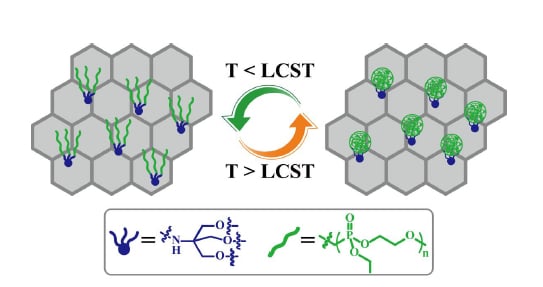
Thermo-Responsive Graphene Oxide/Poly(Ethyl Ethylene Phosphate) Nanocomposite via Ring Opening Polymerization
Abstract
:1. Introduction
2. Experimental
2.1.Characterization
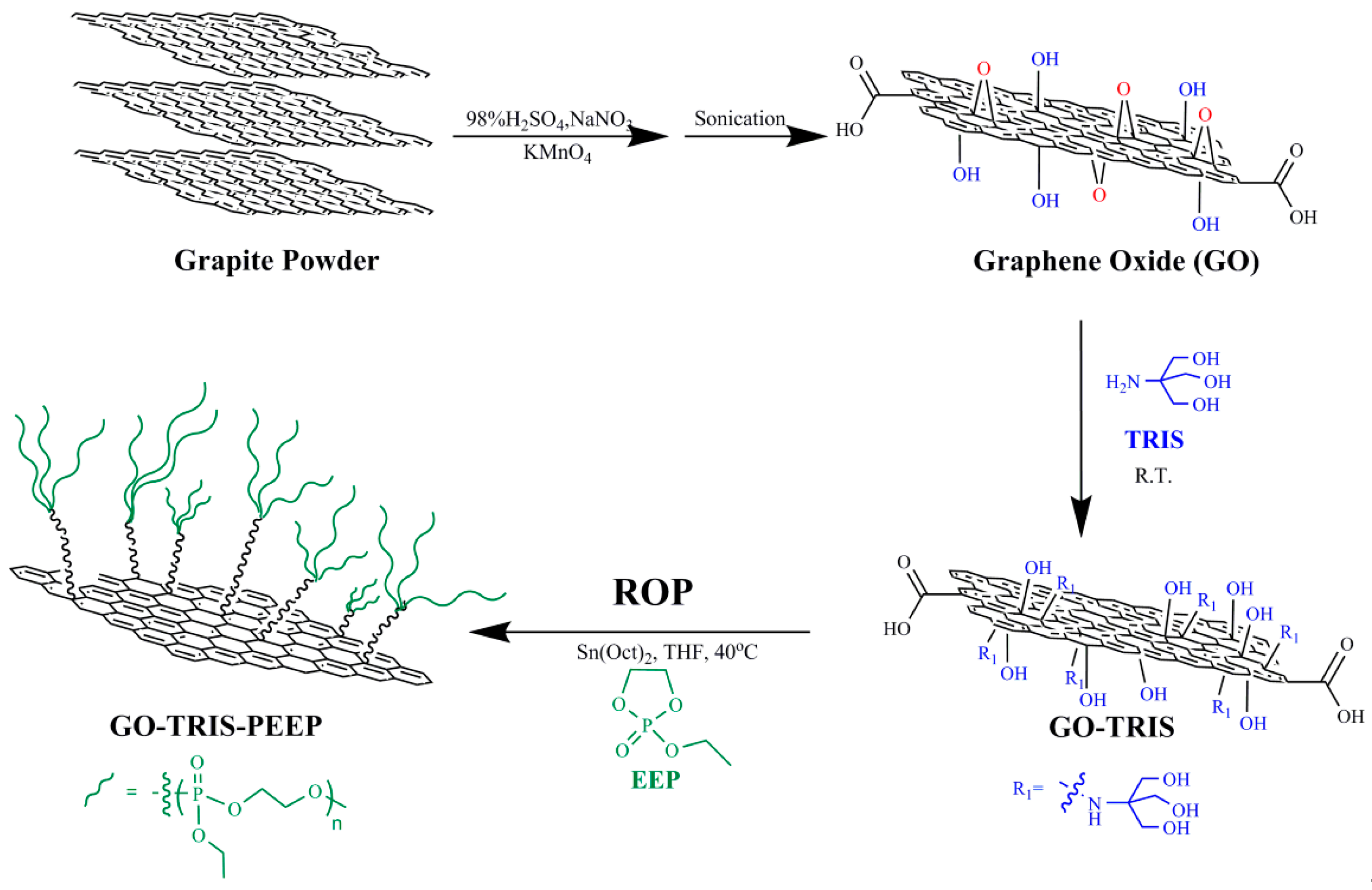
2.2. Preparation of GO Sheets
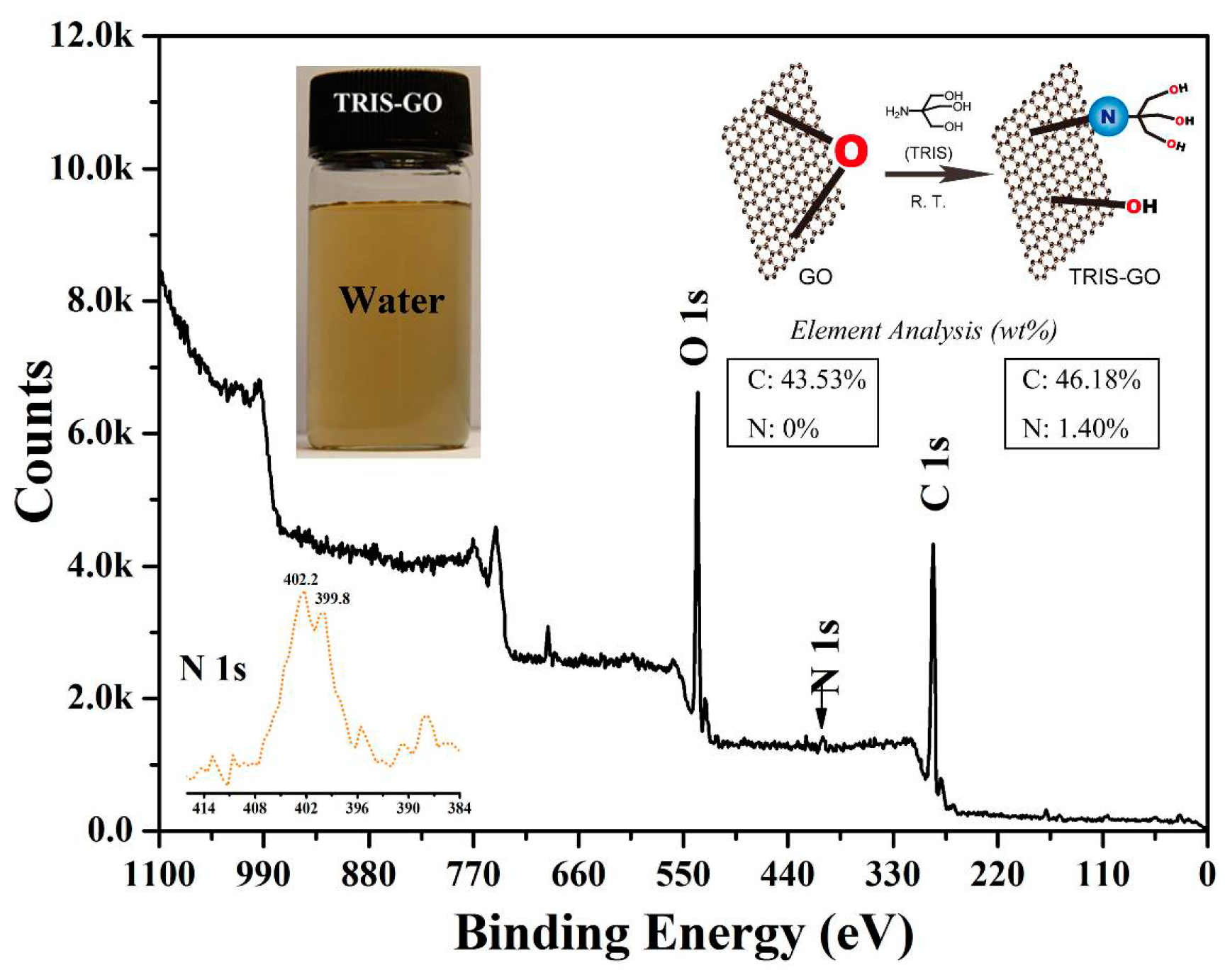
2.3. Preparation of TRIS-Bonded Graphene Sheets (GO-TRIS)
2.4. Preparation of Ethyl Ethylene Phosphate (EEP)
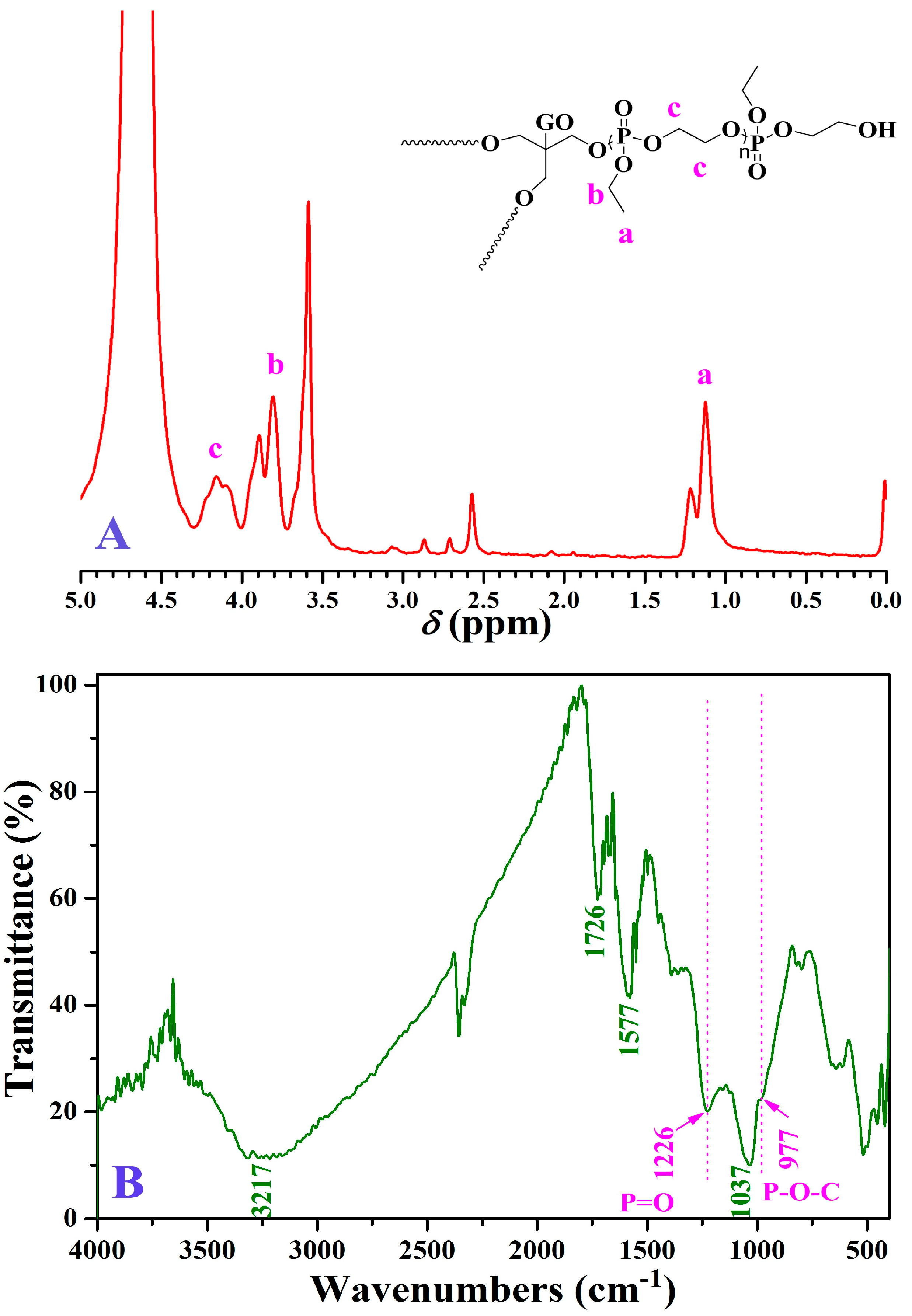
2.5. Preparation of PEEP-Grafted Graphene Sheets (GO-TRIS-PEEP)
3. Results and discussion
3.1. Preparation of GO-TRIS-PEEP
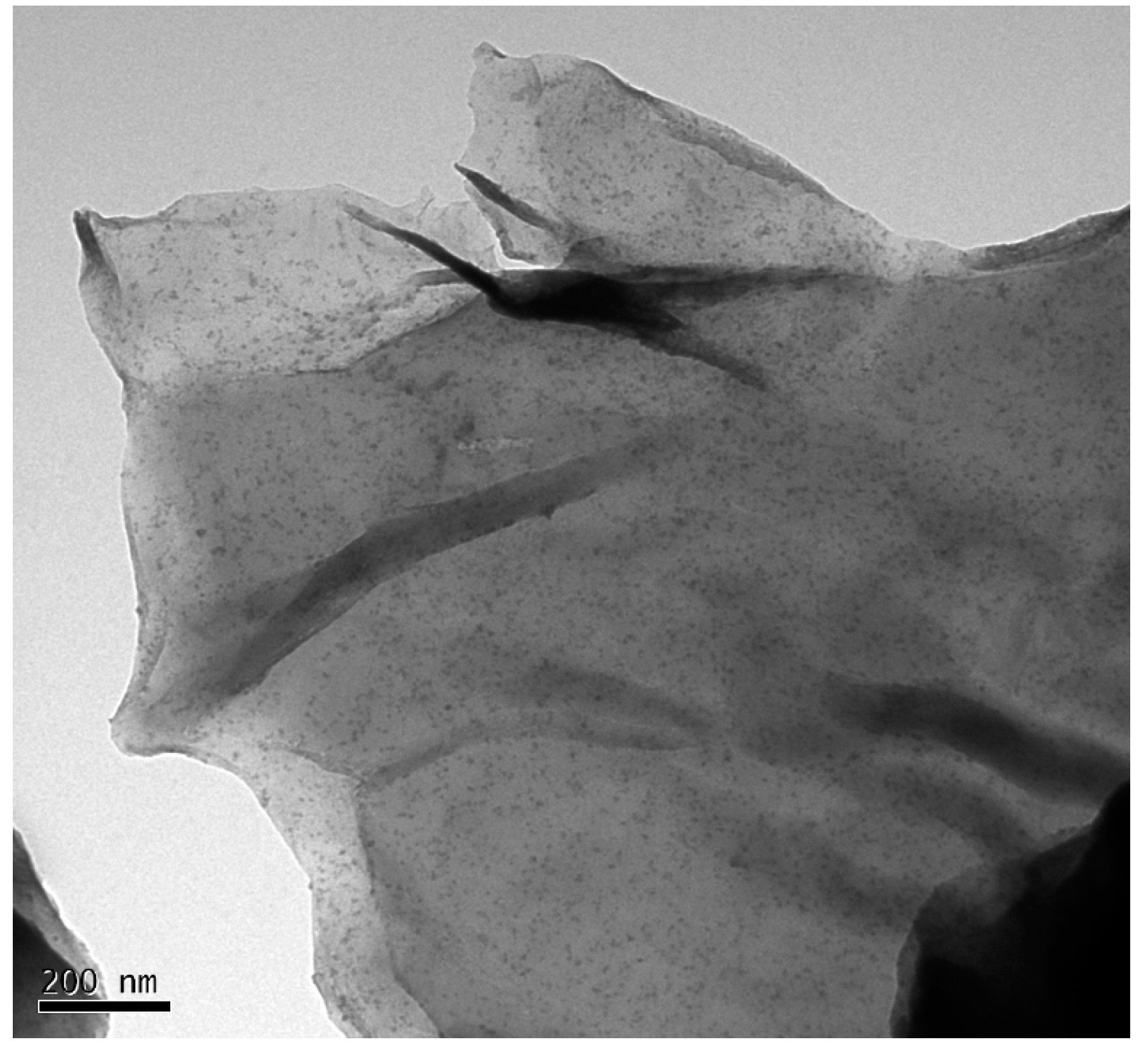
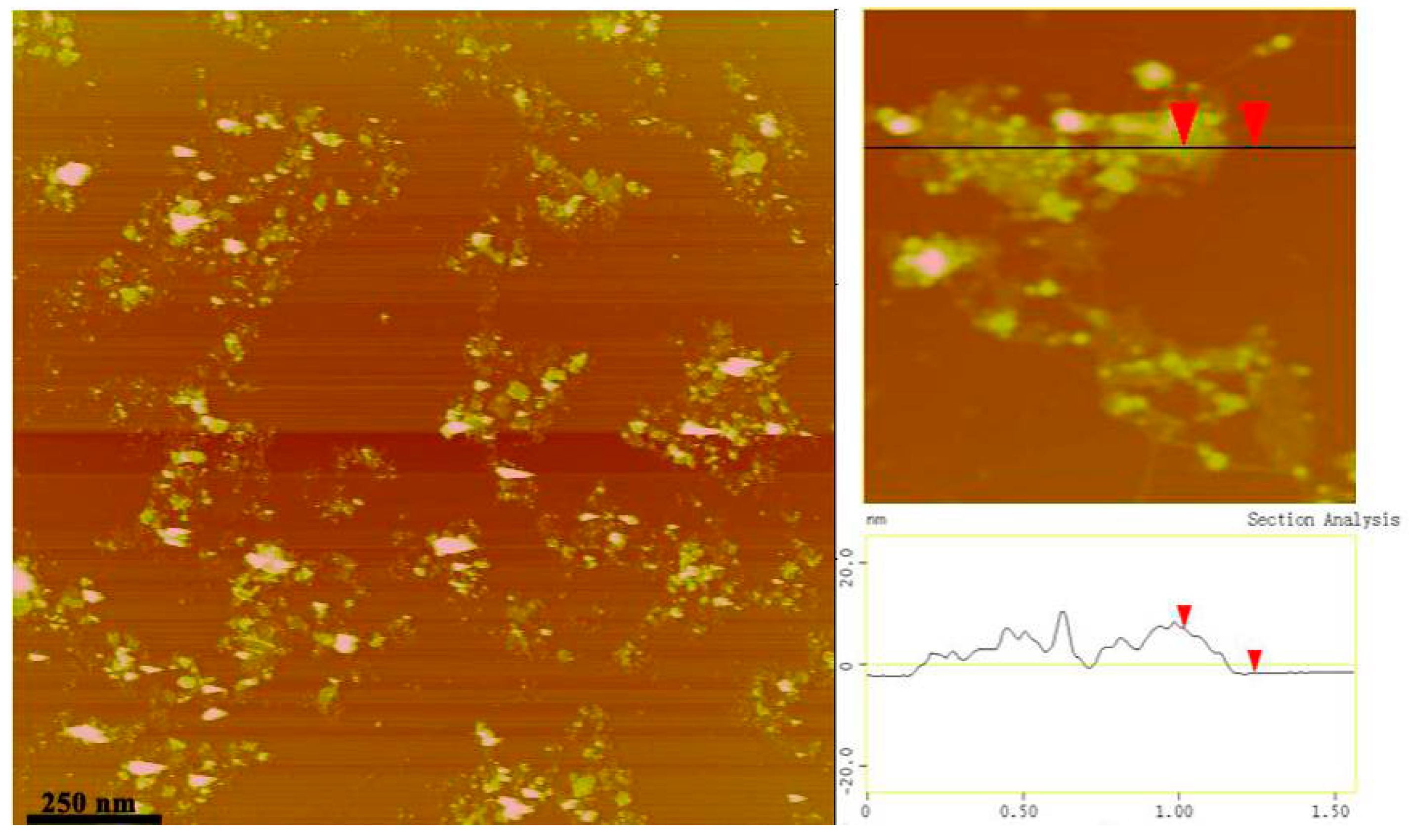
3.2.Surface Morphologies of GO-TRIS-PEEP Sheets
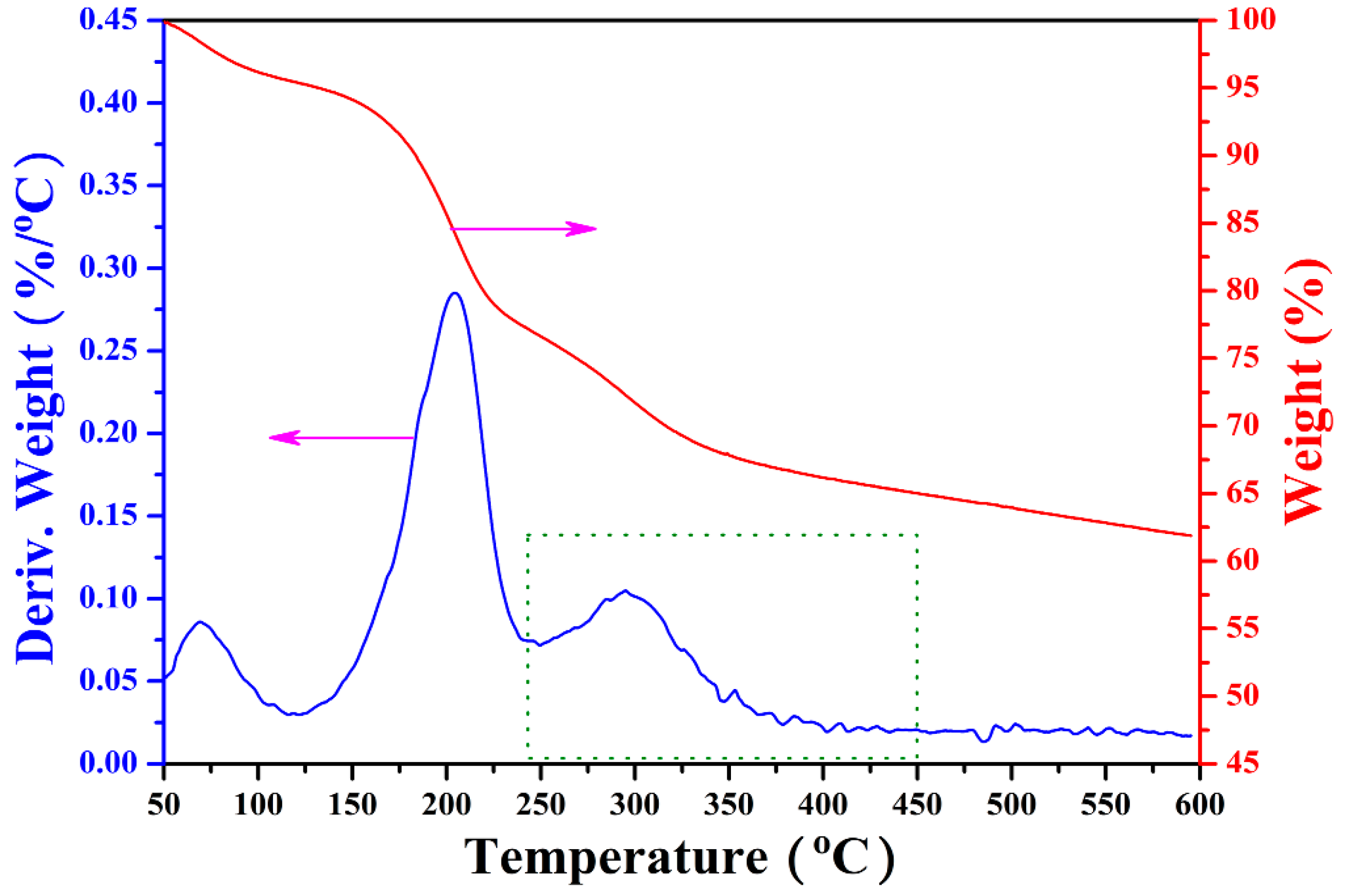
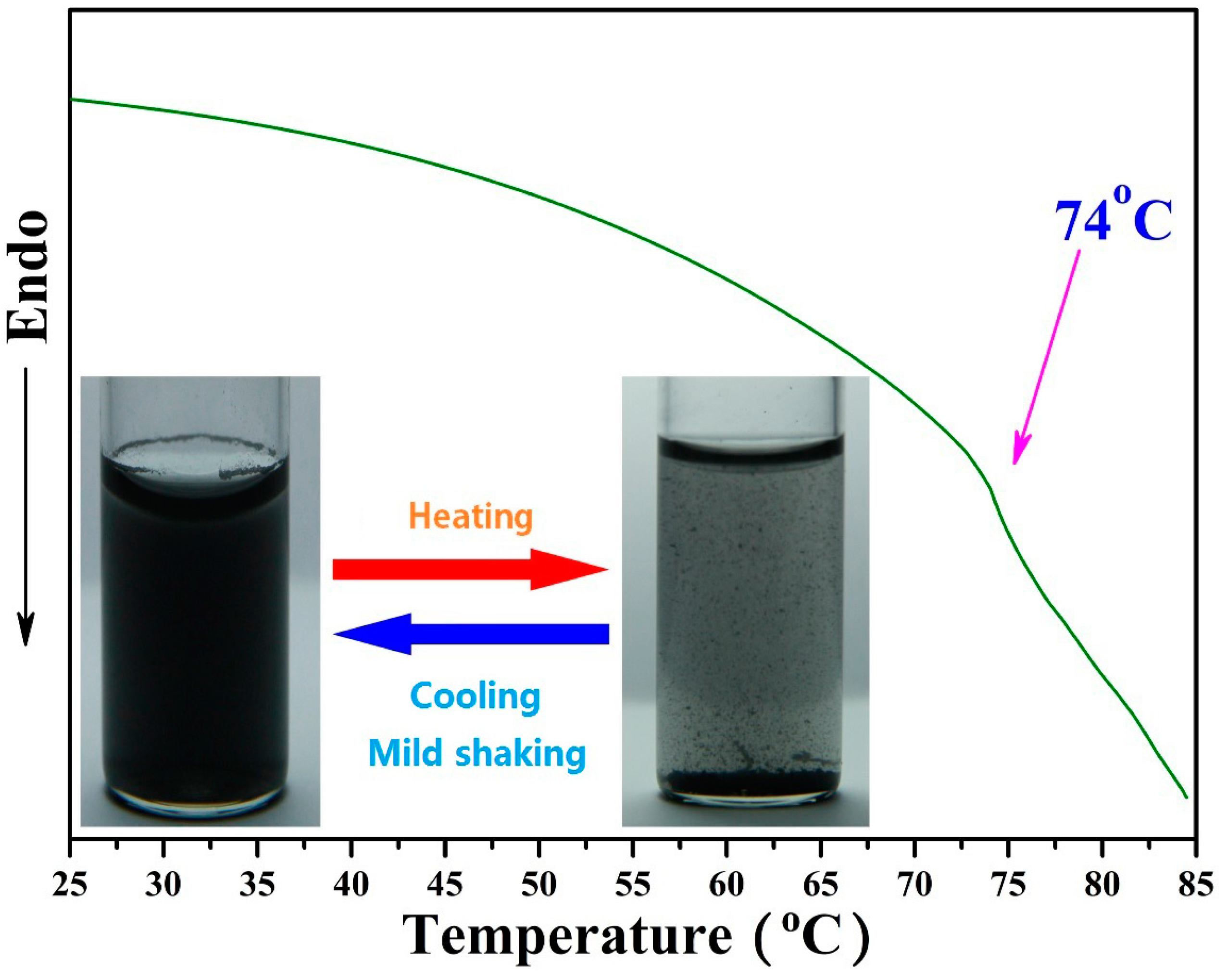
3.3. Thermo-Responsive Behavior of GO-TRIS-PEEP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, T.N.; Gupta, B.K.; Vithayathil, S.A.; Aburto, R.R.; Mani, S.A.; Tijerina, J.T.; Xie, B.; Kaipparettu, B.A.; Torti, S.V.; Ajayan, P.M. Hybrid 2D nanomaterials as dual-mode contrast agents in cellular imaging. Adv. Mater. 2012, 24, 2992–2998. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H.J. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Guan, F.L.; An, F.; Yang, J.; Li, X.; Li, X.H.; Yu, Z.Z. Fiber reinforced three-dimensional graphene aerogels for electrically conductive epoxy composites with enhanced mechanical properties. Chin. J. Polym. Sci. 2017, 35, 1381–1390. