Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
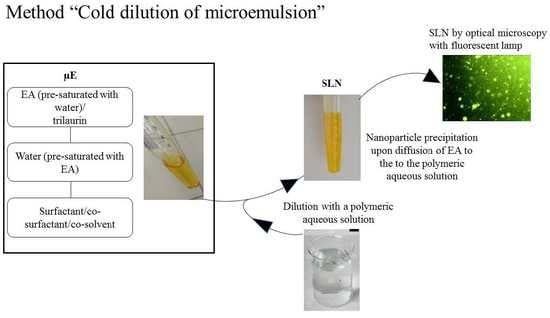
2.2. SLN Preparation
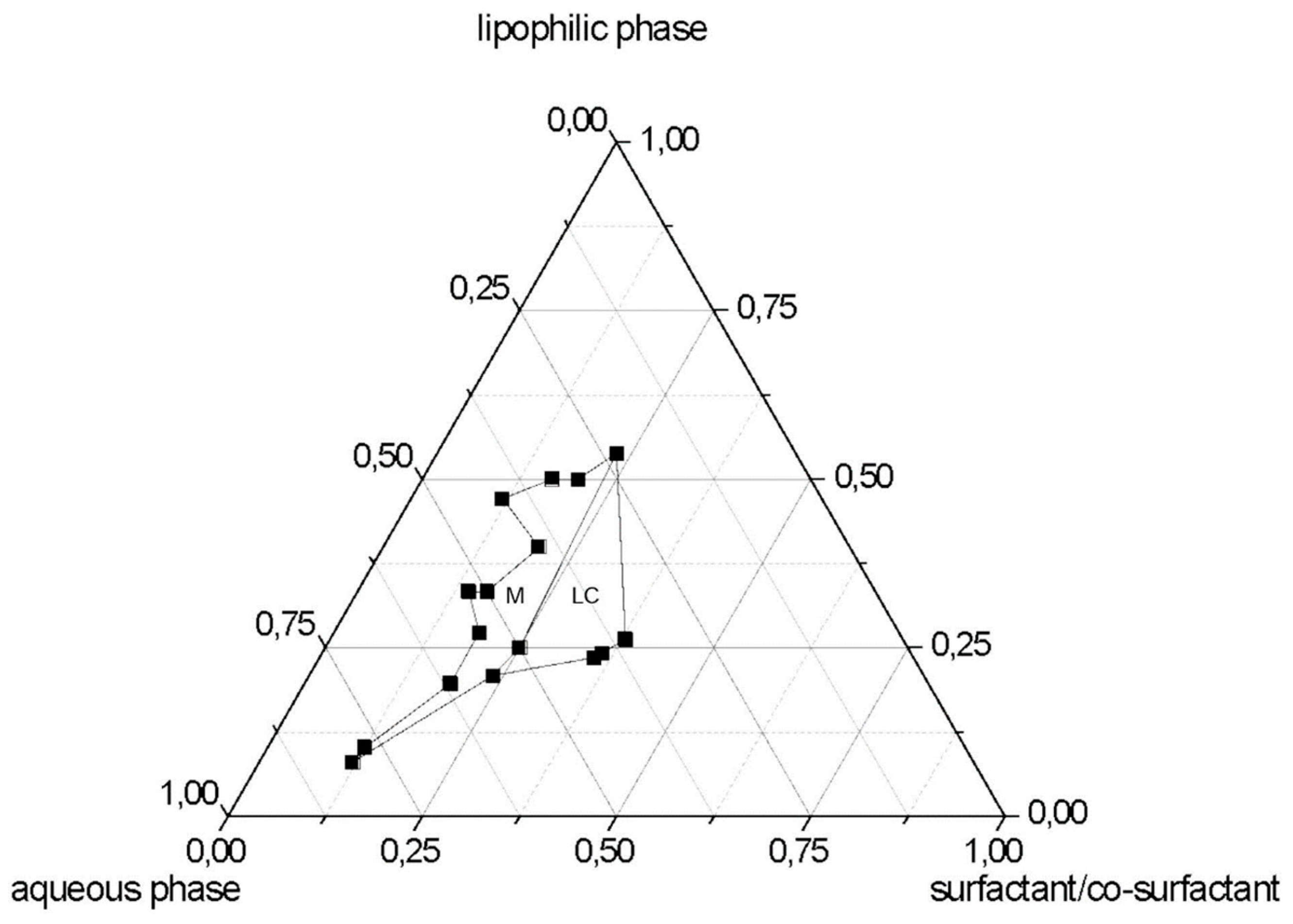
2.3. Pseudo-Ternary Diagrams
2.4. CURC Loaded SLNs
2.5. Gel Filtration of SLNs
2.6. Particle Size and Zeta Potential Determination
2.7. Differential Scanning Calorimetry (DSC)
