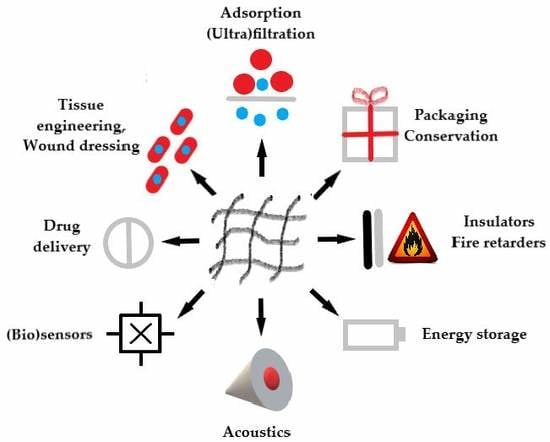
Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing
Abstract
1. Introduction
2. History of Nanocellulose Research with Focus on Biomedical Applications
3. Recent Use of Nanocellulose in Tissue Engineering and Tissue Repair
4. Nanocellulose in Skin Tissue Engineering
4.1. Bacterial Nanocellulose in Skin Tissue Engineering
4.2. Plant- and Algae-Derived Nanocellulose in Skin Tissue Engineering
4.3. Limitations of the Use of Nanocellulose in Skin Tissue Engineering
4.4. Nanocellulose as a Carrier for Cell Delivery into Skin Defects
5. Nanocellulose in Wound Healing
5.1. Bacterial Nanocellulose
5.1.1. Bacterial Nanocellulose without Additives
5.1.2. Bacterial Nanocellulose with Additives
5.2. Plant- and Animal-Derived Nanocellulose in Wound Healing
5.2.1. Plant-Derived Nanocellulose without Additives
5.2.2. Plant-Derived Nanocellulose with Additives
5.2.3. Animal-Derived Nanocellulose
6. Potential Cytotoxicity and Immunogenicity of Nanocellulose
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sano, M.B.; Rojas, A.D.; Gatenholm, P.; Davalos, R.V. Electromagnetically controlled biological assembly of aligned bacterial cellulose nanofibers. Ann. Biomed. Eng. 2010, 38, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ávila, H.; Schwarz, S.; Feldmann, E.-M.; Mantas, A.; von Bomhard, A.; Gatenholm, P.; Rotter, N. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl. Microbiol. Biotech. 2014, 98, 7423–7435. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.S.; Almeida, F.C.G.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Yanamala, N.; Farcas, M.T.; Menas, A.L.; Williams, A.; Fournier, P.M.; Reynolds, J.S.; Gutkin, D.W.; Star, A.; et al. Gender differences in murine pulmonary responses elicited by cellulose nanocrystals. Part. Fibre Toxicol. 2016, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Faradilla, R.H.F.; Lee, G.; Arns, J.-Y.; Roberts, J.; Martens, P.; Stenzel, M.H.; Arcot, J. Characteristics of a free-standing film from banana pseudostem nanocellulose generated from TEMPO-mediated oxidation. Carbohydr. Polym. 2017, 174, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Valentim, R.; Andrade, S.; dos Santos, M.; Santos, A.; Pereira, V.; dos Santos, I.; Dias, C.; dos Reis, M. Composite based on biphasic calcium phosphate (HA/β-TCP) and nanocellulose from the Açaí tegument. Materials 2018, 11, 2213. [Google Scholar] [CrossRef]
- Julie Chandra, C.S.; George, N.; Narayanankutty, S.K. Isolation and characterization of cellulose nanofibrils from arecanut husk fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.G.; Tapia-Blácido, D.R. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced materials through assembly of nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef]
- Novotna, K.; Zajdlova, M.; Suchy, T.; Hadraba, D.; Lopot, F.; Zaloudkova, M.; Douglas, T.E.L.; Munzarova, M.; Juklickova, M.; Stranska, D.; et al. Polylactide nanofibers with hydroxyapatite as growth substrates for osteoblast-like cells. J. Biomed. Mater. Res. 2014, 102, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Commeaux, S.; Troncoso, O. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Bottan, S.; Robotti, F.; Jayathissa, P.; Hegglin, A.; Bahamonde, N.; Heredia-Guerrero, J.A.; Bayer, I.S.; Scarpellini, A.; Merker, H.; Lindenblatt, N.; et al. Surface-structured bacterial cellulose with guided assembly-based biolithography (GAB). ACS Nano 2015, 9, 206–219. [Google Scholar] [CrossRef]
- Khazeni, S.; Hatamian-Zarmi, A.; Yazdian, F.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hosseinzadeh, B.; Noorani, B.; Amoabedini, G.; Soudi, M.R. Production of nanocellulose in miniature-bioreactor: Optimization and characterization. Prep. Biochem. Biotechnol. 2017, 47, 371–378. [Google Scholar] [CrossRef]
- Souza, S.F.; Mariano, M.; Reis, D.; Lombello, C.B.; Ferreira, M.; Sain, M. Cell interactions and cytotoxic studies of cellulose nanofibers from Curauá natural fibers. Carbohydr. Polym. 2018, 201, 87–95. [Google Scholar] [CrossRef]
- Li, W.; Guo, R.; Lan, Y.; Zhang, Y.; Xue, W.; Zhang, Y. Preparation and properties of cellulose nanocrystals reinforced collagen composite films. J. Biomed. Mater. Res. Part A 2014, 102, 1131–1139. [Google Scholar] [CrossRef]
- Yanamala, N.; Farcas, M.T.; Hatfield, M.K.; Kisin, E.R.; Kagan, V.E.; Geraci, C.L.; Shvedova, A.A. In vivo evaluation of the pulmonary toxicity of cellulose nanocrystals: A renewable and sustainable nanomaterial of the future. ACS Sustain. Chem. Eng. 2014, 2, 1691–1698. [Google Scholar] [CrossRef]
- Guglielmo, A.; Sabra, A.; Elbery, M.; Cerveira, M.M.; Ghenov, F.; Sunasee, R.; Ckless, K. A mechanistic insight into curcumin modulation of the IL-1β secretion and NLRP3 S-glutathionylation induced by needle-like cationic cellulose nanocrystals in myeloid cells. Chem. Biol. Interact. 2017, 274, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, G.; Liu, D.; Tian, D.; Zhu, Y.; Chang, Y. Vapor sensing with color-tunable multilayered coatings of cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 39–47. [Google Scholar] [CrossRef]
- Daiyong, Y. Preparation of nanocellulose. Prog. Chem. 2007, 19, 1568–1575. [Google Scholar]
- Klemm, D.; Schumann, D.; Kramer, F.; Heßler, N.; Koth, D.; Sultanova, B. Nanocellulose materials-different cellulose, different functionality. Macromol. Symp. 2009, 280, 60–71. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Edit. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Elder, T.J.; Pu, Y.; Ragauskas, A.J. Facile synthesis of spherical cellulose nanoparticles. Carbohydr. Polym. 2007, 69, 607–611. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, J.; Elder, T.; Deng, Y.; Gatenholm, P.; Ragauskas, A.J. Investigation into nanocellulosics versus acacia reinforced acrylic films. Compos. Part B Eng. 2007, 38, 360–366. [Google Scholar] [CrossRef]
- Chávez-Guerrero, L.; Sepúlveda-Guzmán, S.; Silva-Mendoza, J.; Aguilar-Flores, C.; Pérez-Camacho, O. Eco-friendly isolation of cellulose nanoplatelets through oxidation under mild conditions. Carbohydr. Polym. 2018, 181, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Lindh, J.; Ålander, E.; Strømme, M.; Ferraz, N. On the use of ion-crosslinked nanocellulose hydrogels for wound healing solutions: Physicochemical properties and application-oriented biocompatibility studies. Carbohydr. Polym. 2017, 174, 299–308. [Google Scholar] [CrossRef]
- Basu, A.; Heitz, K.; Strømme, M.; Welch, K.; Ferraz, N. Ion-crosslinked wood-derived nanocellulose hydrogels with tunable antibacterial properties: Candidate materials for advanced wound care applications. Carbohydr. Polym. 2018, 181, 345–350. [Google Scholar] [CrossRef]
- Xin, S.; Li, X.; Ma, Z.; Lei, Z.; Zhao, J.; Pan, S.; Zhou, X.; Deng, H. Cytotoxicity and antibacterial ability of scaffolds immobilized by polysaccharide/layered silicate composites. Carbohydr. Polym. 2013, 92, 1880–1886. [Google Scholar] [CrossRef]
- Mahdavi, M.; Mahmoudi, N.; Rezaie Anaran, F.; Simchi, A. Electrospinning of nanodiamond-modified polysaccharide nanofibers with physico-mechanical properties close to natural skins. Mar. Drugs 2016, 14, 128. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Di, Z.; Shi, Z.; Ullah, M.W.; Li, S.; Yang, G. A transparent wound dressing based on bacterial cellulose whisker and poly (2-hydroxyethyl methacrylate). Int. J. Biol. Macromol. 2017, 105, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Ilves, M.; Järventaus, H.; Hannukainen, K.-S.; Kontturi, E.; Vanhala, E.; Alenius, H.; Savolainen, K.M.; Norppa, H. Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ. Mol. Mutagen. 2015, 56, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Kolehmainen, A.; Visanko, M.; Liimatainen, H.; Niinimäki, J.; Hormi, O.E.O. Strong, self-standing oxygen barrier films from nanocelluloses modified with regioselective oxidative treatments. ACS Appl. Mat. Interfaces 2014, 6, 14384–14390. [Google Scholar] [CrossRef]
- Sharif Hossain, A.B.M.; Uddin, M.M.; Veettil, V.N.; Fawzi, M. Nano-cellulose based nano-coating biomaterial dataset using corn leaf biomass: An innovative biodegradable plant biomaterial. Data Br. 2018, 17, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.; Mohamed-Ahmed, S.; Ojansivu, M.; Berstad, K.; Yassin, M.A.; Kivijärvi, T.; Heggset, E.B.; Syverud, K.; Mustafa, K. Coating 3D printed polycaprolactone scaffolds with nanocellulose promotes growth and differentiation of mesenchymal stem cells. Biomacromolecules 2018, 19, 4307–4319. [Google Scholar] [CrossRef] [PubMed]
