Effect of Hydrophobic and Hydrophilic Metal Oxide Nanoparticles on the Performance of Xanthan Gum Solutions for Heavy Oil Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
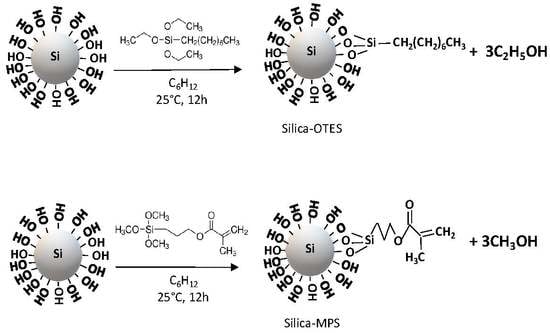
2.2. SiO2 NP Surface Modification
2.3. Modified NP Characterization
2.4. Nanopolymer Sol Preparation
2.5. Colloidal Stability and Particle Size
2.6. Viscosity of the Nanopolymer Sols
2.7. Displacement Test in Linear Sand-Pack
3. Results and Discussion
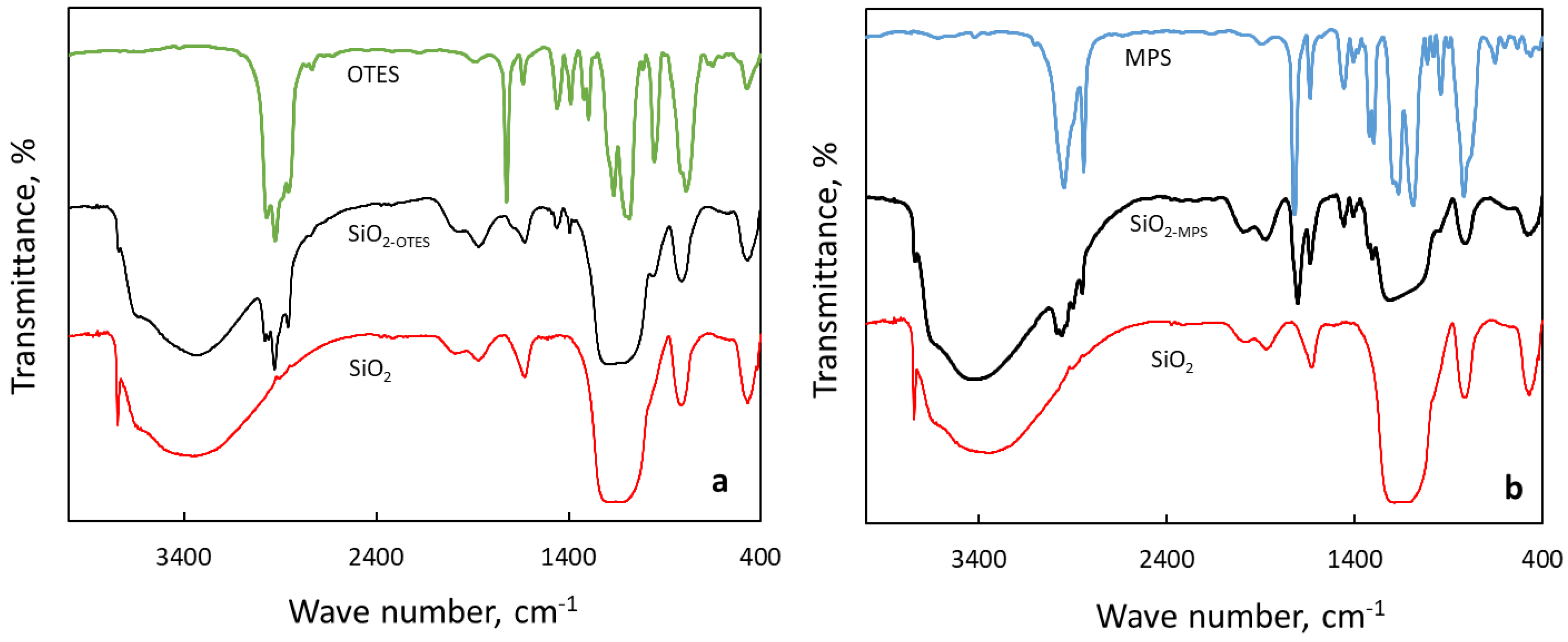
3.1. FTIR and TGA Measurements of the Modified Silica NPs
3.2. Colloidal Stability
3.3. Viscosity Measurements
3.4. Displacement Test in Linear Sand-Pack
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | cross-sectional area of the pack |
| D | darcy |
| FeCl3.6H2O | iron(III) chloride hexahydrate |
| HPAM | hydrolyzed polyacrylamide |
| IFT | interfacial tension |
| K | permeability |
| Kabs | absolute permeability |
| K | consistency factor |
| KBr | potassium bromide |
| L | length of the pack |
| MW | molecular weight |
| n | flow index |
| PV | pore volume |
| PVI | pore volume injected |
| Q | volumetric flow rate |
| SDS | sodium dodecyl sulfate |
| SiO2-MPS | silica modified with 3-(methacryloyloxy)propyl]trimethoxysilane |
| SiO2-OTES | silica modified with octyltriethoxysilane |
| WF | waterflooding |
| XG | xanthan gum |
| ΔP | differential pressure across the sand-pack |
| Φ | porosity |
| shear rate | |
| µ | viscosity of water |
References
- Lutchmansingh, P.M.; Marietta, C.; Ertekin, T.; Abou-Kassem, J.H. Quantitative Analysis of Performance of Polymer Slug Injection. In Proceedings of the SPE Eastern Regional Meeting, Charleston, WV, USA, 1–4 November 1988; pp. 125–131. Available online: http://search.ebscohost.com/login.aspx?direct=true&db=pta&AN=451379&site=ehost-live (accessed on 15 September 2018).
- Sheng, J.J.; Leonhardt, B.; Azri, N. Status of Polymer-Flooding Technology. J. Can. Pet. Technol. 2015, 54, 116–126. [Google Scholar] [CrossRef]
- Kamal, M.S.; Sultan, A.S.; Al-Mubaiyedh, U.A.; Hussein, I.A. Review on Polymer Flooding: Rheology, Adsorption, Stability, and Field Applications of Various Polymer Systems. Polym. Rev. 2015, 55, 491–530. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Gu, D.; Zhou, W.; Xie, T.; Mo, Y. Rheological models for xanthan gum. J. Food Eng. 1996, 27, 203–209. [Google Scholar] [CrossRef]
- Pelletier, E.; Viebke, C.; Meadows, J.; Williams, P.A. A Rheological Study of the Order—Disorder Conformational Transition of xanthan gum. Biopolymers 2001, 59, 339–346. [Google Scholar] [CrossRef]