2.8. Scanning Electron Microscopy (SEM)
2.9. In vitro Quantification of CURC
2.10. CURC Concentration in SLN Suspension and Entrapment Efficiency (%EE)
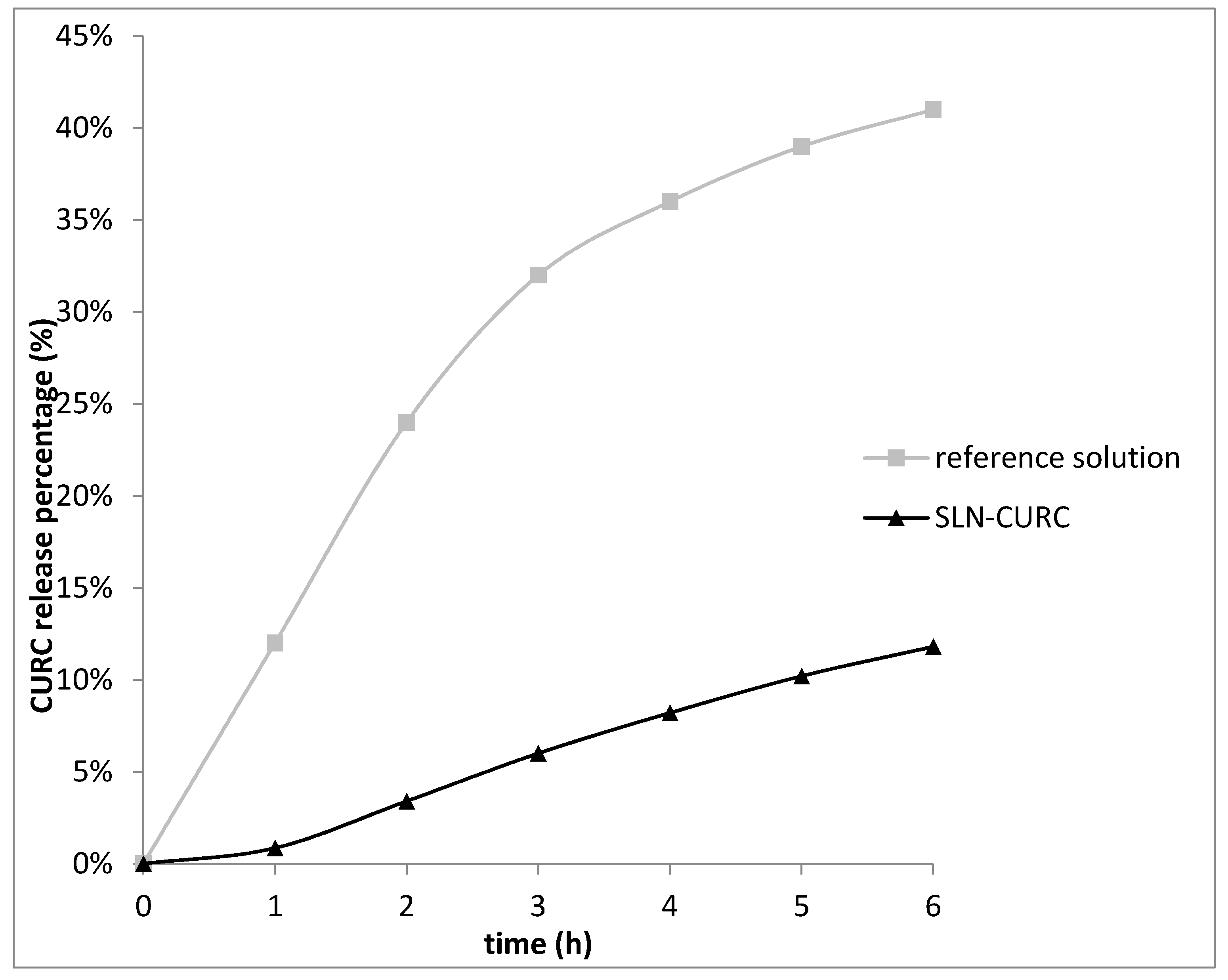
2.11. CURC Release from SLNs
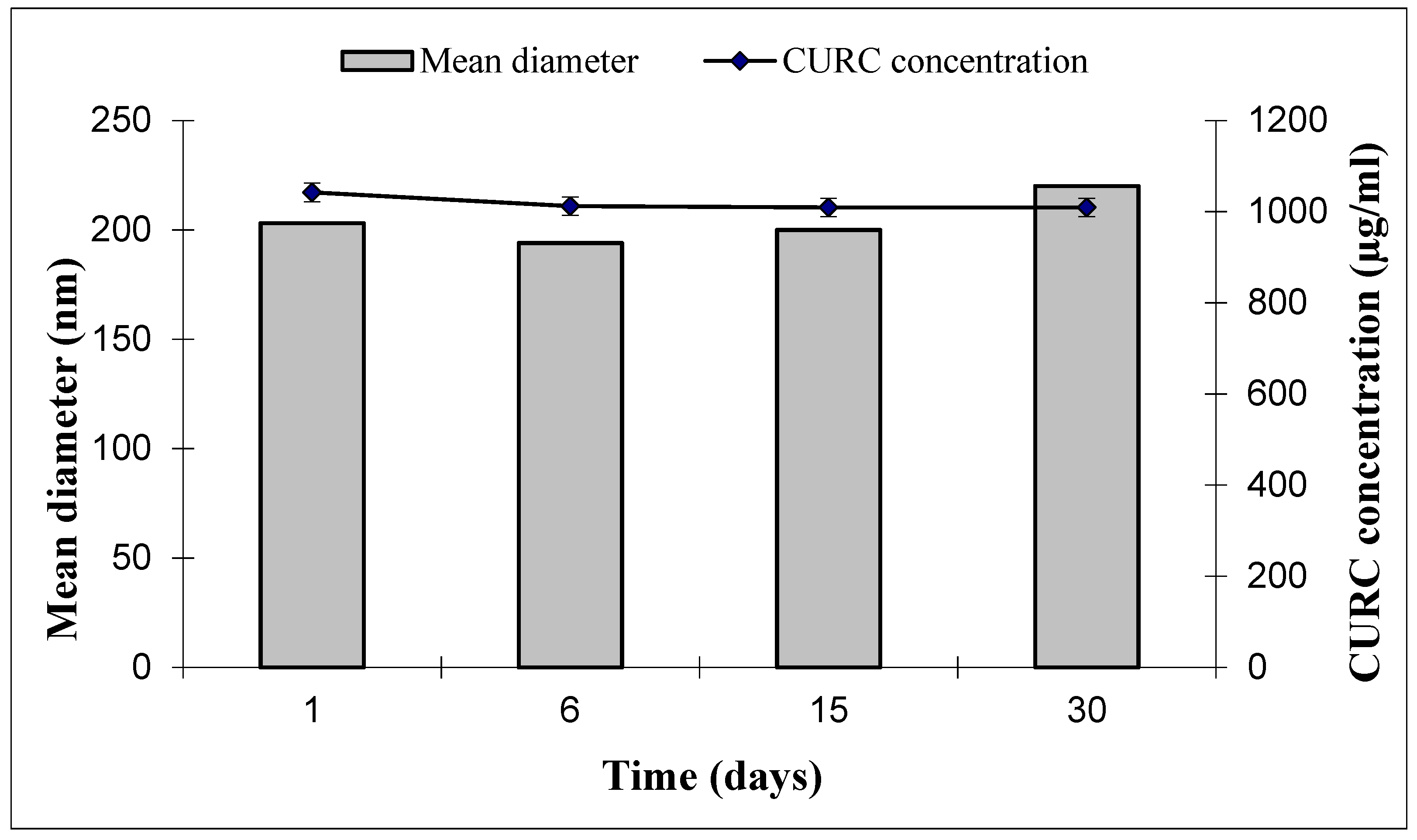
2.12. SLN and CURC Stability
