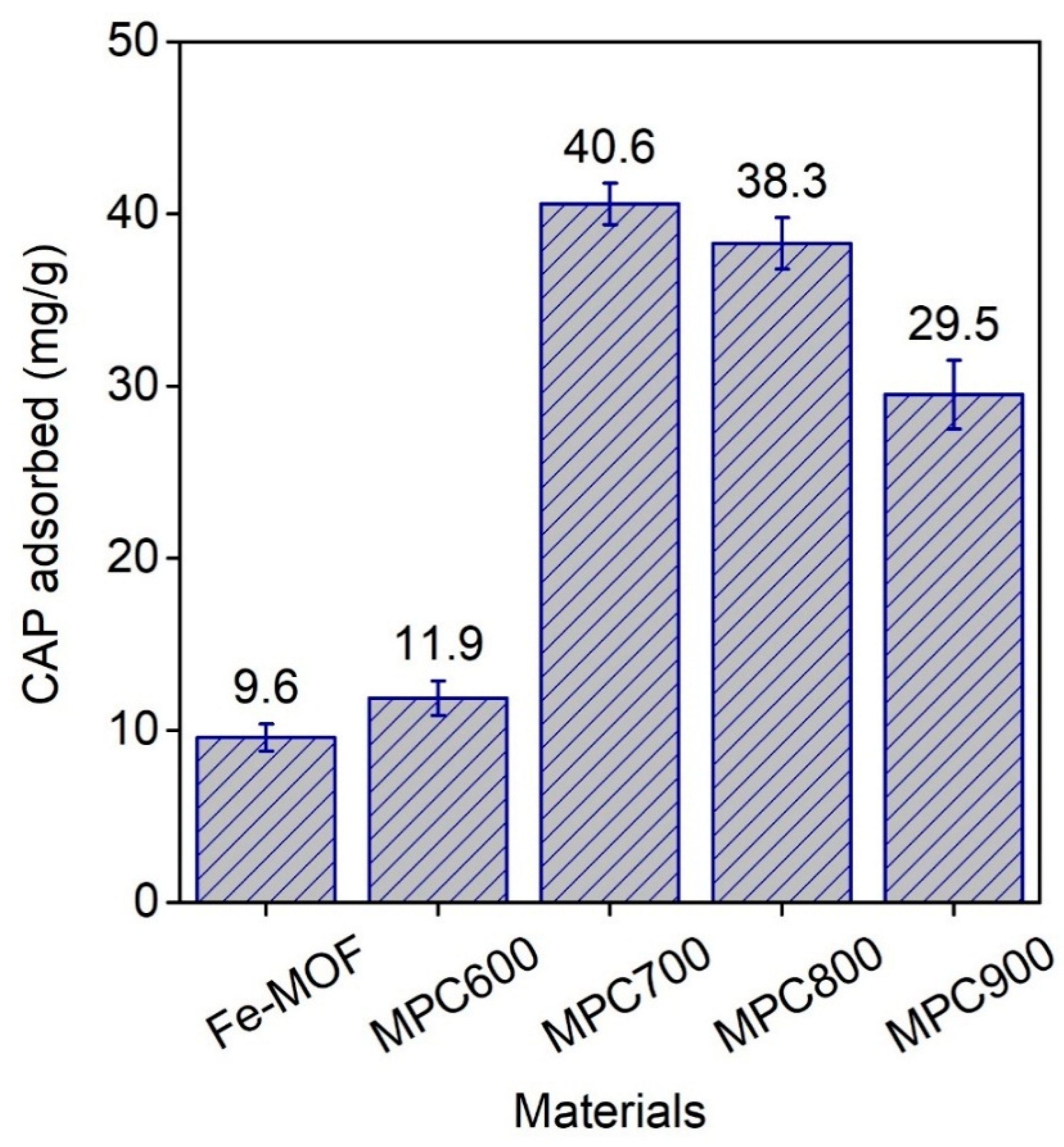
As mentioned, the maximum adsorption capacity values of CAP by MPC700 was so far higher than that by Fe
3O(BDC)
3. These results reflected the importance of surface functional groups towards adsorption of CAP. According to Boehm titration in
Table 3, functional groups including carboxylic, phenolic, and lactonic groups on the surface of MPC700 were found, which provide an abundance of oxygen atoms or hydroxyl groups. Meanwhile, each CAP molecule contains two alcoholic groups that can readily create a type of H-bond with these oxygen atoms or hydroxyl groups. As a result, there was the existence of H-bond in adsorption mechanism.
However, the sorption of CAP onto materials can occur at any pH values possibly due to the existence of other factors such as electrostatic attraction, π–π interaction, and metal–bridging interaction (
Figure 8). While the electrostatic attraction is influenced by the pH values, the kind of latter interactions may be independent on acidity or basicity, making decision on whether the adsorption process in aqueous solution is favorable or not.
Obviously, the π-electronic rich system on mesoporous carbon of MPC700 can create a special interaction with π-electrons of aromatic rings on CAP molecules, called “π–π interaction”, while the bridge between zero-valent iron and oxygen-containing functional groups results in the type of metal-oxygen interaction, as observed in
Figure 8a–c. Otherwise, the electrostatic attraction can be divided into three circumstance.
Firstly, at pH < (pK
a)
CAP in
Figure 8a, the surface functional groups on MPC700 and CAP molecules are all protonated in high acidic solution. Therefore, the formation of electrostatic attraction between negative and positive sites becomes more difficult. The adsorption in this case is not dominant.
Secondly, at (pK
a)
CAP < pH < pH
pzc in
Figure 8b, while the surface of MPC70 is positively charged (pH < pH
pzc = 6.4), the CAP molecules is mainly deprotonated (pH > (pK
a)
CAP = 5.5), resulting in the prevalent presence of negative and positive sites. Thus, the electrostatic attraction is permitted to run, and adsorption of CAP is a favorable process. In fact, the results in
Figure 6e also indicated the best condition for the adsorption of CAP at 6.0, which was highly consistent with given statement.
Thirdly, at pH
pzc < pH in
Figure 8c, both the surface of MPC700 and CAP molecules are all deprotonated in high basic solution (pH > 6.4), and hence, electrostatic attraction is exterminated, leading to a detrimental adsorption process.