- Xu, L.; Dong, M.; Gong, H.; Sun, M.; Li, Y. Effects of inorganic cations on the rheology of aqueous welan, xanthan, gellan solutions and their mixtures. Carbohydr. Polym. 2015, 121, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, G.; Liu, T.; Chen, Y.; Gong, H. The comparison of rheological properties of aqueous welan gum and xanthan gum solutions. Carbohydr. Polym. 2013, 92, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Ghoumrassi-Barr, S.; Aliouche, D. A Rheological Study of Xanthan Polymer for Enhanced Oil Recovery. J. Macromol. Sci. Part B 2016, 55, 793–809. [Google Scholar] [CrossRef]
- Milas, M.; Rinaudo, M. Conformational investigation on the bacterial polysaccharide xanthan. Carbohydr. Res. 1979, 76, 189–196. [Google Scholar] [CrossRef]
- Lambert, F.; Rinaudo, M. On the thermal stability of xanthan gum. Polymer 1985, 26, 1549–1553. [Google Scholar] [CrossRef]
- Brunchi, C.-E.; Bercea, M.; Morariu, S.; Dascalu, M. Some properties of xanthan gum in aqueous solutions: Effect of temperature and pH. J. Polym. Res. 2016, 23, 123. [Google Scholar] [CrossRef]
- Wyatt, N.B.; Liberatore, M.W. The effect of counterion size and valency on the increase in viscosity in polyelectrolyte solutions. Soft Matter 2010, 6, 3346–3352. [Google Scholar] [CrossRef]
- Zhong, L.; Oostrom, M.; Truex, M.J.; Vermeul, V.R.; Szecsody, J.E. Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J. Hazard. Mater. 2013, 244–245, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.H.; Symes, K.C.; Lawson, C.J.; Morris, E.R. Influence of the pyruvate content of xanthan on macromolecular association in solution. Int. J. Biol. Macromol. 1981, 3, 129–134. [Google Scholar] [CrossRef]
- Wellington, S. Biopolymer Solution Viscosity Stabilization—Polymer Degradation and Antioxidant Use. Soc. Pet. Eng. J. 1983, 23, 901–912. [Google Scholar] [CrossRef]
- Ryles, R.G. Chemical Stability Limits of Water-Soluble Polymers Used in Oil Recovery Processes. SPE Reserv. Eng. 1988, 3, 23–34. [Google Scholar] [CrossRef]
- Seright, R.S.; Henrici, B.J. Xanthan Stability at Elevated Temperatures. SPE Reserv. Eng. 1990, 5, 52–60. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. Transportation and interaction of nano and micro size metal particles injected to improve thermal recovery of heavy-oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011; Volume 3, pp. 2257–2268. [Google Scholar] [CrossRef]
- Ponmani, S.; William, J.K.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Formation and characterization of thermal and electrical properties of CuO and ZnO nanofluids in xanthan gum. Colloids Surf. A Physicochem. Eng. Aspects 2014, 443, 37–43. [Google Scholar] [CrossRef]
- William, J.K.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO nanofluids in xanthan gum on thermal, electrical and high-pressure rheology of water-based drilling fluids. J. Pet. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Comba, S.; Dalmazzo, D.; Santagata, E.; Sethi, R. Rheological characterization of xanthan suspensions of nanoscale iron for injection in porous media. J. Hazard. Mater. 2011, 185, 598–605. [Google Scholar] [CrossRef]