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H.J. PEGylated nano-graphene oxide for delivery of water insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.H.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Bitounis, D.; Ali-Boucetta, H.; Hong, B.H.; Min, D.H.; Kostarelos, K. Prospects and challenges of graphene in biomedical applications. Adv. Mater. 2013, 25, 2258–2268. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.X.; Yang, W.Y.; Xiao, C.G.; Wei, M.L. Effects of different amine-functionalized graphene on the mechanical, thermal, and tribological properties of polyimide nanocomposites synthesized by in situ polymerization. Polymer 2018, 140, 56–72. [Google Scholar] [CrossRef]
- Nia, A.S.; Binder, W.H. Graphene as initiator/catalyst in polymerization chemistry. Prog. Polym. Sci. 2017, 67, 48–76. [Google Scholar]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Gracio, J. Surface modification of graphene nanosheets with gold nanoparticles: The role of oxygen moieties at graphene surface on gold nucleation and growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Luan, Y.G.; Zhang, X.A.; Jiang, S.L.; Chen, J.H.; Lyu, Y.F. Self-healing supramolecular polymer composites by hydrogen bonding interactions between hyperbranched polymer and graphene oxide. Chin. J. Polym. Sci. 2018, 36, 584–591. [Google Scholar] [CrossRef]
- Wang, N.; Tian, H.; Zhu, S.Y.; Yan, D.Y.; Mai, Y.Y. Two-dimensional nitrogen-doped mesoporous carbon/graphene nanocomposites from the self-assembly of block copolymer micelles in solution. Chin. J. Polym. Sci. 2018, 36, 266–272. [Google Scholar] [CrossRef]
- Balcioglu, M.; Buyukbekar, B.Z.; Yavuz, M.S.; Yigit, M.V. Smart-polymer-functionalized graphene nanodevices for thermo-switch-controlled biodetection. ACS Biomater. Sci. Eng. 2015, 1, 27–36. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Bao, H.Q.; Sahoo, N.G.; Wu, T.F.; Li, L. Water-soluble poly (N-isopropylacrylamide)-graphene sheets synthesized via click chemistry for drug delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Peng, X.; Liu, T.Q.; Shang, C.; Jiao, C.; Wang, H.L. Mechanically strong Janus poly(N-isopropylacrylamide)/graphene oxide hydrogels as thermo-responsive soft robots. Chin. J. Polym. Sci. 2017, 35, 1268–1275. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. A One-Step Strategy for Thermal- and pH-responsive graphene oxide interpenetrating polymer hydrogel networks. J. Mater. Chem. 2011, 21, 4095–4097. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhang, J.; Liu, J.; Yang, X.; Zhao, H. Exfoliated graphite oxide decorated by PDMAEMA chains and polymer particles. Langmuir 2009, 25, 11808–11814. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.R.; Wang, X.G. Self-assembled graphene/azo polyelectrolyte multilayer film and its application in electrochemical energy storage device. Langmuir 2011, 27, 2007–2013. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of a graphene oxide nanocarrier for dual-drug chemo-phototherapy to overcome drug resistance in cancer. ACS Appl. Mater. Interfaces 2015, 7, 28647–28655. [Google Scholar] [CrossRef]
- Jiang, X.; Deng, Y.; Liu, W.B.; Li, Y.J.; Huang, X.Y. Preparation of graphene/poly(2-acryloxyethyl ferrocenecarboxylate) nanocomposite via a “grafting-onto” strategy. Polym. Chem. 2018, 9, 184–192. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, X.C.; Sun, T.M.; Xiong, M.H.; Wang, J. Functionalized micelles from block copolymer of polyphosphoester and poly(ε-caprolactone) for receptor-mediated drug delivery. J. Control. Release 2008, 128, 32–40. [Google Scholar] [CrossRef]
- Appukutti, N.; Serpell, C.J. High definition polyphosphoesters: between nucleic acids and plastics. Polym. Chem. 2018, 9, 2210–2226. [Google Scholar] [CrossRef]
- Yuan, Y.Y.; Du, Q.; Wang, Y.C.; Wang, J. One-pot syntheses of amphiphilic centipede-like brush copolymers via combination of ring-opening polymerization and “click” chemistry. Macromolecules 2010, 43, 1739–1746. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Wachiralarpphaithoon, C.; Akiyoshi, K. Novel Thermoresponsive polymers having biodegradable phosphoester backbones. Macromolecules 2007, 40, 8136–8138. [Google Scholar] [CrossRef]
