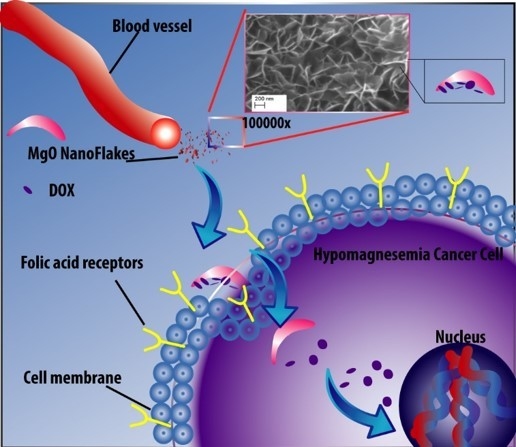
Doxorubicin Loaded Magnesium Oxide Nanoflakes as pH Dependent Carriers for Simultaneous Treatment of Cancer and Hypomagnesemia
Abstract
1. Introduction
2. Materials and Methods
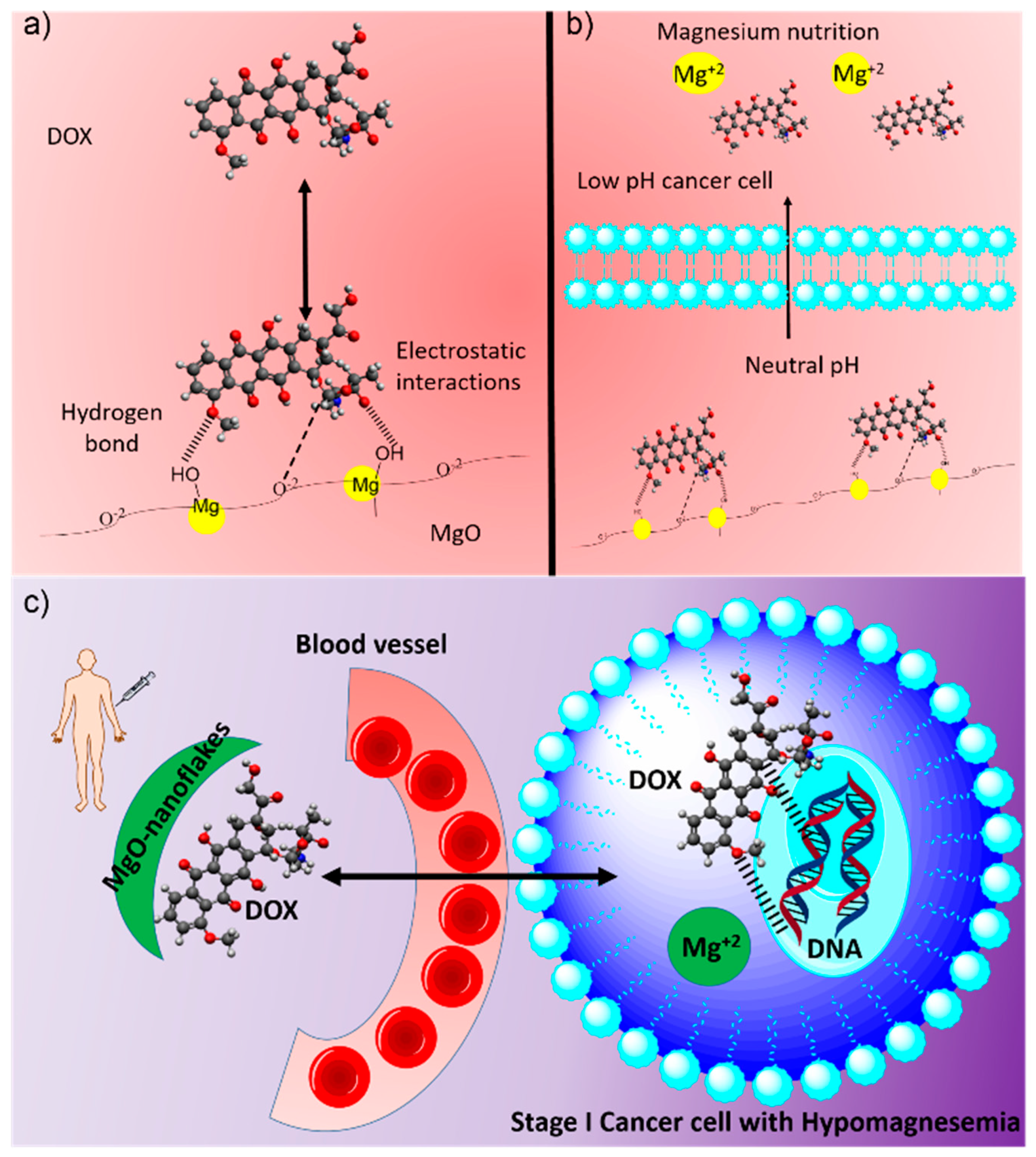
3. Results and Discussion
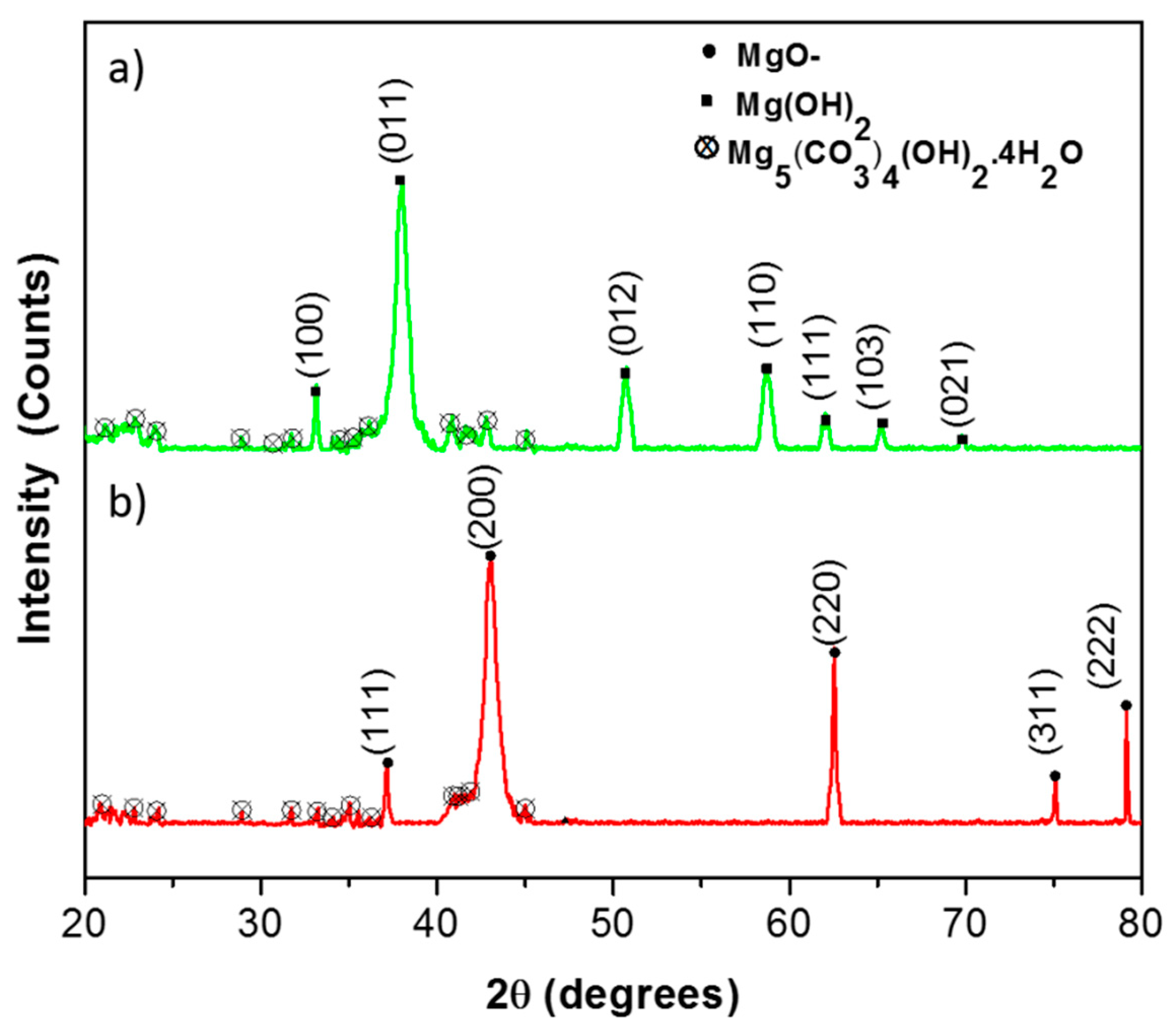
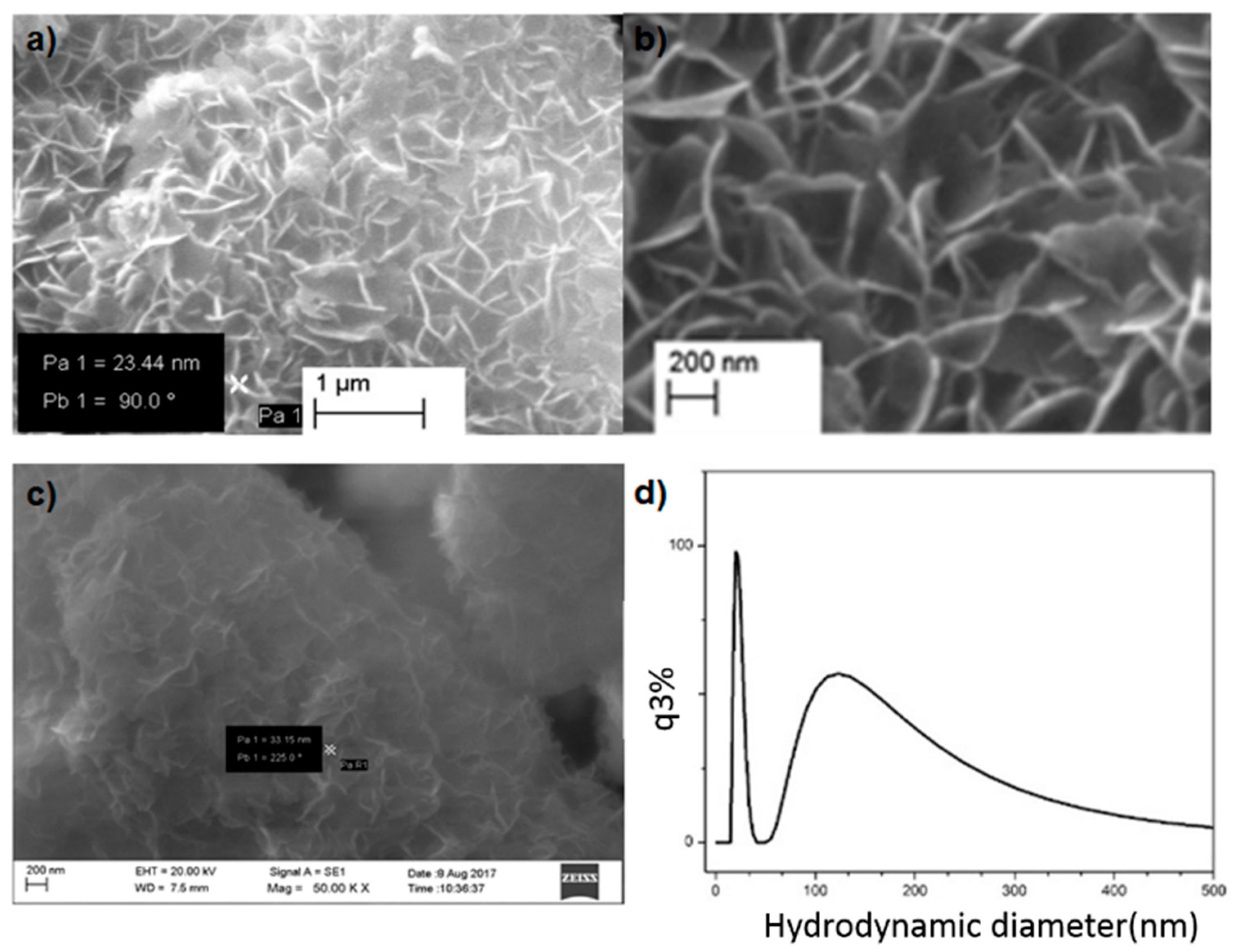
3.1. Synthesis and Characterization of Magnesium Oxide Nanoflake
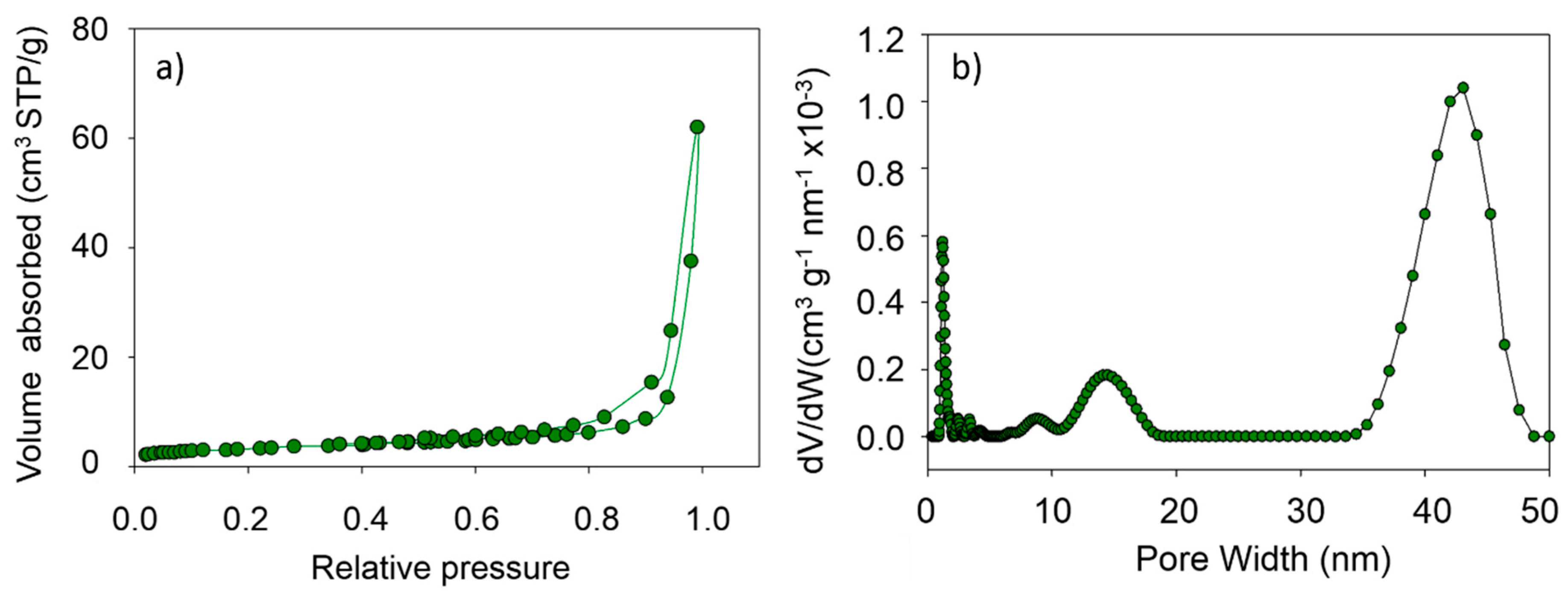
3.2. Pore size Distribution of MgO Nanoflakes
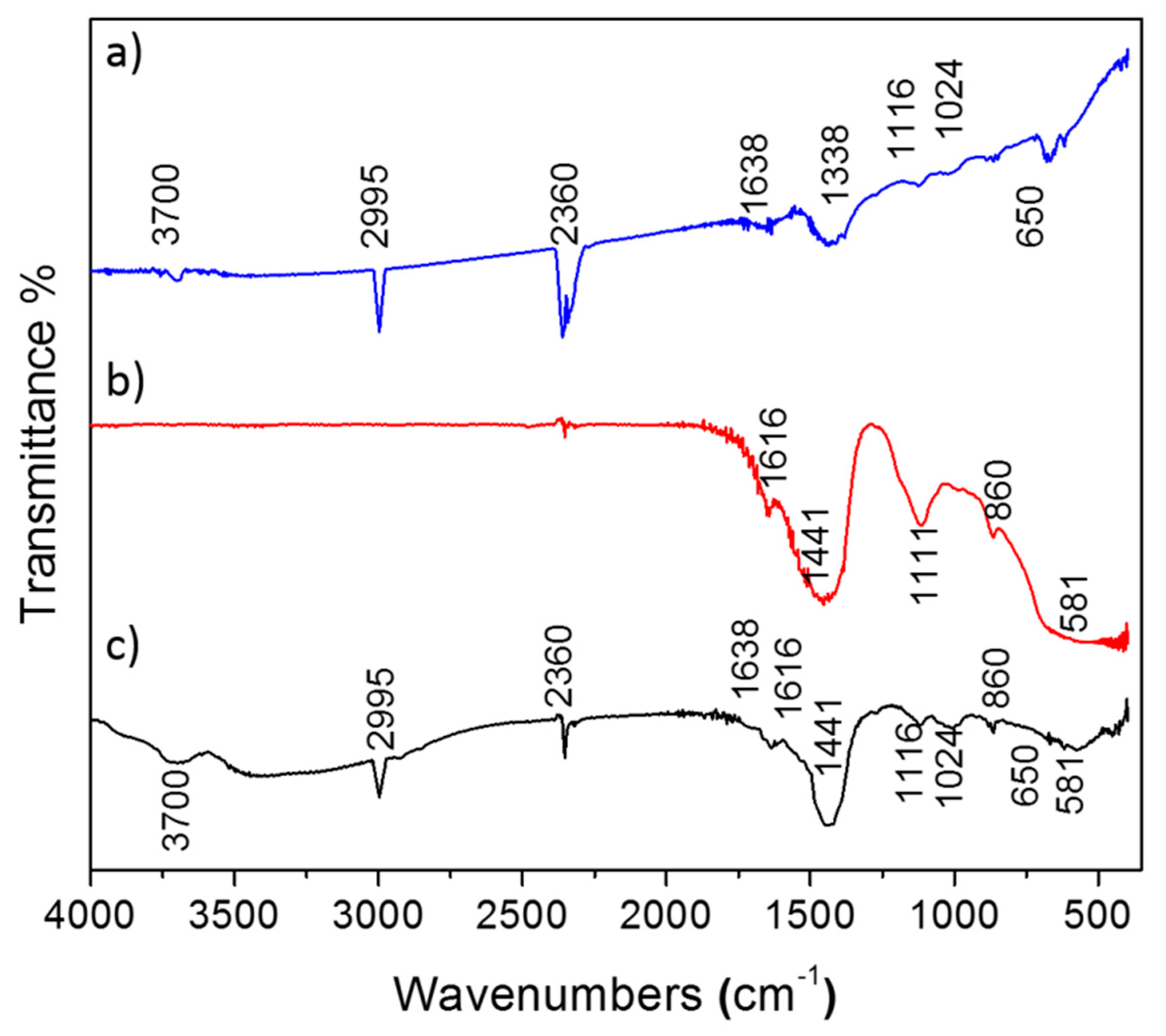
3.3. FTIR Studies for Drug Loaded Nanoparticles
3.4. Drug Loading Capacity (DLC) and Drug Loading Efficiency (DLE)
3.5. Drug Releasing Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7. [Google Scholar] [CrossRef] [PubMed]
- Illouz, F.; Braun, D.; Briet, C.; Schweizer, U.; Rodien, P. Endocrine side-effects of anti-cancer drugs: Thyroid effects of tyrosine kinase inhibitors. Eur. J. Endocrinol. 2014, 171, R91. [Google Scholar] [CrossRef] [PubMed]