2.13. Empty SLN in vitro Toxicity Studies on Podocytes and HUVEC
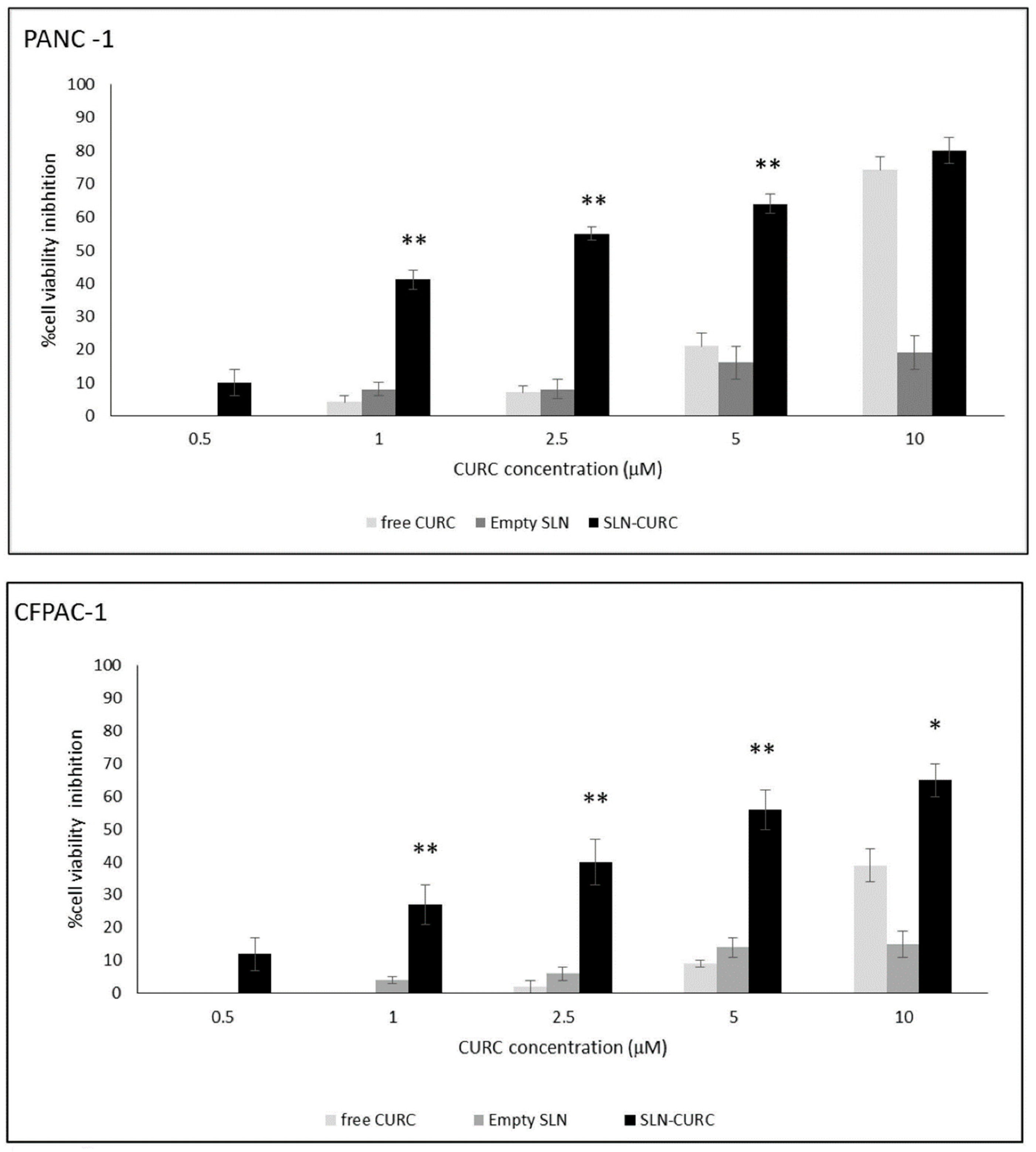
2.14. SLN-CURC in vitro Cytotoxicity Studies
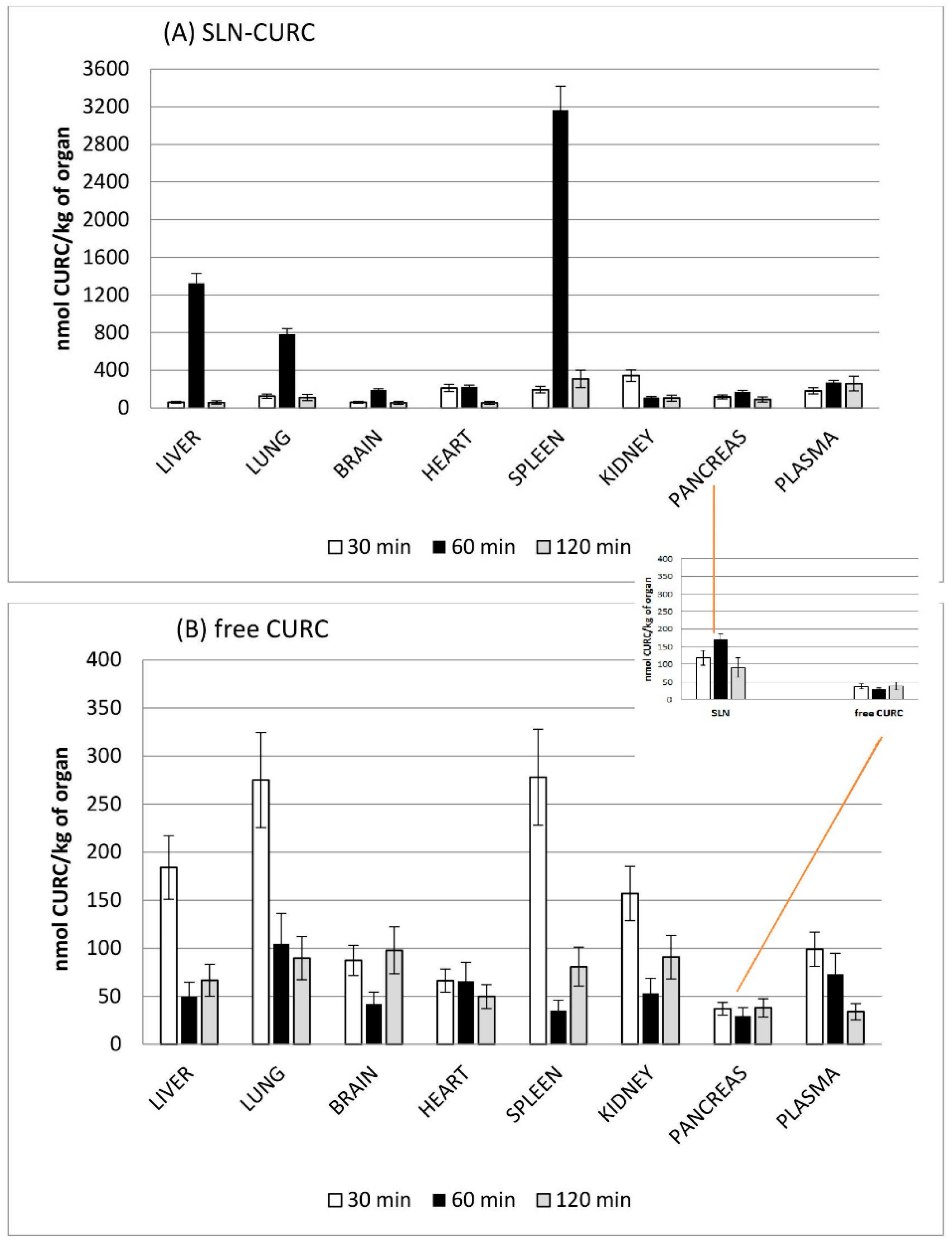
2.15. Biodistribution of SLNs
2.16. Data Analysis
3. Results and Discussion
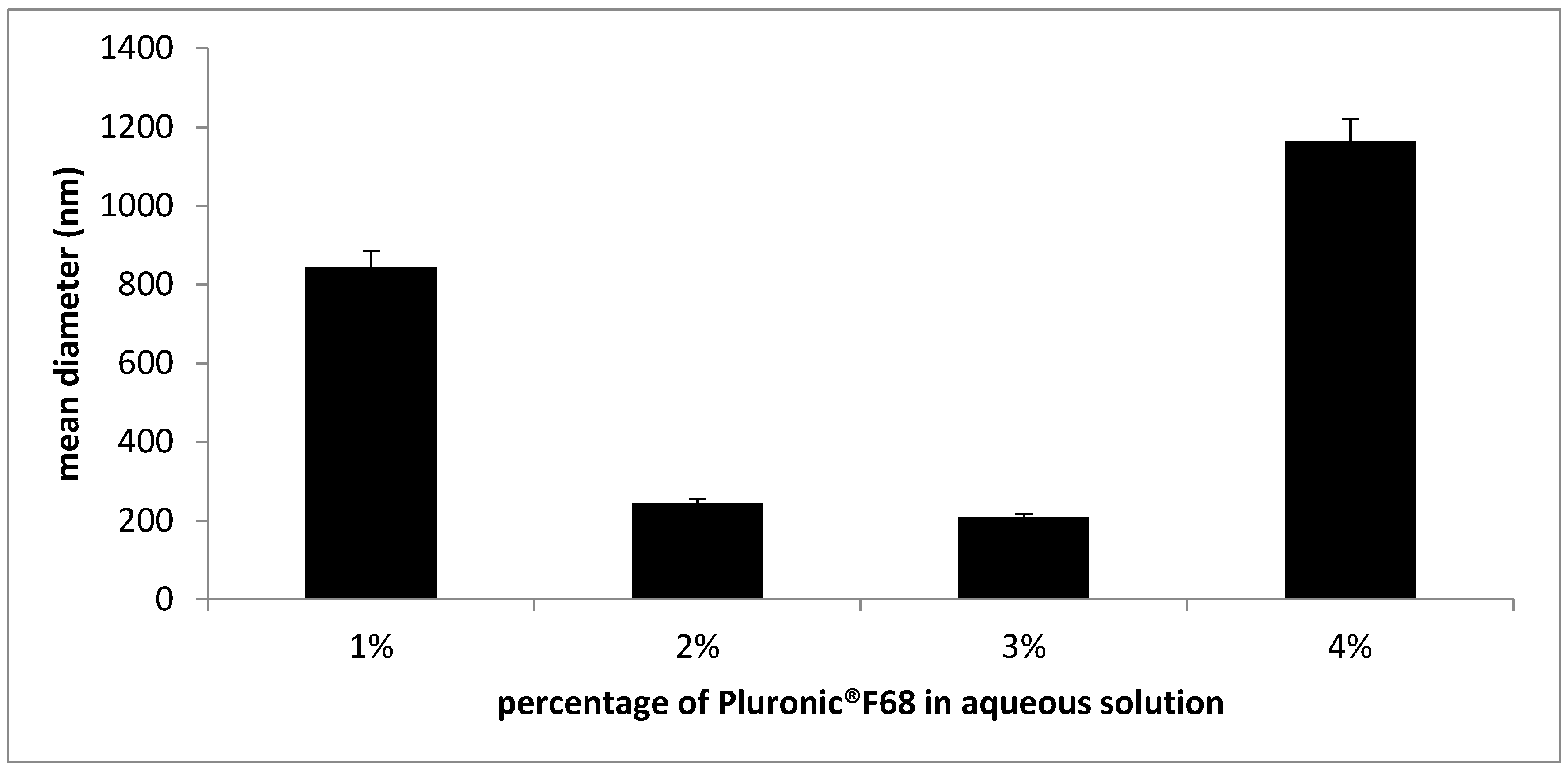
3.1. SLN Formulation and Characterization
3.2. In vitro Cytotoxicity Test
3.3. Biodistribution Studies
4. Conclusions
- the development of a new, solvent free, cold-operating, and simple-to-realize technique, to obtain physiologically compatible SLN;
- the feasibility of the µE dilution method for CURC loading;
- the in vitro efficacy of SLN-CURC on two different pancreatic tumor cell lines;
- the lack of adverse effects of SLNs on rats after intravenous administration
- different biodistribution patterns of CURC when in vivo administered in SLNs and prolonged circulation time when compared with aqueous solution.