- Medhi, P.; Olatunji, O.; Nayak, A.; Uppuluri, C.T.; Olsson, R.T.; Nalluri, B.N.; Das, D.B. Lidocaine-loaded fish scale-nanocellulose biopolymer composite microneedles. AAPS PharmSciTech 2017, 18, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.; Lindh, J.; Hong, J.; Strømme, M.; Mihranyan, A.; Ferraz, N. Blood compatibility of sulfonated cladophora nanocellulose beads. Molecules 2018, 23, 601. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.-Q.; Strømme, M.; Lindh, J. Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye. Carbohydr. Polym. 2018, 181, 200–207. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic nanocellulose aerogels as floating, sustainable, reusable, and recyclable oil absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Hiekkataipale, P.; Malm, J.; Karppinen, M.; Ikkala, O.; Ras, R.H.A. Inorganic hollow nanotube aerogels by atomic layer deposition onto native nanocellulose templates. ACS Nano 2011, 5, 1967–1974. [Google Scholar] [CrossRef]
- Xiao, Y.; Rong, L.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Sui, X. A light-weight and high-efficacy antibacterial nanocellulose-based sponge via covalent immobilization of gentamicin. Carbohydr. Polym. 2018, 200, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.; Fernández-Morales, P.; Gañán, P.; Zuluaga, R.; Kerguelen, H.; Ortiz, I.; Castro, C. Development of novel three-dimensional scaffolds based on bacterial nanocellulose for tissue engineering and regenerative medicine: Effect of processing methods, pore size, and surface area: Development of novel three-dimensional scaffolds. J. Biomed. Mater. Res. Part A 2018, 107, 348–359. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Green bionanocomposite based on kefiran and cellulose nanocrystals produced from beer industrial residues. Int. J. Biol. Macromol. 2015, 77, 85–91. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V. Revalorization of selected municipal solid wastes as new precursors of “green” nanocellulose via a novel one-pot isolation system: A source perspective. Int. J. Biol. Macromol. 2018, 107, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Yuen, J.D.; Walper, S.A.; Melde, B.J.; Daniele, M.A.; Stenger, D.A. Electrolyte-sensing transistor decals enabled by ultrathin microbial nanocellulose. Sci. Rep. 2017, 7, 40867. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ahrem, H.; Pretzel, D.; Endres, M.; Conrad, D.; Courseau, J.; Müller, H.; Jaeger, R.; Kaps, C.; Klemm, D.O.; Kinne, R.W. Laser-structured bacterial nanocellulose hydrogels support ingrowth and differentiation of chondrocytes and show potential as cartilage implants. Acta Biomater. 2014, 10, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, E.M.; Berti, F.V.; Colla, G.; Porto, L.M. Bacterial nanocellulose-IKVAV hydrogel matrix modulates melanoma tumor cell adhesion and proliferation and induces vasculogenic mimicry in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2741–2749. [Google Scholar] [CrossRef]
- Lazarini, S.C.; Yamada, C.; Barud, H.S.; Trovatti, E.; Corbi, P.P.; Lustri, W.R. Influence of chemical and physical conditions in selection of Gluconacetobacter hansenii ATCC 23769 strains with high capacity to produce bacterial cellulose for application as sustained antimicrobial drug-release supports. J. Appl. Microbiol. 2018, 125, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Teixeira, L.N.; de Castro Raucci, L.M.S.; Scarel-Caminaga, R.M.; Franchi, L.P.; dos Santos, R.A.; Santagneli, S.H.; Capela, M.V.; de Oliveira, P.T.; Takahashi, C.S.; et al. Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide for bone regeneration. Int. J. Biol. Macromol. 2017, 103, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Sharma, R.K.; Agarwal, P.; Singh, J.; Sinha, N.; Singh, R.P. From rotten grapes to industrial exploitation: Komagataeibacter europaeus SGP37, a micro-factory for macroscale production of bacterial nanocellulose. Int. J. Biol. Macromol. 2017, 96, 52–60. [Google Scholar] [CrossRef]
- Kaminagakura, K.L.N.; Sue Sato, S.; Sugino, P.; Kataki de Oliveira Veloso, L.; dos Santos, D.C.; Padovani, C.R.; Basmaji, P.; Olyveira, G.; Schellini, S.A. Nanoskin® to treat full thickness skin wounds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, in press. [Google Scholar] [CrossRef]
- Zharikov, A.N.; Lubyansky, V.G.; Gladysheva, E.K.; Skiba, E.A.; Budaeva, V.V.; Semyonova, E.N.; Zharikov, A.A.; Sakovich, G.V. Early morphological changes in tissues when replacing abdominal wall defects by bacterial nanocellulose in experimental trials. J. Mater. Sci. Mater. Med. 2018, 29, 95. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.-C. Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245. [Google Scholar] [CrossRef]
- Kolakovic, R.; Peltonen, L.; Laaksonen, T.; Putkisto, K.; Laukkanen, A.; Hirvonen, J. Spray-dried cellulose nanofibers as novel tablet excipient. AAPS PharmSciTech 2011, 12, 1366–1373. [Google Scholar] [CrossRef]
- Kolakovic, R.; Laaksonen, T.; Peltonen, L.; Laukkanen, A.; Hirvonen, J. Spray-dried nanofibrillar cellulose microparticles for sustained drug release. Int. J. Pharm. 2012, 430, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Controll. Release 2016, 244, 292–301. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Empson, Y.M.; Ekwueme, E.C.; Hong, J.K.; Paynter, D.M.; Kwansa, A.L.; Brown, C.; Pekkanen, A.M.; Roman, M.; Rylander, N.M.; Brolinson, G.P.; et al. High elastic modulus nanoparticles: A novel tool for subfailure connective tissue matrix damage. Transl. Res. 2014, 164, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Hong, J.; Ferraz, N. Hemocompatibility of Ca2+ -crosslinked nanocellulose hydrogels: Toward efficient management of hemostasis. Macromol. Biosci. 2017, 17, 1700236. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Strømme, M.; Ferraz, N. Towards tunable protein-carrier wound dressings based on nanocellulose hydrogels crosslinked with calcium ions. Nanomaterials 2018, 8, 550. [Google Scholar] [CrossRef]
- Powell, L.C.; Khan, S.; Chinga-Carrasco, G.; Wright, C.J.; Hill, K.E.; Thomas, D.W. An investigation of Pseudomonas aeruginosa biofilm growth on novel nanocellulose fibre dressings. Carbohydr. Polym. 2016, 137, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jasmani, L.; Adnan, S. Preparation and characterization of nanocrystalline cellulose from Acacia mangium and its reinforcement potential. Carbohydr. Polym. 2017, 161, 166–171. [Google Scholar] [CrossRef]
- Fu, Q.; Medina, L.; Li, Y.; Carosio, F.; Hajian, A.; Berglund, L.A. Nanostructured wood hybrids for fire-retardancy prepared by clay impregnation into the cell wall. ACS Appl. Mater. Interfaces 2017, 9, 36154–36163. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Patial, V.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Padwad, Y.S.; Yadav, S.K. Cytocompatible anti-microbial dressings of Syzygium cumini cellulose nanocrystals decorated with silver nanoparticles accelerate acute and diabetic wound healing. Sci. Rep. 2017, 7, 10457. [Google Scholar] [CrossRef]
- Elias, N.; Chandren, S.; Razak, F.I.A.; Jamalis, J.; Widodo, N.; Wahab, R.A. Characterization, optimization and stability studies on Candida rugosa lipase supported on nanocellulose reinforced chitosan prepared from oil palm biomass. Int. J. Biol. Macromol. 2018, 114, 306–316. [Google Scholar] [CrossRef]
- Adewuyi, A.; Otuechere, C.A.; Adebayo, O.L.; Anazodo, C.; Pereira, F.V. Renal toxicological evaluations of sulphonated nanocellulose from Khaya sengalensis seed in Wistar rats. Chem. Biol. Interact. 2018, 284, 56–68. [Google Scholar] [CrossRef]
- Matharu, A.S.; de Melo, E.M.; Remón, J.; Wang, S.; Abdulina, A.; Kontturi, E. Processing of citrus nanostructured cellulose: A rigorous design-of-experiment study of the hydrothermal microwave-assisted selective scissoring process. ChemSusChem 2018, 11, 1344–1353. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Ramphul, H.; Bhaw-Luximon, A.; Jhurry, D. Sugar-cane bagasse derived cellulose enhances performance of polylactide and polydioxanone electrospun scaffold for tissue engineering. Carbohydr. Polym. 2017, 178, 238–250. [Google Scholar] [CrossRef]