- Monsuez, J.J.; Charniot, J.C.; Vignat, N.; Artigou, J.Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3. [Google Scholar] [CrossRef] [PubMed]
- Torino, F.; Barnabei, A.; Paragliola, R.M.; Marchetti, P.; Salvatori, R.; Corsello, S.M. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: Clinical evidence and pathogenic hypotheses. Eur. J. Endocrinol. 2013, 169, R153. [Google Scholar] [CrossRef] [PubMed]
- Amoedo, N.D.; Valencia, J.P.; Rodrigues, M.F.; Galina, A.; Rumjanek, F.D. How does the metabolism of tumour cells differ from that of normal cells. Biosci. Rep. 2013, 33, e00080. [Google Scholar] [CrossRef] [PubMed]
- Weerasuriya, D.R.K.; Wijesinghe, W.; Rajapakse, R.M.G. Encapsulation of anticancer drug copper bis(8-hydroxyquinoline) in hydroxyapatite for pH-sensitive targeted delivery and slow release. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 206. [Google Scholar] [CrossRef] [PubMed]
- Dunuweera, S.; Rajapakse, R. Synthesis of Unstable Vaterite Polymorph of Porous Calcium Carbonate Nanoparticles, Encapsulation of Anticancer Drug Cisplatin, Studying Release Kinetics for Safe, Targeted Delivery and Slow Release. J. Nanomed. Biother. Discov. 2017, 7, 2. [Google Scholar]
- Dunuweera, S.; Rajapakse, R. Encapsulation of anticancer drug cisplatin in vaterite polymorph of calcium carbonate nanoparticles for targeted delivery and slow release. Biomed. Phys. Eng. Exp. 2017, 4, 015017. [Google Scholar] [CrossRef]
- Hakeem, A.; Zhan, G.; Xu, Q.; Yong, T.; Yang, X.; Gan, L. Facile synthesis of pH-responsive doxorubicin-loaded layered double hydroxide for efficient cancer therapy. J. Mater. Chem. B 2018, 6, 5768. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Chen, J.; Chen, W.; Xu, Z.P. Co-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticles. Biomaterials 2014, 35, 3331. [Google Scholar] [CrossRef]