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Sousa, E.; Vasconcelos, M.; Pinto, M. Curcumin: A natural lead for potential new drug candidates. Curr. Med. Chem. 2015, 22, 4196–4232. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.D.; Kim, J.A.; Kwak, M.K.; Yoo, B.K.; Yong, C.S.; Choi, H.G. Enhanced oral bioavailability of curcumin via a solid lipid-based self-emulsifying drug delivery system using a spray-drying technique. Biol. Pharm. Bull. 2011, 34, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Gallarate, M.; Peira, E.; Battaglia, L.; Serpe, L.; Trotta, M. Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J. Microencapsul. 2011, 28, 537–548. [Google Scholar] [CrossRef]
- Larrañeta, E.; Barturen, L.; Ervine, M.; Donnelly, R.F. Hydrogels based on poly(methyl vinyl ether-co-maleic acid) and Tween 85for sustained delivery of hydrophobic drugs. Int. J. Pharm. 2018, 538, 147–158. [Google Scholar] [CrossRef]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Peng, H.; Li, Y.; Xiong, J.; Xu, Z. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mat. Sci. Engin. C 2013, 33, 4802–4808. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of nanoparticle-based carriers for targeted drug delivery. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Carbone, C.; Cupri, S.; Leonardi, A.; Puglisi, G.; Pignatello, R. Lipid-based nanocarriers for drug delivery and targeting: A patent survey of methods of production and characterization. Pharm. Pat. Anal. 2013, 2, 665–677. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–167. [Google Scholar] [CrossRef]
- Gasco, M.R. Method for Producing Solid Lipid Microspheres Having a Narrow Size Distribution. U.S. Patent 5,250,236, 5 October 1993. [Google Scholar]
- Schubert, M.A.; Müller-Goymann, C.C. Solvent injection as a new approach for manufacturing lipid nanoparticles—Evaluation of the method and process parameters. Eur. J. Pharm. Biopharm. 2003, 55, 125–131. [Google Scholar] [CrossRef]
- Trotta, M.; Debernardi, F.; Caputo, O. Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int. J. Pharm. 2003, 257, 153–160. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M.; Cavalli, R.; Trotta, M. Solid lipid nanoparticles produced through a coacervation method. J. Microencapsul. 2010, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Peira, E.; Sapino, S.; Dianzani, C.; Barge, A.; Muntoni, E.; Morel, S.; Gallarate, M. Stearoyl-chitosan coated nanoparticles obtained by microemulsion cold dilution technique. J. Mol. Sci. 2018, 19, 3833. [Google Scholar] [CrossRef] [PubMed]
- Peira, E.; Carlotti, M.E.; Trotta, C.; Cavalli, R.; Trotta, M. Positively charged microemulsions for topical application. Int. J. Pharm. 2008, 346, 119–123. [Google Scholar] [CrossRef]
- Siekmann, B.; Westesen, K. Melt-homogenized solid lipid nanoparticles stabilized by the non-ionic surfactant tylxapol. I. Preparation and particle size determination. Pharm Pharmacol Lett. 1994, 3, 194–197. [Google Scholar]