- Liu, J.Y.; Huang, W.; Pang, Y.; Yan, D.Y. Hyperbranched polyphosphates: synthesis, functionalization and biomedical applications. Chem. Soc. Rev. 2015, 44, 3942–3952. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Sun, Y.; Zhang, M.; He, J.L.; Ni, P.H. Polyphosphoester-camptothecin prodrug with reduction-response prepared via Michael addition polymerization and click reaction. ACS Appl. Mater. Interfaces 2017, 9, 13939–13949. [Google Scholar] [CrossRef] [PubMed]
- Hindi, K.M.; Ditto, A.J.; Panzner, M.J.; Medvetz, D.A.; Han, D.S.; Hovis, C.E.; Hilliard, J.K.; Taylor, J.B.; Yun, Y.H.; Cannon, C.L.; et al. The antimicrobial efficacy of sustained release silver-carbine complex-loaded l-tyrosine polyphosphate nanoparticles: characterization, in vitro and in vivo Studies. Biomaterials 2009, 30, 3771–3779. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, J.; Mao, H.Q.; Leong, K.W. Polyphosphoesters in drug and gene delivery. Adv. Drug Deliv. Rev. 2003, 55, 483–499. [Google Scholar] [CrossRef]
- Gu, Z.; Xie, H.; Li, L.; Zhang, X.; Liu, F.; Yu, X. Application of strontium-doped calcium polyphosphate scaffold on angiogenesis for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Tang, L.Y.; Li, Y.; Wang, J. Thermoresponsive block copolymers of poly (ethylene glycol) and polyphosphoester: thermo-induced self-assembly, biocompatibility, and hydrolytic degradation. Biomacromolecules 2009, 10, 66–73. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Kreiser-Saunders, I.; Stricker, A. Polylactones 48. SnOct2-initiated polymerizations of lactide: A mechanistic study. Macromolecules 2000, 33, 702–709. [Google Scholar] [CrossRef]
- Shen, J.F.; Hu, Y.Z.; Shi, M.; Li, N.; Ma, H.W.; Ye, M.X. One step synthesis of graphene oxide-magnetic nanoparticle composite. J. Phys. Chem. C 2010, 114, 1498–1503. [Google Scholar] [CrossRef]
- Bui, T.N.H.; Verhage, J.J.; Irgum, K. Tris(hydroxymethyl)aminomethane-functionalized silica particles and their application for hydrophilic interaction chromatography. J. Sep. Sci. 2010, 33, 2965–2976. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Abdolmaleki, A.; Khalesi, Z.; Borandeh, S. Surface functionalization of GO, preparation and characterization of PVA/TRIS-GO nanocomposites. Polymer 2015, 81, 140–150. [Google Scholar] [CrossRef]
- Yang, H.F.; Shan, C.S.; Li, F.H.; Han, D.X.; Zhang, Q.X.; Niu, L. Covalent functionalization of polydisperse chemically-converted graphene sheets with amine-terminated ionic liquid. Chem. Commun. 2009, 3880–3882. [Google Scholar] [CrossRef] [PubMed]