- Dhal, J.P.; Sethi, M.; Mishra, B.G.; Hota, G. MgO nanomaterials with different morphologies and their sorption capacity for removal of toxic dyes. Mater. Lett. 2015, 141, 267. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Pahari, S.K.; Pal, P.; Bajaj, H.C.; Mukhopadhyay, I.; Panda, A.B. Controlled Synthesis of Different Morphologies of MgO and Their Use as Solid Base Catalysts. J. Phys. Chem. C 2011, 115, 12308. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human Health Risk Assessment for Aluminium, Aluminium Oxide, and Aluminium Hydroxide. J. Toxicol. Environ. Health B 2007, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26. [Google Scholar] [CrossRef] [PubMed]
- D’Erasmo, E.; Celi, F.S.; Acca, M.; Minisola, S.; Aliberti, G.; Mazzuoli, G.F. Hypocalcemia and hypomagnesemia in cancer patients. Biomed. Pharmacother. 1991, 45, 315. [Google Scholar] [CrossRef]
- Lopez-Saca, J.M.; Lopez-Picazo, J.M.; Larumbe, A.; Urdiroz, J.; Centeno, C. Hypomagnesemia as a possible explanation behind episodes of severe pain in cancer patients receiving palliative care. Support. Care Cancer 2013, 21, 649. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1. [Google Scholar] [CrossRef]
- Mantilaka, M.; Pitawala, H.; Karunaratne, D.; Rajapakse, R. Nanocrystalline magnesium oxide from dolomite via poly (acrylate) stabilized magnesium hydroxide colloids. Colloid. Surf. A-Physicochem. Eng. Asp. 2014, 443, 201. [Google Scholar] [CrossRef]
- Mantilaka, M.M.M.G.P.G.; Rajapakse, R.M.G.; Karunaratne, D.G.G.P.; Pitawala, H.M.T.G.A. Preparation of amorphous calcium carbonate nanoparticles from impure dolomitic marble with the aid of poly(acrylic acid) as a stabilizer. Adv. Powder Technol. 2014, 25, 591. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907. [Google Scholar] [CrossRef]