- Xue, D.; Sethi, R. Viscoelastic gels of guar and xanthan gum mixtures provide long-term stabilization of iron micro- and nanoparticles. J. Nanopart. Res. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Attia, A.M.; Musa, H. Effect of Sodium Magnesium Silicate Nanoparticles on Rheology of Xanthan Gum Polymer. Int. J. Sci. Eng. Res. 2015, 6, 1349–1364. [Google Scholar]
- Kennedy, J.; Kent, K.; Brown, J. Rheology of dispersions of xanthan gum, locust bean gum and mixed biopolymer gel with silicon dioxide nanoparticles. Mater. Sci. Eng. C 2015, 48, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Yang-Chuan, K.; Guang-Yao, W.; Yi, W. Preparation, morphology and properties of nanocomposites of polyacrylamide copolymers with monodisperse silica. Eur. Polym. J. 2008, 44, 2448–2457. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. An experimental investigation of silica nanoparticles effect on the rheological behavior of polyacrylamide solution to enhance heavy oil recovery. Pet. Sci. Technol. 2013, 31, 500–508. [Google Scholar] [CrossRef]
- Maghzi, A.; Kharrat, R.; Mohebbi, A.; Ghazanfari, M.H. The impact of silica nanoparticles on the performance of polymer solution in presence of salts in polymer flooding for heavy oil recovery. Fuel 2014, 123, 123–132. [Google Scholar] [CrossRef]
- Zeyghami, M.; Kharrat, R.; Ghazanfari, M.H. Investigation of the Applicability of Nano Silica Particles as a Thickening Additive for Polymer Solution Applied in EOR Processes. Energy Sources Part A 2014, 36, 1315–1324. [Google Scholar] [CrossRef]
- Zhu, D.; Han, Y.; Zhang, J.; Li, X.; Feng, Y. Enhancing rheological properties of hydrophobically associative polyacrylamide aqueous solutions by hybriding with silica nanoparticles. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Yousefvand, H.; Jafari, A. Enhanced Oil Recovery Using Polymer/nanosilica. Procedia Mater. Sci. 2015, 11, 565–570. [Google Scholar] [CrossRef]
- Maurya, N.K.; Mandal, A. Studies on behavior of suspension of silica nanoparticle in aqueous polyacrylamide solution for application in enhanced oil recovery. Pet. Sci. Technol. 2016, 34, 429–436. [Google Scholar] [CrossRef]
- Cheraghian, G. Application of nano-fumed silica in heavy oil recovery. Pet. Sci. Technol. 2016, 34, 12–18. [Google Scholar] [CrossRef]
- Sharma, T.; Iglauer, S.; Sangwai, J.S. Silica Nanofluids in an Oilfield Polymer Polyacrylamide: Interfacial Properties, Wettability Alteration, and Applications for Chemical Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2016, 55, 12387–12397. [Google Scholar] [CrossRef]