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus Giganteus: A commercially viable sustainable source of cellulose nanocrystals. Carbohydr. Polym. 2017, 155, 230–241. [Google Scholar] [CrossRef]
- De Carvalho Benini, K.C.C.; Voorwald, H.J.C.; Cioffi, M.O.H.; Rezende, M.C.; Arantes, V. Preparation of nanocellulose from Imperata brasiliensis grass using Taguchi method. Carbohydr. Polym. 2018, 192, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Soni, S.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Patial, V.; Padwad, Y.S.; Yadav, S.K. In situ functionalized nanobiocomposites dressings of bamboo cellulose nanocrystals and silver nanoparticles for accelerated wound healing. Carbohydr. Polym. 2017, 155, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef]
- Tarrés, Q.; Deltell, A.; Espinach, F.X.; Pèlach, M.À.; Delgado-Aguilar, M.; Mutjé, P. Magnetic bionanocomposites from cellulose nanofibers: Fast, simple and effective production method. Int. J. Biol. Macromol. 2017, 99, 29–36. [Google Scholar] [CrossRef]
- Varanasi, S.; Henzel, L.; Sharman, S.; Batchelor, W.; Garnier, G. Producing nanofibres from carrots with a chemical-free process. Carbohydr. Polym. 2018, 184, 307–314. [Google Scholar] [CrossRef]
- Song, L.; Li, Y.; Xiong, Z.; Pan, L.; Luo, Q.; Xu, X.; Lu, S. Water-Induced shape memory effect of nanocellulose papers from sisal cellulose nanofibers with graphene oxide. Carbohydr. Polym. 2018, 179, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bonné, M.J.; Edler, K.J.; Buchanan, J.G.; Wolverson, D.; Psillakis, E.; Helton, M.; Thielemans, W.; Marken, F. Thin-film modified electrodes with reconstituted cellulose−PDDAC films for the accumulation and detection of triclosan. J. Phys. Chem. C 2008, 112, 2660–2666. [Google Scholar] [CrossRef]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A size-exclusion nanocellulose filter paper for virus removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef]
- Hua, K.; Rocha, I.; Zhang, P.; Gustafsson, S.; Ning, Y.; Strømme, M.; Mihranyan, A.; Ferraz, N. Transition from bioinert to bioactive material by tailoring the biological cell response to carboxylated nanocellulose. Biomacromolecules 2016, 17, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Willför, S.; Mihranyan, A. On importance of impurities, potential leachables and extractables in algal nanocellulose for biomedical use. Carbohydr. Polym. 2017, 172, 11–19. [Google Scholar] [CrossRef]
- Gustafsson, S.; Manukyan, L.; Mihranyan, A. Protein–nanocellulose interactions in paper filters for advanced separation applications. Langmuir 2017, 33, 4729–4736. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, O.; Gustafsson, S.; Manukyan, L.; Mihranyan, A. Significance of brownian motion for nanoparticle and virus capture in nanocellulose-based filter paper. Membranes 2018, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Zarei, S.; Niad, M.; Raanaei, H. The removal of mercury ion pollution by using Fe3O4-nanocellulose: Synthesis, characterizations and DFT studies. J. Hazard. Mater. 2018, 344, 258–273. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.E.; Lee, Y.J.; Kwak, M.H.; Sung, G.Y.; Kwon, S.H.; Son, H.J.; Lee, H.S.; Jung, Y.J.; Hwang, D.Y. Cellulose film regenerated from Styela clava tunics have biodegradability, toxicity and biocompatibility in the skin of SD rats. J. Mater. Sci. Mater. Med. 2014, 25, 1519–1530. [Google Scholar] [CrossRef]
- Song, S.H.; Seong, K.Y.; Kim, J.E.; Go, J.; Koh, E.K.; Sung, J.E.; Son, H.J.; Jung, Y.J.; Kim, H.S.; Hong, J.T.; et al. Effects of different cellulose membranes regenerated from Styela clava tunics on wound healing. Int. J. Mol. Med. 2017, 39, 1173–1187. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.E.; Koh, E.K.; Sung, J.E.; Lee, H.A.; Yun, W.B.; Hong, J.T.; Hwang, D.Y. Selenium-loaded cellulose film derived from Styela clava tunic accelerates the healing process of cutaneous wounds in streptozotocin-induced diabetic Sprague–Dawley rats. J. Dermatol. Treat. 2018, 29, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Zhan, H.; Peng, N.; Lei, X.; Huang, Y.; Li, D.; Tao, R.; Chang, C. UV-induced self-cleanable TiO2/nanocellulose membrane for selective separation of oil/water emulsion. Carbohydr. Polym. 2018, 201, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, N.; Mashayekhy Rad, F.; Mace, A.; Ansari, F.; Akhtar, F.; Nilsson, U.; Berglund, L.; Bergström, L. Nanocellulose–zeolite composite films for odor elimination. ACS Appl. Mater. Interfaces 2015, 7, 14254–14262. [Google Scholar] [CrossRef]
- Li, J.; Zuo, K.; Wu, W.; Xu, Z.; Yi, Y.; Jing, Y.; Dai, H.; Fang, G. Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu (II) and Pb (II). Carbohydr. Polym. 2018, 196, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Jodeh, S.; Hamed, O.; Melhem, A.; Salghi, R.; Jodeh, D.; Azzaoui, K.; Benmassaoud, Y.; Murtada, K. Magnetic nanocellulose from olive industry solid waste for the effective removal of methylene blue from wastewater. Environ. Sci. Pollut. Res. 2018, 25, 22060–22074. [Google Scholar] [CrossRef]
- Rathod, M.; Moradeeya, P.G.; Haldar, S.; Basha, S. Nanocellulose/TiO2 composites: Preparation, characterization and application in the photocatalytic degradation of a potential endocrine disruptor, mefenamic acid, in aqueous media. Photochem. Photobiol. Sci. 2018, 17, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Adelantado, C.; Ríos, Á.; Zougagh, M. Magnetic nanocellulose hybrid nanoparticles and ionic liquid for extraction of neonicotinoid insecticides from milk samples prior to determination by liquid chromatography-mass spectrometry. Food Addit. Contam. Part A 2018, 35, 1755–1766. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, M.; Liu, S.; Wang, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Xu, B.; Sui, X. Copper-loaded nanocellulose sponge as a sustainable catalyst for regioselective hydroboration of alkynes. Carbohydr. Polym. 2018, 191, 17–24. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Rejeena, S.R. Adsorption and hydrolytic activity of trypsin on a carboxylate-functionalized cation exchanger prepared from nanocellulose. J. Coll. Interface Sci. 2012, 381, 125–136. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Hong, F.F.; Zhu, M. Evaluation of nanocellulose carriers produced by four different bacterial strains for laccase immobilization. Carbohydr. Polym. 2018, 196, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, M.; Okshevsky, M.; van de Ven, T.G.M.; Tufenkji, N. Developing antibacterial nanocrystalline cellulose using natural antibacterial agents. ACS Appl. Mater. Interfaces 2018, 10, 33827–33838. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Sohal, I.S.; Lorente, L.R.; Molina, R.M.; Pyrgiotakis, G.; Stevanovic, A.; Zhang, R.; McClements, D.J.; Geitner, N.K.; Bousfield, D.W.; et al. Reducing intestinal digestion and absorption of fat using a nature-derived biopolymer: Interference of triglyceride hydrolysis by nanocellulose. ACS Nano 2018, 12, 6469–6479. [Google Scholar] [CrossRef]
- Razaq, A.; Nyström, G.; Strømme, M.; Mihranyan, A.; Nyholm, L. High-capacity conductive nanocellulose paper sheets for electrochemically controlled extraction of DNA oligomers. PLoS ONE 2011, 6, e29243. [Google Scholar] [CrossRef]
- Xu, T.; Jiang, Q.; Ghim, D.; Liu, K.-K.; Sun, H.; Derami, H.G.; Wang, Z.; Tadepalli, S.; Jun, Y.-S.; Zhang, Q.; et al. Catalytically active bacterial nanocellulose-based ultrafiltration membrane. Small 2018, 14, 1704006. [Google Scholar] [CrossRef]
- Ferraz, N.; Carlsson, D.O.; Hong, J.; Larsson, R.; Fellstrom, B.; Nyholm, L.; Stromme, M.; Mihranyan, A. Haemocompatibility and ion exchange capability of nanocellulose polypyrrole membranes intended for blood purification. J. R. Soc. Interface 2012, 9, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Asper, M.; Hanrieder, T.; Quellmalz, A.; Mihranyan, A. Removal of xenotropic murine leukemia virus by nanocellulose based filter paper. Biologicals 2015, 43, 452–456. [Google Scholar] [CrossRef]
- El-Samahy, M.A.; Mohamed, S.A.A.; Abdel Rehim, M.H.; Mohram, M.E. Synthesis of hybrid paper sheets with enhanced air barrier and antimicrobial properties for food packaging. Carbohydr. Polym. 2017, 168, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Kolman, K.; Bridarolli, A.; Odlyha, M.; Bozec, L.; Oriola, M.; Campo-Francés, G.; Persson, M.; Holmberg, K.; Bordes, R. On the potential of using nanocellulose for consolidation of painting canvases. Carbohydr. Polym. 2018, 194, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Song, J.; Zhao, X.; Yang, Z.; Pastel, G.; Xu, S.; Jia, C.; Dai, J.; Chen, C.; Gong, A.; et al. Anisotropic, lightweight, strong, and super thermally insulating nanowood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, eaar3724. [Google Scholar] [CrossRef] [PubMed]