- Kamba, S.A.; Ismail, M.; Hussein-Al-Ali, S.H.; Ibrahim, T.A.T.; Zakaria, Z.A.B. In Vitro Delivery and Controlled Release of Doxorubicin for Targeting Osteosarcoma Bone Cancer. Molecules 2013, 18, 10580. [Google Scholar] [CrossRef] [PubMed]
- Chandrappa, K.; Venkatesha, T.; Praveen, B.; Shylesha, B. Generation of nanostructured MgO particles by solution phase method. Res. J. Chem. Sci. 2015, 2231, 606X. [Google Scholar]
- Wu, X.-F.; Hu, G.-S.; Wang, B.-B.; Yang, Y.-F. Synthesis and characterization of superfine magnesium hydroxide with monodispersity. J. Cryst. Growth 2008, 310, 457. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranathunge, T.A.; Karunaratne, D.G.G.P.; Rajapakse, R.M.G.; Watkins, D.L. Doxorubicin Loaded Magnesium Oxide Nanoflakes as pH Dependent Carriers for Simultaneous Treatment of Cancer and Hypomagnesemia. Nanomaterials 2019, 9, 208. https://doi.org/10.3390/nano9020208
Ranathunge TA, Karunaratne DGGP, Rajapakse RMG, Watkins DL. Doxorubicin Loaded Magnesium Oxide Nanoflakes as pH Dependent Carriers for Simultaneous Treatment of Cancer and Hypomagnesemia. Nanomaterials. 2019; 9(2):208. https://doi.org/10.3390/nano9020208
Chicago/Turabian StyleRanathunge, Tharindu A., D.G.G.P. Karunaratne, R.M.G. Rajapakse, and Davita L. Watkins. 2019. "Doxorubicin Loaded Magnesium Oxide Nanoflakes as pH Dependent Carriers for Simultaneous Treatment of Cancer and Hypomagnesemia" Nanomaterials 9, no. 2: 208. https://doi.org/10.3390/nano9020208
APA StyleRanathunge, T. A., Karunaratne, D. G. G. P., Rajapakse, R. M. G., & Watkins, D. L. (2019). Doxorubicin Loaded Magnesium Oxide Nanoflakes as pH Dependent Carriers for Simultaneous Treatment of Cancer and Hypomagnesemia. Nanomaterials, 9(2), 208. https://doi.org/10.3390/nano9020208