- Sharma, T.; Sangwai, J.S. Silica nanofluids in polyacrylamide with and without surfactant: Viscosity, surface tension, and interfacial tension with liquid paraffin. J. Pet. Sci. Eng. 2017. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri RV, S.; Tiwari, P. Silica Nanoparticle Assisted Polymer Flooding of Heavy Crude Oil: Emulsification, Rheology, and Wettability Alteration Characteristics. Ind. Eng. Chem. Res. 2018, 57, 6364–6376. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. SPE 160847. Enhanced Oil Recovery Using Nanoparticles. In Proceedings of the SPE Technical Symposium and Exhibition, Al-Khobar, SAU, 8–11 April 2012; pp. 1–9. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2014, 5, 181–199. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V. Enhanced Heavy Oil Recovery Using TiO2 Nanoparticles: Investigation of Deposition during Transport in Core Plug. Energy Fuels 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Negin, C.; Ali, S.; Xie, Q. Application of nanotechnology for enhancing oil recovery—A review. Petroleum 2016, 2, 324–333. [Google Scholar] [CrossRef]
- Sondi, I.; Fedynyshyn, T.H.; Sinta, R.; Matijević, E. Encapsulation of nanosized silica by in situ polymerization of tert-butyl acrylate monomer. Langmuir 2000, 16, 9031–9034. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R’’Si(OR’)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Moral, L.M. Development of Functionalized Aerogels for Applications as Catalyst and Hydrides Matrix. Ph.D. Thesis, Universidad de Valladolid, Valladolid, Spain, 2016. [Google Scholar]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Aspects 2000, 173, 1–38. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Methods of Test for Zeta Potential of Colloids in Water and Waste Water; ASTM D4187-82; American Society for Testing and Materials: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Gun’ko, V.M.; Zarko, V.I.; Leboda, R.; Chibowski, E. Aqueous suspension of fumed oxides: Particle size distribution and zeta potential. Adv. Colloid Interface Sci. 2001, 91, 1–112. [Google Scholar] [CrossRef]
- Colic, M.; Fisher, M.L.; Franks, G.V. Influence of Ion Size on Short-Range Repulsive Forces between Silica Surfaces. Langmuir 1998, 14, 6107–6112. [Google Scholar] [CrossRef]
- Singh, B.P.; Menchavez, R.; Takai, C.; Fuji, M.; Takahashi, M. Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface Sci. 2005, 291, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Husein, M. Preparation of nanoscale organosols and hydrosols via the phase transfer route. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Somasundaran, P.; Fuerstenau, D.W. Mechanisms of Alkyl Sulfonate Adsorption at the Alumina-Water Interface 1. J. Phys. Chem. 1966, 70, 90–96. [Google Scholar] [CrossRef]
- Pomogailo, A.D.; Kestelman, V.N. Metallopolymer Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Wyatt, N.B.; Liberatore, M.W. Rheology and Viscosity Scaling of the Polyelectrolyte Xanthan Gum. J. Appl. Polym. Sci. 2009, 114, 4076–4084. [Google Scholar] [CrossRef]