- Nechyporchuk, O.; Bordes, R.; Köhnke, T. Wet spinning of flame-retardant cellulosic fibers supported by interfacial complexation of cellulose nanofibrils with silica nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 39069–39077. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, R.; Ruan, C.; Edström, K.; Strømme, M.; Nyholm, L. Redox-active separators for lithium-ion batteries. Adv. Sci. 2018, 5, 1700663. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Xu, X.; Sun, R.; Wang, Z.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Nanocellulose modified polyethylene separators for lithium metal batteries. Small 2018, 14, 1704371. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Y.; Du, C.; Ren, Y.; Li, X.; Zuo, P.; Yin, G.; Ma, Y.; Cheng, X.; Gao, Y. Free-standing sandwich-type graphene/nanocellulose/silicon laminar anode for flexible rechargeable lithium ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 29638–29646. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Chun, S.-J.; Lee, S.-S.; Kim, B.-Y.; Kim, J.H.; Chung, H.; Lee, S.-Y.; Kim, W. All-solid-state flexible supercapacitors fabricated with bacterial nanocellulose papers, carbon nanotubes, and triblock-copolymer ion gels. ACS Nano 2012, 6, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, D.A.; Pai, A.R.; Pottathara, Y.B.; Pasquini, D.; Carlos de Morais, L.; Luke, M.; Kalarikkal, N.; Grohens, Y.; Thomas, S. Cellulose nanofiber-based polyaniline flexible papers as sustainable microwave absorbers in the X-band. ACS Appl. Mater. Interfaces 2018, 10, 20032–20043. [Google Scholar] [CrossRef]
- Xiong, Z.; Lin, M.; Lin, H.; Huang, M. Facile synthesis of cellulose nanofiber nanocomposite as a SERS substrate for detection of thiram in juice. Carbohydr. Polym. 2018, 189, 79–86. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, R.; Mahmoud, M.A.; Quigley, E.N.; Chang, H.; El-Sayed, M.; Tsukruk, V.V. Dual-excitation nanocellulose plasmonic membranes for molecular and cellular SERS detection. ACS Appl. Mater. Interfaces 2018, 10, 18380–18389. [Google Scholar] [CrossRef]
- Abbasi-Moayed, S.; Golmohammadi, H.; Hormozi-Nezhad, M.R. A nanopaper-based artificial tongue: A ratiometric fluorescent sensor array on bacterial nanocellulose for chemical discrimination applications. Nanoscale 2018, 10, 2492–2502. [Google Scholar] [CrossRef]
- Abbasi-Moayed, S.; Golmohammadi, H.; Bigdeli, A.; Hormozi-Nezhad, M.R. A rainbow ratiometric fluorescent sensor array on bacterial nanocellulose for visual discrimination of biothiols. The Analyst 2018, 143, 3415–3424. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Pircher, N.; Liebner, F.; Condon, B.D.; Qureshi, H.; Yager, D. Designing cellulosic and nanocellulosic sensors for interface with a protease sequestrant wound-dressing prototype: Implications of material selection for dressing and protease sensor design. J. Biomater. Appl. 2017, 32, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Deepa, J.R. Binussreejayan electrochemical sensing of cholesterol by molecularly imprinted polymer of silylated graphene oxide and chemically modified nanocellulose polymer. Mater. Sci. Eng. C 2018, 92, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, J.; Waterhouse, G.I.N.; Cheng, Z.; Ai, S. Electrochemical immunosensor with nanocellulose-Au composite assisted multiple signal amplification for detection of avian leukosis virus subgroup J. Biosens. Bioelectron. 2018, 101, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mangayil, R.; Rajala, S.; Pammo, A.; Sarlin, E.; Luo, J.; Santala, V.; Karp, M.; Tuukkanen, S. Engineering and characterization of bacterial nanocellulose films as low cost and flexible sensor material. ACS Appl. Mater. Interfaces 2017, 9, 19048–19056. [Google Scholar] [CrossRef] [PubMed]
- Rajala, S.; Siponkoski, T.; Sarlin, E.; Mettänen, M.; Vuoriluoto, M.; Pammo, A.; Juuti, J.; Rojas, O.J.; Franssila, S.; Tuukkanen, S. Cellulose nanofibril film as a piezoelectric sensor material. ACS Appl. Mater. Interfaces 2016, 8, 15607–15614. [Google Scholar] [CrossRef]
- Hänninen, A.; Sarlin, E.; Lyyra, I.; Salpavaara, T.; Kellomäki, M.; Tuukkanen, S. Nanocellulose and chitosan based films as low cost, green piezoelectric materials. Carbohydr. Polym. 2018, 202, 418–424. [Google Scholar] [CrossRef]
- Jung, M.; Kim, K.; Kim, B.; Lee, K.-J.; Kang, J.-W.; Jeon, S. Vertically stacked nanocellulose tactile sensor. Nanoscale 2017, 9, 17212–17219. [Google Scholar] [CrossRef]
- Zhou, J.; Hsieh, Y.-L. Conductive polymer protonated nanocellulose aerogels for tunable and linearly responsive strain sensors. ACS Appl. Mater. Interfaces 2018, 10, 27902–27910. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Ferreira Cury, B.S.; dos Santos, A.M.; Franco, D.F.; Barud, H.S.; da Silva Filho, E.C. Resistant starch/pectin free-standing films reinforced with nanocellulose intended for colonic methotrexate release. Carbohydr. Polym. 2017, 157, 1013–1023. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Rodrigues, A.F.; Almeida, I.F.; Costa, P.C.; Rosado, C.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial cellulose membranes as transdermal delivery systems for diclofenac: In vitro dissolution and permeation studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef]
- Bodhibukkana, C.; Srichana, T.; Kaewnopparat, S.; Tangthong, N.; Bouking, P.; Martin, G.P.; Suedee, R. Composite membrane of bacterially-derived cellulose and molecularly imprinted polymer for use as a transdermal enantioselective controlled-release system of racemic propranolol. J. Controll. Release 2006, 113, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Brassolatti, P.; Kido, H.W.; Bossini, P.S.; Gabbai-Armelin, P.R.; Otterço, A.N.; Almeida-Lopes, L.; Zanardi, L.M.; Napolitano, M.A.; de Avó, L.R.d.S.; Forato, L.A.; et al. Bacterial cellulose membrane used as biological dressings on third-degree burns in rats. Biomed. Mater. Eng. 2018, 29, 29–42. [Google Scholar]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, R.; Heuberger, L.; Siqueira, G.; Gutt, B.; Zimmermann, T.; Maniura-Weber, K.; Salentinig, S.; Faccio, G. Enhanced antimicrobial activity and structural transitions of a nanofibrillated cellulose–nisin biocomposite suspension. ACS Appl. Mater. Interfaces 2018, 10, 20170–20181. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Tsivintzelis, I.; Panayiotou, C.; Papageorgiou, V.P. Electrospun fiber mats containing shikonin and derivatives with potential biomedical applications. Int. J. Pharm. 2011, 409, 216–228. [Google Scholar] [CrossRef]
- Asabuwa Ngwabebhoh, F.; Ilkar Erdagi, S.; Yildiz, U. Pickering emulsions stabilized nanocellulosic-based nanoparticles for coumarin and curcumin nanoencapsulations: In vitro release, anticancer and antimicrobial activities. Carbohydr. Polym. 2018, 201, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Mohd Amin, M.C.I. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef]
- Rodrigues, C.; de Assis, A.M.; Moura, D.J.; Halmenschlager, G.; Saffi, J.; Xavier, L.L.; da Cruz Fernandes, M.; Wink, M.R. New therapy of skin repair combining adipose-derived mesenchymal stem cells with sodium carboxymethylcellulose scaffold in a pre-clinical rat model. PLoS ONE 2014, 9, e96241. [Google Scholar] [CrossRef]