- Freitas, C.; Müller, R.H. Correlation between long-term stability of solid lipid nanoparticles (SLNTM) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Trotta, M.; Peira, E.; Carlotti, M.E.; Gallarate, M. Deformable liposomes for dermal administration of methotrexate. Int. J. Pharm. 2004, 270, 119–125. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and chemical stability of curcumin in aqueous solutions and emulsions: impact of pH, temperature, and molecular environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Gallarate, M.; Pattarino, F.; Marengo, E.; Gasco, M.R. Microemulsions containing lecithin and bile salts: Evaluation of the role of bile salts as co-surfactants by partial least squares regression analysis. STP Pharma Sci. 1993, 3, 413–418. [Google Scholar]
- Lim, S.J.; Kim, C.K. Formulation parameters determining the physicochemical characteristics of solid lipid nanoparticles loaded with all-trans retinoic acid. Int. J. Pharm. 2002, 243, 135–146. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery. II. Drug incorporation and physicochemical characterization. J. Microencapsul. 1999, 16, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Windbergs, M.; Strachan, C.J.; Kleinebudde, P. Investigating the principles of recrystallization from glyceride melts. AAPS Pharm. Sci. Tech. 2009, 10, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.Q.; Jiang, S.P.; Du, Y.Z.; Yuan, H.; Ye, Y.Q.; Zeng, S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces 2005, 45, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Li, X.; Szewczuk, M.R.; Malardier-Jugroot, C. Folic acid-conjugated amphiphilic alternating copolymer as a new active tumor targeting drug delivery platform. Drug Des. Dev. Ther. 2016, 10, 4101–4110. [Google Scholar] [CrossRef]
- Miglietta, A.; Cavalli, R.; Bocca, C.; Gabriel, L.; Gasco, M.R. Cellular uptake and cytotoxicity of solid lipid nanospheres (SLN) incorporating doxorubicin or paclitaxel. Int. J. Pharm. 2000, 210, 61–67. [Google Scholar] [CrossRef]
- Martins, S.; Costa-Lima, S.; Carneiroa, T.; Cordeiro-da-Silva, A.; Souto, E.B.; Ferreira, D.C. Solid lipid nanoparticles as intracellular drug transporters: An investigation of the uptake mechanism and pathway. Int. J. Pharm. 2012, 430, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Gelperina, S.E.; Khalansky, A.S.; Skidan, I.N.; Smirnova, Z.S.; Bobruskin, A.I.; Severin, S.E.; Turowski, B.; Zanella, F.E.; Kreuter, J. Toxicological studies of doxorubicin bound to polysorbate 80-coated poly(butyl cyanoacrylate) nanoparticles in healthy rats and rats with intracranial glioblastoma. Toxicol. Lett. 2002, 126, 131–141. [Google Scholar] [CrossRef]
- Sutaria, D.; Grandhi, B.K.; Thakkar, A.; Wang, J.; Prabhu, S. Chemoprevention of pancreatic cancer using solid-lipid nanoparticulate delivery of a novel aspirin, curcumin and sulforaphane drug combination regimen. Int. J. Oncol. 2012, 41, 2260–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 500 nm).