- Khan, M.Y.; Samanta, A.; Ojha, K.; Mandal, A. Interaction between aqueous solutions of polymer and surfactant and its effect on physicochemical properties. Asia-Pac. J. Chem. Eng. 2008, 3, 579–585. [Google Scholar] [CrossRef]
- Nedjhioui, M.; Moulai-Mostefa, N.; Canselier, J.P.; Bensmaili, A. Investigation of combined effects of xanthan gum, sodium dodecyl sulphate, and salt on some physicochemical properties of their mixtures using a response surface method. J. Dispers. Sci. Technol. 2009, 30, 1333–1341. [Google Scholar] [CrossRef]
- Corredor-Rojas, L.M.; Hemmati-Sarapardeh, A.; Husein, M.M.; Dong, M.; Maini, B.B. Rheological Behavior of Surface Modified Silica Nanoparticles Dispersed in Partially Hydrolyzed Polyacrylamide and Xanthan Gum Solutions: Experimental Measurements, Mechanistic Understanding, and Model Development. Energy Fuels 2018. [Google Scholar] [CrossRef]
- Caldelas, F.; Murphy, M.J.; Huh, C.; Bryant, S.L. SPE 142305 Factors Governing Distance of Nanoparticle Propagation in Porous Media. In Proceedings of the SPE Production & Operations Symposium, Oklahoma City, OK, USA, 27–29 March 2011; pp. 1–16. [Google Scholar] [CrossRef]
- Mishra, S.; Bera, A.; Mandal, A.; Mishra, S.; Bera, A.; Mandal, A. Effect of Polymer Adsorption on Permeability Reduction in Enhanced Oil Recovery. J. Pet. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef]



| NaCl Concentration, wt % | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.3 | 1 | ||||
| NP Type | Dispersion Medium | ζ-Potential | pH | ζ-Potential | pH | pH | pH |
| SiO2 | DI water | −17.5 | 6.89 | - | - | - | - |
| DI water–SDS | −25.1 | 6.73 | - | - | - | - | |
| XG–SDS | −65.7 | 6.62 | −56.3 | 6.44 | 6.73 | 6.89 | |
| SiO2-OTES | DI water | −20.7 | 7.01 | - | - | - | - |
| DI water–SDS | −28.3 | 6.89 | - | - | - | - | |
| XG–SDS | −65.9 | 6.55 | −57.8 | 6.38 | 6.60 | 6.75 | |
| SiO2-MPS | DI water | −20.3 | 7.06 | - | - | - | - |
| DI water–SDS | −29.4 | 6.77 | - | - | - | - | |
| XG–SDS | −65.7 | 6.35 | −59.1 | 6.22 | 6.61 | 6.85 | |
| TiO2 | DI water | −25.3 | 6.95 | - | - | - | - |
| DI water–SDS | −28.8 | 6.78 | - | - | - | - | |
| XG–SDS | −53.8 | 7.04 | −46.4 | 6.87 | 7.41 | 8.15 | |
| Al2O3 | DI water | 16.4 | 6.75 | - | - | - | - |
| DI water–SDS | −26.9 | 6.54 | - | - | - | - | |
| XG–SDS | −67.7 | 6.32 | −59.5 | 6.04 | 6.48 | 6.64 | |
| Fe(OH)3 | XG | - | - | - | - | 10.5 | 10.62 |
| Sample | K, Pa.sn | n | r2 |
|---|---|---|---|
| XG | 2.8601 | 0.188 | 0.9972 |
| XG–0.3NaCl | 2.7642 | 0.199 | 0.9986 |
| XG–1.0NaCl | 2.8308 | 0.193 | 0.9982 |
| XG–0.2SiO2 | 2.6939 | 0.237 | 0.9903 |
| XG–0.2SiO2–0.3NaCl | 2.8466 | 0.212 | 0.9989 |
| XG–0.2SiO2–1.0NaCl | 3.0761 | 0.195 | 0.9989 |
| XG–0.2SiO2–OTES | 3.2163 | 0.186 | 0.9988 |