- Kramer, F.; Klemm, D.; Schumann, D.; Heßler, N.; Wesarg, F.; Fried, W.; Stadermann, D. Nanocellulose polymer composites as innovative pool for (bio)material development. Macromol. Symp. 2006, 244, 136–148. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Kramer, F.; Heßler, N.; Hornung, M.; Schmauder, H.-P.; Marsch, S. Nanocelluloses as innovative polymers in research and application. In Polysaccharides II; Klemm, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 205, pp. 49–96. ISBN 978-3-540-37102-1. [Google Scholar]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Nordli, H.R.; Pukstad, B.; Kristofer Gamstedt, E.; Chinga-Carrasco, G. Mechanical characteristics of nanocellulose-PEG bionanocomposite wound dressings in wet conditions. J. Mech. Behav. Biomed. Mater. 2017, 69, 377–384. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-based antibacterial materials. Adv. Healthc. Mater. 2018, 7, 1800334. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Ahola, S.; Salmi, J.; Johansson, L.-S.; Laine, J.; Österberg, M. Model films from native cellulose nanofibrils. preparation, swelling, and surface interactions. Biomacromolecules 2008, 9, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.; Brackmann, C.; Puchades, M.; Brattås, K.; Ewing, A.; Gatenholm, P.; Enejder, A. Neuronal networks on nanocellulose scaffolds. Tissue Eng. Part C Methods 2015, 21, 1162–1170. [Google Scholar] [CrossRef]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Skogberg, A.; Mäki, A.-J.; Mettänen, M.; Lahtinen, P.; Kallio, P. Cellulose nanofiber alignment using evaporation-induced droplet-casting, and cell alignment on aligned nanocellulose surfaces. Biomacromolecules 2017, 18, 3936–3953. [Google Scholar] [CrossRef]
- Hua, K.; Ålander, E.; Lindström, T.; Mihranyan, A.; Strømme, M.; Ferraz, N. Surface chemistry of nanocellulose fibers directs monocyte/macrophage response. Biomacromolecules 2015, 16, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Novotna, K.; Sopuch, T.; Havelka, P. Cell interaction with cellulose-based scaffolds for tissue engineering -A Review. In Cellulose and Cellulose Derivatives: Synthesis, Modification, Nanostructure and Applications; Mondal, I.H., Ed.; Nova Science Publishers, Inc. Hauppauge: New York, NY, USA, 2015; pp. 341–375. ISBN 978-1-63483-571-8. [Google Scholar]
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of nanocellulose with a xyloglucan–RGD conjugate enhances adhesion and proliferation of endothelial cells: Implications for tissue engineering. Biomacromolecules 2007, 8, 3697–3704. [Google Scholar] [CrossRef]
- Juntaro, J.; Pommet, M.; Mantalaris, A.; Shaffer, M.; Bismarck, A. Nanocellulose enhanced interfaces in truly green unidirectional fibre reinforced composites. Compos. Interfaces 2007, 14, 753–762. [Google Scholar] [CrossRef]
- Auad, M.L.; Contos, V.S.; Nutt, S.; Aranguren, M.I.; Marcovich, N.E. Characterization of nanocellulose-reinforced shape memory polyurethanes. Polym. Int. 2008, 57, 651–659. [Google Scholar] [CrossRef]
- Lönnberg, H.; Fogelström, L.; Berglund, L.; Malmström, E.; Hult, A. Surface grafting of microfibrillated cellulose with poly(ε-caprolactone)—Synthesis and characterization. Eur. Polym. J. 2008, 44, 2991–2997. [Google Scholar] [CrossRef]
- Gardner, D.J.; Oporto, G.S.; Mills, R.; Samir, M.A.S.A. Adhesion and surface issues in cellulose and nanocellulose. J. Adhes. Sci. Technol. 2008, 22, 545–567. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.P.; Sain, M. Novel bionanocomposites: Processing, properties and potential applications. Plast. Rubber Compos. 2009, 38, 396–405. [Google Scholar] [CrossRef]
- Heßler, N.; Klemm, D. Alteration of bacterial nanocellulose structure by in situ modification using polyethylene glycol and carbohydrate additives. Cellulose 2009, 16, 899–910. [Google Scholar] [CrossRef]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Österberg, M.; Wågberg, L. Nanoscale cellulose films with different crystallinities and mesostructures—Their surface properties and interaction with water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef]
- Saxena, A.; Elder, T.J.; Pan, S.; Ragauskas, A.J. Novel nanocellulosic xylan composite film. Compos. Part B Eng. 2009, 40, 727–730. [Google Scholar] [CrossRef]
- Gatenholm, P.; Klemm, D. Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Uribe-Calderon, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and properties of nanocellulose-reinforced methylcellulose-based biodegradable films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Mattoso, L.H.C.; Avena-Bustillos, R.J.; Filho, G.C.; Munford, M.L.; Wood, D.; McHugh, T.H. Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci. 2010, 75, N1–N7. [Google Scholar] [CrossRef] [PubMed]
- Kralisch, D.; Hessler, N.; Klemm, D.; Erdmann, R.; Schmidt, W. White biotechnology for cellulose manufacturing–The HoLiR concept. Biotechnol. Bioeng. 2010, 105, 740–747. [Google Scholar] [PubMed]
- Nyström, G.; Mihranyan, A.; Razaq, A.; Lindström, T.; Nyholm, L.; Strømme, M. A Nanocellulose polypyrrole composite based on microfibrillated cellulose from wood. J. Phys. Chem. B 2010, 114, 4178–4182. [Google Scholar] [CrossRef]
- Iseli, A.M.; Kwen, H.-D.; Ul-Alam, M.; Balasubramanian, M.; Rajagopalan, S. Enhanced contaminated human remains pouch: Initial development and preliminary performance assessments. Am. J. Disaster Med. 2011, 6, 31–38. [Google Scholar] [PubMed]
- Wesarg, F.; Schlott, F.; Grabow, J.; Kurland, H.-D.; Heßler, N.; Kralisch, D.; Müller, F.A. In situ synthesis of photocatalytically active hybrids consisting of bacterial nanocellulose and anatase nanoparticles. Langmuir 2012, 28, 13518–13525. [Google Scholar] [CrossRef] [PubMed]
- Schütz, C.; Sort, J.; Bacsik, Z.; Oliynyk, V.; Pellicer, E.; Fall, A.; Wågberg, L.; Berglund, L.; Bergström, L.; Salazar-Alvarez, G. Hard and transparent films formed by nanocellulose–TiO2 nanoparticle hybrids. PLoS ONE 2012, 7, e45828. [Google Scholar] [CrossRef]
- Loranger, E.; Piché, A.-O.; Daneault, C. Influence of high shear dispersion on the production of cellulose nanofibers by ultrasound-assisted TEMPO-oxidation of kraft pulp. Nanomaterials 2012, 2, 286–297. [Google Scholar] [CrossRef]
- Orelma, H.; Teerinen, T.; Johansson, L.-S.; Holappa, S.; Laine, J. CMC-modified cellulose biointerface for antibody conjugation. Biomacromolecules 2012, 13, 1051–1058. [Google Scholar] [CrossRef]
- Díez, I.; Eronen, P.; Österberg, M.; Linder, M.B.; Ikkala, O.; Ras, R.H.A. Functionalization of nanofibrillated cellulose with silver nanoclusters: Fluorescence and antibacterial activity. Macromol. Biosci. 2011, 11, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Bäckdahl, H.; Risberg, B.; Gatenholm, P. Observations on bacterial cellulose tube formation for application as vascular graft. Mater. Sci. Eng. C 2011, 31, 14–21. [Google Scholar] [CrossRef]
- Zimmermann, K.A.; LeBlanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 2011, 31, 43–49. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.-R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; GuGuen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Controll. Release 2012, 164, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Silva, J.P.; Rambo, C.R.; Barra, G.M.O.; Dourado, F.; Gama, F.M. Neuronal cells’ behavior on polypyrrole coated bacterial nanocellulose three-dimensional (3D) scaffolds. J. Biomater. Sci. Polym. Edit. 2013, 24, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 3, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Nimeskern, L.; Martínez Ávila, H.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, E.-M.; Sundberg, J.; Bobbili, B.; Schwarz, S.; Gatenholm, P.; Rotter, N. Description of a novel approach to engineer cartilage with porous bacterial nanocellulose for reconstruction of a human auricle. J. Biomater. Appl. 2013, 28, 626–640. [Google Scholar] [CrossRef]
- Pretzel, D.; Linss, S.; Ahrem, H.; Endres, M.; Kaps, C.; Klemm, D.; Kinne, R.W. A novel in vitro bovine cartilage punch model for assessing the regeneration of focal cartilage defects with biocompatible bacterial nanocellulose. Arthritis Res. Ther. 2013, 15, R59. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, P.; Zhang, S.; Yang, G. Evaluation of bacterial nanocellulose-based uniform wound dressing for large area skin transplantation. Mater. Sci. Eng. C 2013, 33, 2995–3000. [Google Scholar] [CrossRef]
- Kuzmenko, V.; Sämfors, S.; Hägg, D.; Gatenholm, P. Universal method for protein bioconjugation with nanocellulose scaffolds for increased cell adhesion. Mater. Sci. Eng. C 2013, 33, 4599–4607. [Google Scholar] [CrossRef]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Gumrah Dumanli, A. Nanocellulose and its composites for biomedical applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef]
- Halib, N.; Perrone, F.; Cemazar, M.; Dapas, B.; Farra, R.; Abrami, M.; Chiarappa, G.; Forte, G.; Zanconati, F.; Pozzato, G.; et al. Potential applications of nanocellulose-containing materials in the biomedical field. Materials 2017, 10, 977. [Google Scholar] [CrossRef]