- Ryner, M.; Stridsberg, K.; Albertsson, A.C. Mechanism of ring-opening polymerization of 1,5-dioxepan-2-oneandl-lactide with stannous 2-ethylhexanoate. A theoretical study. Macromolecules 2001, 34, 3877–3881. [Google Scholar] [CrossRef]
- Nakajima, T.; Mabuchi, A.; Hagiwara, R. A new structure model of graphite oxide. Carbon 1988, 26, 357–361. [Google Scholar] [CrossRef]
- Teng, C.Y.; Yeh, T.F.; Lin, K.I.; Chen, S.J.; Yoshimura, M.; Teng, H. Synthesis of graphene oxide dots for excitation-wavelength independent photoluminescence at high quantum yields. J. Mater. Chem. C 2015, 3, 4553–4562. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, J.Z.; Li, Y.J.; Hu, J.H.; Yang, D.; Huang, X.Y. Thermoresponsive graphene oxide-PNIPAM nanocomposites with controllable grafting polymer chains via moderate in situ SET-LRP. J. Polym. Sci. Polym. Chem. 2012, 50, 4451–4458. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Y.J.; Dai, J.; Lang, M.D.; Huang, X.Y. Functionalization of graphene oxide towards thermo-sensitive nanocomposites via moderate in situ SET-LRP. J. Polym. Sci. Polym. Chem. 2011, 49, 4747–4755. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.G.; Lu, H.B.; Yang, Y.L.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ Polymerization approach to graphene-reinforced nylon-6 composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.G.; Lu, H.B.; Yang, Y.L.; Nutt, S. Single-layer graphene nanosheets with controlled grafting of polymer chains. J. Mater. Chem. 2010, 20, 1982–1992. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; Vicente, J.D.; Nardecchia, S.; Marchal, J.A.; Boulaiz, H. Thermo-sensitive nanomaterials: recent advance in synthesis and biomedical applications. Nanomaterials 2018, 8, 935. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Lu, G.; Huang, X.; Li, Y.; Cao, F.; Chen, H.; Liu, W. Thermo-Responsive Graphene Oxide/Poly(Ethyl Ethylene Phosphate) Nanocomposite via Ring Opening Polymerization. Nanomaterials 2019, 9, 207. https://doi.org/10.3390/nano9020207
Jiang X, Lu G, Huang X, Li Y, Cao F, Chen H, Liu W. Thermo-Responsive Graphene Oxide/Poly(Ethyl Ethylene Phosphate) Nanocomposite via Ring Opening Polymerization. Nanomaterials. 2019; 9(2):207. https://doi.org/10.3390/nano9020207
Chicago/Turabian StyleJiang, Xue, Guolin Lu, Xiaoyu Huang, Yu Li, Fangqi Cao, Hong Chen, and Wenbin Liu. 2019. "Thermo-Responsive Graphene Oxide/Poly(Ethyl Ethylene Phosphate) Nanocomposite via Ring Opening Polymerization" Nanomaterials 9, no. 2: 207. https://doi.org/10.3390/nano9020207
APA StyleJiang, X., Lu, G., Huang, X., Li, Y., Cao, F., Chen, H., & Liu, W. (2019). Thermo-Responsive Graphene Oxide/Poly(Ethyl Ethylene Phosphate) Nanocomposite via Ring Opening Polymerization. Nanomaterials, 9(2), 207. https://doi.org/10.3390/nano9020207