| XG–0.2SiO2–OTES–0.3NaCl | 3.3465 | 0.181 | 0.9987 |
| XG–0.2SiO2–OTES–1.0NaCl | 3.1808 | 0.187 | 0.9991 |
| XG–0.2SiO2–MPS | 3.1175 | 0.198 | 0.9991 |
| XG–0.2SiO2–MPS–0.3NaCl | 3.0886 | 0.193 | 0.9991 |
| XG–0.2SiO2–MPS–1.0NaCl | 2.8506 | 0.186 | 0.9991 |
| XG–0.2Fe(OH)3–0.3NaCl | 2.5509 | 0.197 | 0.9992 |
| XG–0.2Fe(OH)3–1.0NaCl | 2.5132 | 0.195 | 0.9992 |
| XG–0.2Al2O3 | 2.6541 | 0.189 | 0.9985 |
| XG–0.2Al2O3–0.3NaCl | 2.8708 | 0.182 | 0.9989 |
| XG–0.2Al2O3–1.0NaCl | 2.9945 | 0.179 | 0.9989 |
| XG–0.2TiO2 | 2.6801 | 0.19 | 0.9996 |
| XG–0.2TiO2–0.3NaCl | 2.7115 | 0.192 | 0.9993 |
| XG–0.2TiO2–1.0NaCl | 2.9036 | 0.232 | 0.9993 |
| Experiment | Kabs, D | Ф | ΔP after Polymer Flooding, psi/ft | ΔP after Waterflooding, psi/ft |
|---|---|---|---|---|
| Water | 6.25 | 0.35 | - | 1.9 |
| XG | 5.93 | 0.31 | 8.2 | 4.5 |
| XG–0.3NaCl | 6.49 | 0.34 | 35.7 | 5.0 |
| XG–1.0NaCl | 5.99 | 0.34 | 120.0 | 10.1 |
| XG–0.2SiO2 | 5.65 | 0.33 | 156.0 | 17.4 |
| XG–0.2SiO2–0.3NaCl | 5.96 | 0.34 | 161.0 | 25.4 |
| XG–0.2SiO2–1.0NaCl | 6.56 | 0.36 | 202.0 | 35.2 |
| XG–0.2SiO2–OTES | 6.09 | 0.35 | 82.0 | 26.0 |
| XG–0.2SiO2–OTES–0.3NaCl | 6.66 | 0.36 | 92.0 | 30.6 |
| XG–0.2SiO2–OTES–1.0NaCl | 5.64 | 0.34 | 185.0 | 32.0 |
| XG–0.2SiO2–MPS | 5.78 | 0.33 | 98.0 | 22.0 |
| XG–0.2SiO2–MPS–0.3NaCl | 6.49 | 0.36 | 173.0 | 43.4 |
| XG–0.2SiO2–MPS–1.0NaCl | 6.06 | 0.35 | 196.0 | 57.0 |
| XG–0.2Al2O3 | 6.16 | 0.35 | 127.5 | 40.0 |
| XG–0.2Al2O3–0.3NaCl | 6.52 | 0.35 | 136.1 | 53.5 |
| XG–0.2Al2O3–1.0NaCl | 6.77 | 0.37 | 230.9 | 65.9 |
| XG–0.2TiO2 | 5.96 | 0.32 | 56.0 | 24.0 |
| XG–0.2TiO2–0.3NaCl | 6.02 | 0.33 | 99.0 | 27.1 |
| XG–0.2TiO2–1.0NaCl | 6.67 | 0.36 | 136.9 | 37.5 |
| XG–0.2Fe(OH)3–0.3NaCl | 5.78 | 0.31 | 150.0 | 65.0 |
| XG–0.2Fe(OH)3–1.0NaCl | 5.94 | 0.32 | 154.0 | 88.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corredor, L.M.; Husein, M.M.; Maini, B.B. Effect of Hydrophobic and Hydrophilic Metal Oxide Nanoparticles on the Performance of Xanthan Gum Solutions for Heavy Oil Recovery. Nanomaterials 2019, 9, 94. https://doi.org/10.3390/nano9010094
Corredor LM, Husein MM, Maini BB. Effect of Hydrophobic and Hydrophilic Metal Oxide Nanoparticles on the Performance of Xanthan Gum Solutions for Heavy Oil Recovery. Nanomaterials. 2019; 9(1):94. https://doi.org/10.3390/nano9010094
Chicago/Turabian StyleCorredor, Laura M., Maen M. Husein, and Brij B. Maini. 2019. "Effect of Hydrophobic and Hydrophilic Metal Oxide Nanoparticles on the Performance of Xanthan Gum Solutions for Heavy Oil Recovery" Nanomaterials 9, no. 1: 94. https://doi.org/10.3390/nano9010094
APA StyleCorredor, L. M., Husein, M. M., & Maini, B. B. (2019). Effect of Hydrophobic and Hydrophilic Metal Oxide Nanoparticles on the Performance of Xanthan Gum Solutions for Heavy Oil Recovery. Nanomaterials, 9(1), 94. https://doi.org/10.3390/nano9010094