- Pötzinger, Y.; Kralisch, D.; Fischer, D. Bacterial nanocellulose: The future of controlled drug delivery? Ther. Deliv. 2017, 8, 753–761. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Sandler, N.; Willför, S.; Xu, C. Three-dimensional printing of wood-derived biopolymers: A review focused on biomedical applications. ACS Sustain. Chem. Eng. 2018, 6, 5663–5680. [Google Scholar] [CrossRef] [PubMed]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D culturing and differentiation of SH-SY5Y neuroblastoma cells on bacterial nanocellulose scaffolds. Artif. Cells Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, V.; Karabulut, E.; Pernevik, E.; Enoksson, P.; Gatenholm, P. Tailor-made conductive inks from cellulose nanofibrils for 3D printing of neural guidelines. Carbohydr. Polym. 2018, 189, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ávila, H.; Feldmann, E.-M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Müller, R.; Hendriks, J.; Rotter, N.; et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 2015, 44, 122–133. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Deepa, B.; Mathew, A.P.; Oksman, K.; Girandon, L. Nanocellulose-based interpenetrating polymer network (IPN) hydrogels for cartilage applications. Biomacromolecules 2016, 17, 3714–3723. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A.; Biggs, M.J. Nanocellulose reinforced gellan-gum hydrogels as potential biological substitutes for annulus fibrosus tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 897–908. [Google Scholar] [CrossRef]
- Malinen, M.M.; Kanninen, L.K.; Corlu, A.; Isoniemi, H.M.; Lou, Y.-R.; Yliperttula, M.L.; Urtti, A.O. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 2014, 35, 5110–5121. [Google Scholar] [CrossRef] [PubMed]
- Krontiras, P.; Gatenholm, P.; Hägg, D.A. Adipogenic differentiation of stem cells in three-dimensional porous bacterial nanocellulose scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, I.; Gatenholm, P.; Hägg, D.A. Increased lipid accumulation and adipogenic gene expression of adipocytes in 3D bioprinted nanocellulose scaffolds. Biofabrication 2017, 9, 015022. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-R.; Kanninen, L.; Kuisma, T.; Niklander, J.; Noon, L.A.; Burks, D.; Urtti, A.; Yliperttula, M. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 2014, 23, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Wei, B.; Chen, L. Preliminary study on biosynthesis of bacterial nanocellulose tubes in a novel double-silicone-tube bioreactor for potential vascular prosthesis. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Weber, C.; Reinhardt, S.; Eghbalzadeh, K.; Wacker, M.; Guschlbauer, M.; Maul, A.; Sterner-Kock, A.; Wahlers, T.; Wippermann, J.; Scherner, M. Patency and in vivo compatibility of bacterial nanocellulose grafts as small-diameter vascular substitute. J. Vasc. Surg. 2018, 68, 177S–187S.e1. [Google Scholar] [CrossRef]
- Echeverry-Rendon, M.; Reece, L.M.; Pastrana, F.; Arias, S.L.; Shetty, A.R.; Pavón, J.J.; Allain, J.P. Bacterial nanocellulose magnetically functionalized for neuro-endovascular treatment. Macromol. Biosci. 2017, 17, 1600382. [Google Scholar] [CrossRef] [PubMed]
- Pavón, J.J.; Allain, J.P.; Verma, D.; Echeverry-Rendón, M.; Cooper, C.L.; Reece, L.M.; Shetty, A.R.; Tomar, V. In situ study unravels bio-nanomechanical behavior in a magnetic bacterial nano-cellulose (MBNC) hydrogel for neuro-endovascular reconstruction. Macromol. Biosci. 2018, 1800225. [Google Scholar] [CrossRef] [PubMed]
- Vielreicher, M.; Kralisch, D.; Völkl, S.; Sternal, F.; Arkudas, A.; Friedrich, O. Bacterial nanocellulose stimulates mesenchymal stem cell expansion and formation of stable collagen-I networks as a novel biomaterial in tissue engineering. Sci Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rendon, J.G.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M.; et al. Bioactive gyroid scaffolds formed by sacrificial templating of nanocellulose and nanochitin hydrogels as instructive platforms for biomimetic tissue engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef]
- Sundberg, J.; Götherström, C.; Gatenholm, P. Biosynthesis and in vitro evaluation of macroporous mineralized bacterial nanocellulose scaffolds for bone tissue engineering. Biomed. Mater. Eng. 2015, 25, 39–52. [Google Scholar]
- Si, J.; Cui, Z.; Wang, Q.; Liu, Q.; Liu, C. Biomimetic composite scaffolds based on mineralization of hydroxyapatite on electrospun poly (ɛ-caprolactone)/nanocellulose fibers. Carbohydr. Polym. 2016, 143, 270–278. [Google Scholar] [CrossRef]
- Chen, Q.; Garcia, R.P.; Munoz, J.; Pérez de Larraya, U.; Garmendia, N.; Yao, Q.; Boccaccini, A.R. Cellulose nanocrystals—Bioactive glass hybrid coating as bone substitutes by electrophoretic co-deposition: In situ control of mineralization of bioactive glass and enhancement of osteoblastic performance. ACS Appl. Mater. Interfaces 2015, 7, 24715–24725. [Google Scholar] [CrossRef]
- Huang, J.-W.; Lv, X.-G.; Li, Z.; Song, L.-J.; Feng, C.; Xie, M.-K.; Li, C.; Li, H.-B.; Wang, J.-H.; Zhu, W.-D.; et al. Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 2015, 10, 055005. [Google Scholar] [CrossRef]
- Lv, X.; Feng, C.; Liu, Y.; Peng, X.; Chen, S.; Xiao, D.; Wang, H.; Li, Z.; Xu, Y.; Lu, M. A smart bilayered scaffold supporting keratinocytes and muscle cells in micro/nano-scale for urethral reconstruction. Theranostics 2018, 8, 3153–3163. [Google Scholar] [CrossRef]
- Goldschmidt, E.; Cacicedo, M.; Kornfeld, S.; Valinoti, M.; Ielpi, M.; Ajler, P.M.; Yampolsky, C.; Rasmussen, J.; Castro, G.R.; Argibay, P. Construction and in vitro testing of a cellulose dura mater graft. Neurol. Res. 2016, 38, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Merkel, E.; Fuchs, F.; Schumann, D.; Klemm, D.; Kramer, F.; Mayer-Wagner, S.; Schroeder, C.; Freudenthal, F.; Netz, H.; et al. Bacterial nanocellulose as a new patch material for closure of ventricular septal defects in a pig model. Eur. J. Cardiothorac. Surg. 2015, 47, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Tummala, G.K.; Joffre, T.; Rojas, R.; Persson, C.; Mihranyan, A. Strain-induced stiffening of nanocellulose-reinforced poly (vinyl alcohol) hydrogels mimicking collagenous soft tissues. Soft Matter 2017, 13, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, R.V.; Valente, F.L.; Reis, E.C.C.; Araújo, F.R.; Eleotério, R.B.; Queiroz, P.V.S.; Borges, A.P.B.; Universidade Federal De Viçosa. Brazil bacterial cellulose and bacterial cellulose/polycaprolactone composite as tissue substitutes in rabbits’ cornea. Pesquisa Veterinária Brasileira 2016, 36, 986–992. [Google Scholar] [CrossRef]
- Ludwicka, K.; Kolodziejczyk, M.; Gendaszewska-Darmach, E.; Chrzanowski, M.; Jedrzejczak-Krzepkowska, M.; Rytczak, P.; Bielecki, S. Stable composite of bacterial nanocellulose and perforated polypropylene mesh for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Lee, D.; Hyun, J. Nanocellulose-alginate hydrogel for cell encapsulation. Carbohydr. Polym. 2015, 116, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Shin, S.; Cheng, J.; Hyun, J. Nanocellulose based asymmetric composite membrane for the multiple functions in cell encapsulation. Carbohydr. Polym. 2017, 158, 133–140. [Google Scholar] [CrossRef]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264. [Google Scholar] [CrossRef]
- Sanchavanakit, N.; Sangrungraungroj, W.; Kaomongkolgit, R.; Banaprasert, T.; Pavasant, P.; Phisalaphong, M. Growth of human keratinocytes and fibroblasts on bacterial cellulose film. Biotechnol. Prog. 2006, 22, 1194–1199. [Google Scholar] [CrossRef]
- Kingkaew, J.; Jatupaiboon, N.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Biocompatibility and growth of human keratinocytes and fibroblasts on biosynthesized cellulose–chitosan film. J. Biomater. Sci. Polym. Edit. 2010, 21, 1009–1021. [Google Scholar] [CrossRef]
- Keskin, Z.; Sendemir Urkmez, A.; Hames, E.E. Novel keratin modified bacterial cellulose nanocomposite production and characterization for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 1144–1153. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: A potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Chen, X.; Han, H.; Yang, G. Double network bacterial cellulose hydrogel to build a biology–Device interface. Nanoscale 2014, 6, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.R.; Yang, X.; Zhang, J.; Cao, X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater. Sci. Eng. C 2018, 82, 372–383. [Google Scholar] [CrossRef]