500 nm).
 500 nm).
500 nm).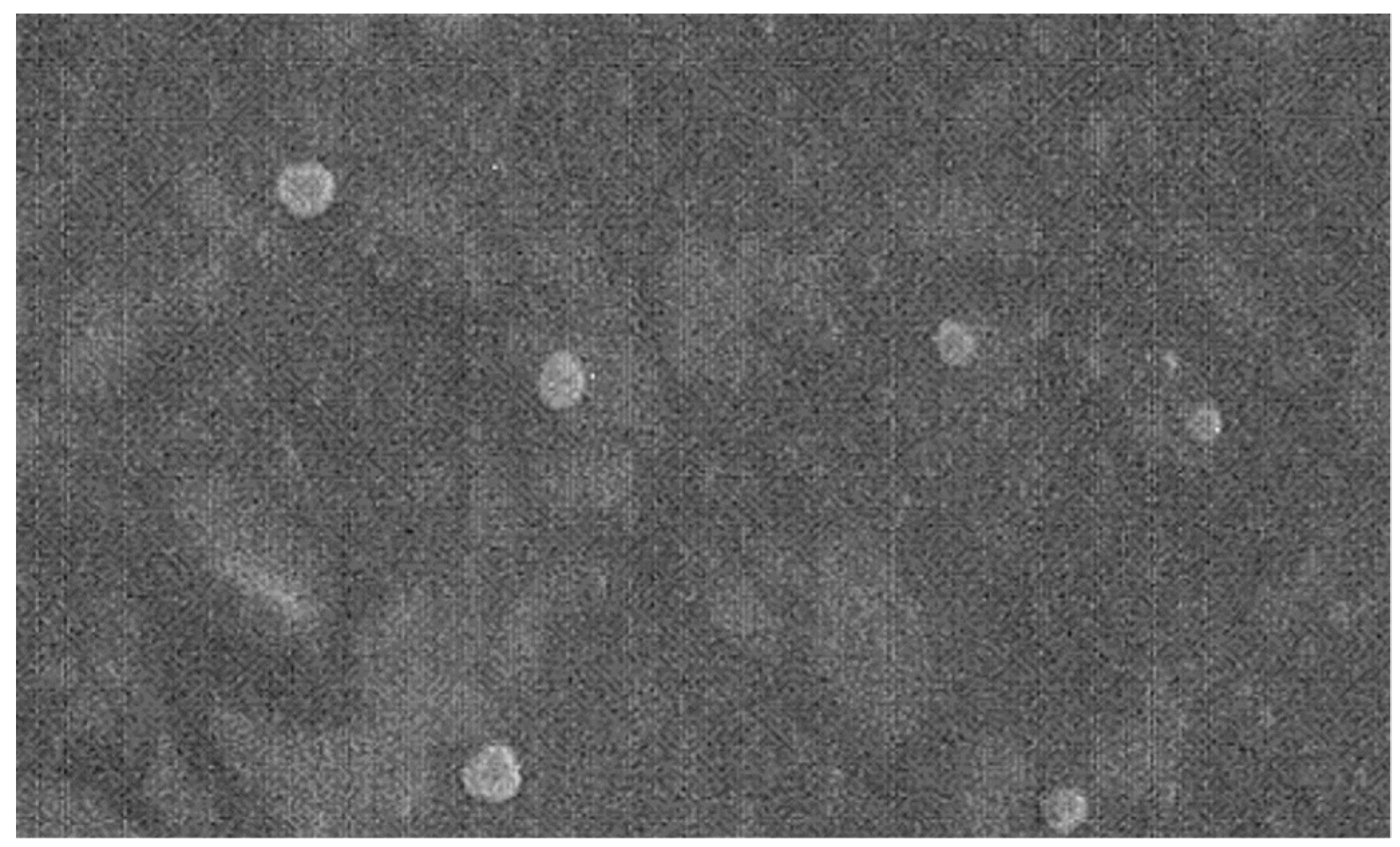
| Solvent | Solubility of TL in Water Saturated Solvent (mg/mL) | Solvent Water Solubility (g/100 mL) |
|---|---|---|
| TA | <300 | 6.1 |
| BL | <300 | 4.2 |
| EA | >300 | 8.7 |
| BenzOH | <300 | 4.3 |
| Components | µE1 Composition (w/w %) | µE2 Composition (w/w %) |
|---|---|---|
| TL | 3.33 | 4.35 |
| EAs | 14.97 | 13.06 |
| Epikuron® 200 | 12.48 | 10.88 |
| Cremophor® RH60 | 4.16 | 3.63 |
| Ws | 58.22 | 50.80 |
| Bile salt | 2.49 | 2.18 |
| BenzOH | 4.35 | – |
| 1,2 Propanediol | – | 15.10 |
| Samples | Mean Diameter (nm) ± S.E. (PI) |
|---|---|
| SLNs in PVA® 9000 | 200.2 ± 2.2 (0.191) |
| SLNs in Cremophor® RH60 | 223.9 ± 2.4 (0.224) |
| SLNs in Pluronic® F68 | 151.9 ± 3.4 (0.210) |
| SLNs in PVA® 14000 | 282.2 ± 11.5 (0.139) |
| SLN | Mean Diameter (nm) ± S.E. | pH of SLN Suspension |
|---|---|---|
| SLNs with Na TdC | 332 ± 1.4 | 4.43 |
| SLNs with Na TC | 152 ± 2.4 | 3.89 |
| SLNs with Na GC | 334 ± 1.5 | 4.57 |
| SLNs with Na C | 230 ± 1.7 | 6.49 |
| mg of CURC Added | Mean Diameter (nm) ± S.E. (PI) | Zeta Potential (mV) ± S.E. | %EE ± S.E. |
|---|---|---|---|
| 3 mg in SLN | 206.2 ± 4.4 (0.220) | −9. 89 ± 1.98 | 87 ± 1.1 |
| 5 mg in SLN | 169.8 ± 4.7 (0.255) | −10.02 ± 2.66 | 78 ± 1.3 |
| 7 mg in SLN | 185.9 ± 2.9 (0.265) | −10.20 ± 2.10 | 74 ± 1.8 |
| 9 mg in SLN | 203.5 ± 5.9 (0.259) | −10.06 ± 2.66 | 75 ± 1.0 |
| 11 mg in SLN | 215.3 ± 5.4 (0.222) | −18.96 ± 2.88 | 70 ± 2.1 |
| Mean Diameter (nm) ± S.E. (PI) Before Elution in GF | Mean Diameter (nm) ± S.E. (PI) After Elution in GF and Concentration under N2 | Mean Diameter (nm) ± S.E. (PI) After Elution in GF and Concentration Freeze-Drying | |
|---|---|---|---|
| SLN | 152 ± 2.4 (0.21) | 193 ± 0.6 (0.134) | 302 ± 2.1 (0.198) |
| SLN-CURC | 204 ± 5.9 (0.26) | 173 ± 1.1 (0.123) | 468 ± 1.9 (0.188) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.L.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials 2019, 9, 230. https://doi.org/10.3390/nano9020230
Chirio D, Peira E, Dianzani C, Muntoni E, Gigliotti CL, Ferrara B, Sapino S, Chindamo G, Gallarate M. Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials. 2019; 9(2):230. https://doi.org/10.3390/nano9020230
Chicago/Turabian StyleChirio, Daniela, Elena Peira, Chiara Dianzani, Elisabetta Muntoni, Casimiro Luca Gigliotti, Benedetta Ferrara, Simona Sapino, Giulia Chindamo, and Marina Gallarate. 2019. "Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution" Nanomaterials 9, no. 2: 230. https://doi.org/10.3390/nano9020230
APA StyleChirio, D., Peira, E., Dianzani, C., Muntoni, E., Gigliotti, C. L., Ferrara, B., Sapino, S., Chindamo, G., & Gallarate, M. (2019). Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution. Nanomaterials, 9(2), 230. https://doi.org/10.3390/nano9020230