- Trovatti, E.; Tang, H.; Hajian, A.; Meng, Q.; Gandini, A.; Berglund, L.A.; Zhou, Q. Enhancing strength and toughness of cellulose nanofibril network structures with an adhesive peptide. Carbohydr. Polym. 2018, 181, 256–263. [Google Scholar] [CrossRef]
- Shefa, A.A.; Amirian, J.; Kang, H.J.; Bae, S.H.; Jung, H.-I.; Choi, H.; Lee, S.Y.; Lee, B.-T. In vitro and in vivo evaluation of effectiveness of a novel TEMPO-oxidized cellulose nanofiber-silk fibroin scaffold in wound healing. Carbohydr. Polym. 2017, 177, 284–296. [Google Scholar] [CrossRef]
- Skogberg, A. (Tampere University of Technology, Tampere, Finland). Personal communication, 2018, unpublished data.
- Kim, M.; Kim, G. 3D multi-layered fibrous cellulose structure using an electrohydrodynamic process for tissue engineering. J. Colloid Interface Sci. 2015, 457, 180–187. [Google Scholar] [CrossRef]
- Vatankhah, E.; Prabhakaran, M.P.; Jin, G.; Mobarakeh, L.G.; Ramakrishna, S. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J. Biomater. Appl. 2014, 28, 909–921. [Google Scholar] [CrossRef]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Nouri Khorasani, S.; Razavi, M.; Hashemi Beni, B.; Heydari, F.; Tamayol, A. Nanofibrous scaffolds with biomimetic structure. J. Biomed. Mater. Res. Part A 2018, 106, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Chantre, C.O.; Gannon, A.R.; Lind, J.U.; Campbell, P.H.; Grevesse, T.; O’Connor, B.B.; Parker, K.K. Soy protein/cellulose nanofiber scaffolds mimicking skin extracellular matrix for enhanced wound healing. Adv. Healthc. Mater. 2018, 7, 1701175. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, F.H.; Jahir Hussain, F.S.; Abdull Rasad, M.S.B.; Mohd Yusoff, M. Improved cellular response of chemically crosslinked collagen incorporated hydroxyethyl cellulose/poly(vinyl) alcohol nanofibers scaffold. J. Biomater. Appl. 2015, 29, 1014–1027. [Google Scholar] [CrossRef]
- Zulkifli, F.H.; Hussain, F.S.J.; Zeyohannes, S.S.; Rasad, M.S.B.A.; Yusuff, M.M. A facile synthesis method of hydroxyethyl cellulose-silver nanoparticle scaffolds for skin tissue engineering applications. Mater. Sci. Eng. C 2017, 79, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Guo, R.; Liu, J.; Lan, Y.; Zhang, Y.; Xue, W.; Zhang, Y. Preparation and properties of PLGA nanofiber membranes reinforced with cellulose nanocrystals. Colloids Surf. B Biointerfaces 2015, 132, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Catchmark, J.M. In vitro biodegradability and mechanical properties of bioabsorbable bacterial cellulose incorporating cellulases. Acta Biomater. 2011, 7, 2835–2845. [Google Scholar] [CrossRef]
- Hu, Y.; Catchmark, J.M. Integration of cellulases into bacterial cellulose: Toward bioabsorbable cellulose composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97B, 114–123. [Google Scholar] [CrossRef]
- Yadav, V.; Paniliatis, B.J.; Shi, H.; Lee, K.; Cebe, P.; Kaplan, D.L. Novel in vivo-degradable cellulose-chitin copolymer from metabolically engineered Gluconacetobacter xylinus. App. Environ. Microbiol. 2010, 76, 6257–6265. [Google Scholar] [CrossRef]
- Yadav, V.; Sun, L.; Panilaitis, B.; Kaplan, D.L. In vitro chondrogenesis with lysozyme susceptible bacterial cellulose as a scaffold. J. Tissue Eng. Regen. Med. 2015, 9, E276–E288. [Google Scholar] [CrossRef]
- Elçin, A.E. In vitro and in vivo degradation of oxidized acetyl- and ethyl-cellulose sponges. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 407–418. [Google Scholar] [CrossRef] [PubMed]
- RoyChowdhury, P.; Kumar, V. Fabrication and evaluation of porous 2,3-dialdehydecellulose membrane as a potential biodegradable tissue-engineering scaffold. J. Biomed. Mater. Res. Part A 2006, 76A, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.M.C.O.; Mesquita, L.A.F.; Souza, D.; Irioda, A.C.; Francisco, J.C.; Souza, C.F.; Guarita-Souza, L.C.; Sierakowski, M.-R.; Carvalho, K.A.T. Regeneration of skin tissue promoted by mesenchymal stem cells seeded in nanostructured membrane. Transpl. Proc. 2014, 46, 1882–1886. [Google Scholar] [CrossRef]
- Mertaniemi, H.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Gandía, C.; Mäkitie, A.; Partanen, J.; Ikkala, O.; Yliperttula, M. Human stem cell decorated nanocellulose threads for biomedical applications. Biomaterials 2016, 82, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, M.; Pajorova, J.; Sopuch, T.; Bacakova, L. Fibrin-modified cellulose as a promising dressing for accelerated wound healing. Materials 2018, 11, 2314. [Google Scholar] [CrossRef]
- Kwak, M.H.; Kim, J.E.; Go, J.; Koh, E.K.; Song, S.H.; Son, H.J.; Kim, H.S.; Yun, Y.H.; Jung, Y.J.; Hwang, D.Y. Bacterial cellulose membrane produced by Acetobacter sp. A10 for burn wound dressing applications. Carbohydr. Polym. 2015, 122, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Huang, R.; Huang, Z.; Hu, B.; Zheng, W.; Yang, G.; Jiang, X. Evaluation of the effect of the structure of bacterial cellulose on full thickness skin wound repair on a microfluidic chip. Biomacromolecules 2015, 16, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Zieger, M.; Hipler, U.C.; Lademann, J.; Albrecht, V.; Bueckle, R.; Meß, C.; Kaatz, M.; Huck, V. Multiphotonic staging of chronic wounds and evaluation of sterile, optical transparent bacterial nanocellulose covering: A diagnostic window into human skin. Skin Res. Technol. 2018, 25, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Zhang, Q.; Hong, F.F. Using in situ dynamic cultures to rapidly biofabricate fabric-reinforced composites of chitosan/bacterial nanocellulose for antibacterial wound dressings. Front. Microbiol. 2016, 7, 260. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N. The characteristics of bacterial nanocellulose gel releasing silk sericin for facial treatment. BMC Biotechnol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Lamboni, L.; Li, Y.; Liu, J.; Yang, G. Silk sericin-functionalized bacterial cellulose as a potential wound-healing biomaterial. Biomacromolecules 2016, 17, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hu, L.; Gong, N.; Tang, Q.; Du, L.; Chen, L. The effects of macrophage-stimulating protein on the migration, proliferation, and collagen synthesis of skin fibroblasts in vitro and in vivo. Tissue Eng. Part A 2015, 21, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Sierakowski, M.R.; Woehl, M.A.; Ono, L.; Cofré, A.R.; Vanin, L.P.; Pontarolo, R.; De Freitas, R.A. Lysozyme-triggered epidermal growth factor release from bacterial cellulose membranes controlled by smart nanostructured films. J. Pharm. Sci. 2014, 103, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.D.; Freire, C.S.R.; Sousa Lobo, J.M.; Costa, P.C. Bacterial cellulose membranes as drug delivery systems: An in vivo skin compatibility study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Luan, J.; Wu, J.; Zheng, Y.; Song, W.; Wang, G.; Guo, J.; Ding, X. Impregnation of silver sulfadiazine into bacterial cellulose for antimicrobial and biocompatible wound dressing. Biomed. Mater. 2012, 7, 065006. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver nanoparticle/bacterial cellulose gel membranes for antibacterial wound dressing: investigation in vitro and in vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Zuhainis Saad, W.; Navaderi, M.; Arulselvan, P.; Mohamad, R. Molecular study of wound healing after using biosynthesized BNC/Fe3O4 nanocomposites assisted with a bioinformatics approach. Int. J. Nanomed. 2018, 13, 2955–2971. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Zheng, W.; Feng, Y.; Huang, R.; Shao, J.; Tang, R.; Wang, P.; Jia, Y.; Zhang, J.; et al. Composites of bacterial cellulose and small molecule-decorated gold nanoparticles for treating gram-negative bacteria-infected wounds. Small 2017, 13, 1700130. [Google Scholar] [CrossRef]
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.-C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Żywicka, A.; Fijałkowski, K.; Junka, A.F.; Grzesiak, J.; El Fray, M. Modification of bacterial cellulose with quaternary ammonium compounds based on fatty acids and amino acids and the effect on antimicrobial activity. Biomacromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ataide, J.A.; de Carvalho, N.M.; de Rebelo, M.A.; Chaud, M.V.; Grotto, D.; Gerenutti, M.; Rai, M.; Mazzola, P.G.; Jozala, A.F. Bacterial nanocellulose loaded with bromelain: Assessment of antimicrobial, antioxidant and physical-chemical properties. Sci. Rep. 2017, 7, 18031. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.; Spasojević, D.; Orlovska, I.; Kozyrovska, N.; Soković, M.; Glamočlija, J.; Dmitrović, S.; Matović, B.; Tasić, N.; Maksimović, V.; et al. Bacterial cellulose-lignin composite hydrogel as a promising agent in chronic wound healing. Int. J. Biol. Macromol. 2018, 118, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Khamrai, M.; Banerjee, S.L.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2018, 122, 940–953. [Google Scholar] [CrossRef]
- Kumavat, S.D.; Chaudhari, Y.S.; Borole, P.; Mishra, P.; Shenghani, K.; Duvvuri, P. Degradation studies of curcumin. Int. J. Pharm. Rev. Res. 2013, 3, 50–55. [Google Scholar]
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893. [Google Scholar] [CrossRef]
- Kolarova, K. (University of Chemistry and Technology Prague, Prague, Czech Republic). Personal communication, 2018, unpublished data.
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and anticancer properties of bacterial cellulose films containing ethanolic extract of mangosteen peel. J. Biomater. Sci. Polym. Edit. 2014, 25, 907–922. [Google Scholar] [CrossRef]
- Vosmanska, V.; Kolarova, K.; Rimpelova, S.; Svorcik, V. Surface modification of oxidized cellulose haemostat by argon plasma treatment. Cellulose 2014, 21, 2445–2456. [Google Scholar] [CrossRef]
- Jack, A.A.; Nordli, H.R.; Powell, L.C.; Powell, K.A.; Kishnani, H.; Johnsen, P.O.; Pukstad, B.; Thomas, D.W.; Chinga-Carrasco, G.; Hill, K.E. The interaction of wood nanocellulose dressings and the wound pathogen P. aeruginosa. Carbohydr. Polym. 2017, 157, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Poonguzhali, R.; Khaleel Basha, S.; Sugantha Kumari, V. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2018, 112, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, F.; Liu, J.; Smått, J.-H.; Gepperth, D.; Lastusaari, M.; Xu, C.; Hupa, L. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016, 46, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Werrett, M.V.; Herdman, M.E.; Brammananth, R.; Garusinghe, U.; Batchelor, W.; Crellin, P.K.; Coppel, R.L.; Andrews, P.C. Bismuth phosphinates in Bi-nanocellulose composites and their efficacy towards multi-drug resistant bacteria. Chem. A Eur. J. 2018, 24, 12938–12949. [Google Scholar] [CrossRef]
- Evdokimova, O.; Svensson, F.; Agafonov, A.; Håkansson, S.; Seisenbaeva, G.; Kessler, V. Hybrid drug delivery patches based on spherical cellulose nanocrystals and colloid titania—Synthesis and antibacterial properties. Nanomaterials 2018, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a nonionic surfactant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef]
- Suwannateep, N.; Wanichwecharungruang, S.; Fluhr, J.; Patzelt, A.; Lademann, J.; Meinke, M.C. Comparison of two encapsulated curcumin particular systems contained in different formulations with regard to in vitro skin penetration. Skin Res. Technol. 2013, 19, 1–9. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Mo, Y.; Guo, R.; Zhang, Y.; Xue, W.; Cheng, B.; Zhang, Y. Controlled dual delivery of angiogenin and curcumin by electrospun nanofibers for skin regeneration. Tissue Eng. Part A 2017, 23, 597–608. [Google Scholar] [CrossRef]
- Guo, R.; Lan, Y.; Xue, W.; Cheng, B.; Zhang, Y.; Wang, C.; Ramakrishna, S. Collagen-cellulose nanocrystal scaffolds containing curcumin-loaded microspheres on infected full-thickness burns repair. J. Tissue Eng. Regen. Med. 2017, 11, 3544–3555. [Google Scholar] [CrossRef] [PubMed]
- Vosmanská, V.; Kolářová, K.; Rimpelová, S.; Kolská, Z.; Švorčík, V. Antibacterial wound dressing: Plasma treatment effect on chitosan impregnation and in situ synthesis of silver chloride on cellulose surface. RSC Adv. 2015, 5, 17690–17699. [Google Scholar] [CrossRef]
- Dong, S.; Cho, H.J.; Lee, Y.W.; Roman, M. Synthesis and cellular uptake of folic acid-conjugated cellulose nanocrystals for cancer targeting. Biomacromolecules 2014, 15, 1560–1567. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Y.; Huang, W.; Mo, Y.; Lan, Y.; Guo, R.; Cheng, B. Neurotensin-loaded PLGA/CNC composite nanofiber membranes accelerate diabetic wound healing. Artif. Cells Nanomed. Biotechnol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Mohammadi, M. The use of cellulose nanocrystals for potential application in topical delivery of hydroquinone. Chem. Biol. Drug Des. 2015, 86, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-Y.; Wang, B.; Ma, M.-G.; Zhu, J.-F. Cellulose-based nanocarriers as platforms for cancer therapy. Curr. Pharm. Des. 2017, 23, 5292–5300. [Google Scholar] [CrossRef]
- Edwards, J.; Fontenot, K.; Liebner, F.; Condon, B. Peptide-cellulose conjugates on cotton-based materials have protease sensor/sequestrant activity. Sensors 2018, 18, 2334. [Google Scholar] [CrossRef]
- Edwards, J.; Fontenot, K.; Liebner, F.; Pircher, N.; French, A.; Condon, B. Structure/function analysis of cotton-based peptide-cellulose conjugates: Spatiotemporal/kinetic asessment of protease aerogels compared to nanocrystalline and paper cellulose. Int. J. Mol. Sci. 2018, 19, 840. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Kiliç, G.; Costa, P.M.; Fadeel, B. Cytotoxicity screening and cytokine profiling of nineteen nanomaterials enables hazard ranking and grouping based on inflammogenic potential. Nanotoxicology 2017, 11, 809–826. [Google Scholar]
- Menas, A.L.; Yanamala, N.; Farcas, M.T.; Russo, M.; Friend, S.; Fournier, P.M.; Star, A.; Iavicoli, I.; Shurin, G.V.; Vogel, U.B.; et al. Fibrillar vs crystalline nanocellulose pulmonary epithelial cell responses: Cytotoxicity or inflammation? Chemosphere 2017, 171, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Khaliullin, T.O.; Shurin, M.R.; Kisin, E.R.; Yanamala, N.; Fadeel, B.; Chang, J.; Shvedova, A.A. Fibrous nanocellulose, crystalline nanocellulose, carbon nanotubes, and crocidolite asbestos elicit disparate immune responses upon pharyngeal aspiration in mice. J. Immunotoxicol. 2018, 15, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bacher, M.; Rosenau, T.; Willför, S.; Mihranyan, A. Potentially immunogenic contaminants in wood-based and bacterial nanocellulose: Assessment of endotoxin and (1,3)-β-d-glucan levels. Biomacromolecules 2018, 19, 150–157. [Google Scholar] [CrossRef] [PubMed]
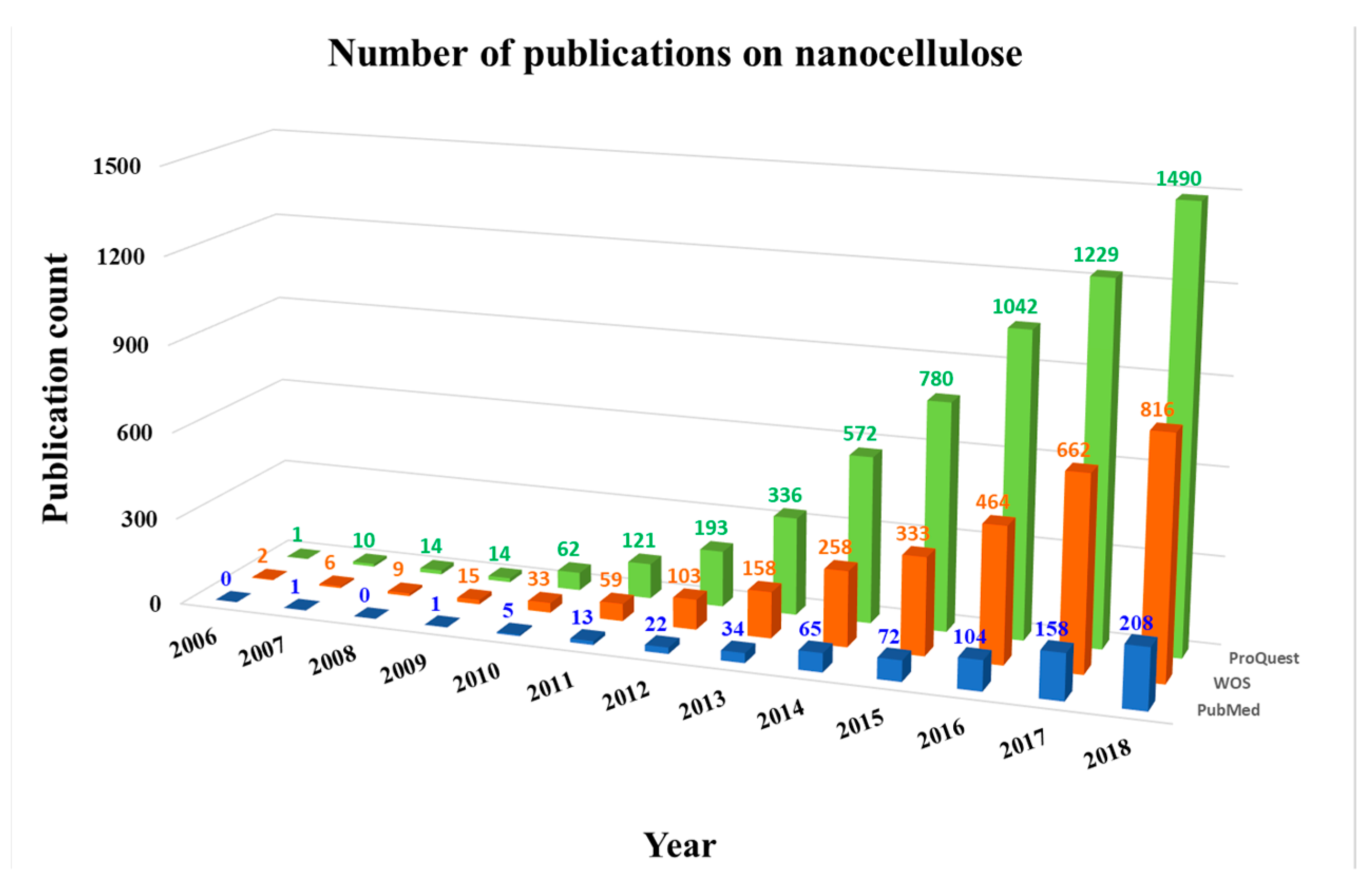
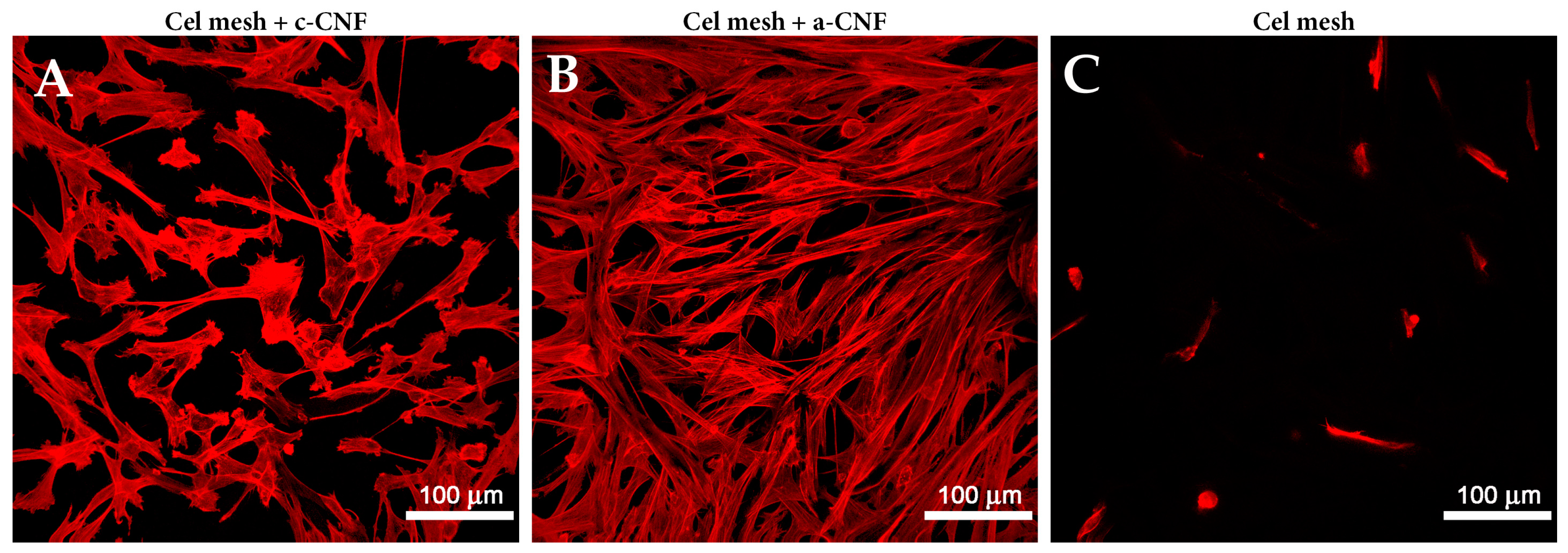
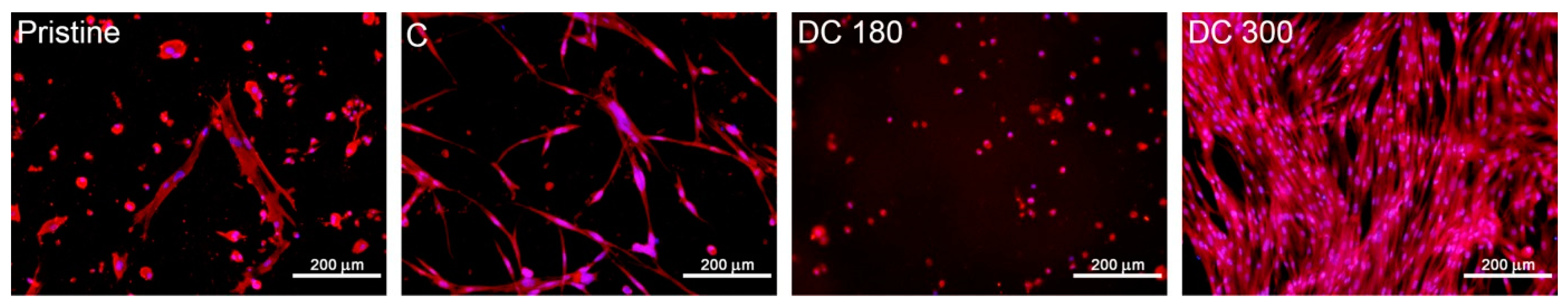
| Nanocellulose structures | Example | Dimensions | Reference |
|---|---|---|---|
| Nanofibrils | In bacterial cellulose | Diameter from 70 to 140 nm, length in µm | [2] |
| In wood-derived cellulose | Diameter 3–5 nm, length several µm, form 20–50 nm thick aggregates | [26,27] | |
| Nanofibers | Created by electrospinning | Cellulose acetate: average diameterabout 400 nm | [28] |
| Bacterial cellulose (33 wt. %) with chitosan: diameters from 80 to 170 nm | [29] | ||
| Isolated from pineapple | Width 6.4 ± 4.6 nm, length in µm | [15] | |
| Nanowhiskers | Pine kraft pulp | Diameter 5–15 nm, length 100–250 nm | [24] |
| Kenaf bast | Diameter 10–15 nm, length hundreds nm | [30] | |
| Bacterial cellulose | Diameter 10–100 nm, length 100–1000 nm | [31] | |
| Nanocrystals | Cotton-derived | Mean width 7.3 nm, mean length 135 nm | [32] |
| Nanorods | Grass-derived | Width 15 ± 3 nm, length 120 ± 15 nm | [19] |
| Nanoballs | Wood-derived | Diameter 80-85 nm | [23,24] |
| Nanoplatelets | Agave-derived | Thickness 80 nm, other dimensions in µm | [25] |
| Application | Specification | Example | Reference |
|---|---|---|---|
| Adsorption | Air purification | Odor removal (in combination with zeolites) | [94] |
| Removal of pollutants from aqueous solutions | Heavy metal ions (Cu2+, Pb2+, Hg2+) | [88,95] | |
| Toxic dyes (methylene blue, Congo Red) | [38,96] | ||
| Mefenamic acid (a nonsteroidal anti-inflammatory drug, a potential endocrine disruptor) | [97] | ||
| Oily substances | [39,93] | ||
| Insecticides (neonicotinoids in milk) | [98] | ||
| Immobilization of atoms and (bio) molecules | Metal catalysts (copper) | [99] | |
| Proteins (bovine serum albumin, lysozyme, γ-globulin, and human IgG | [86] | ||
| Enzymes (trypsin, laccase, lysozyme, lipase) | [69,100,101,102] | ||
| Ingested lipids (obesity management) | [103] | ||
| DNA oligomers | [104] | ||
| (Ultra)filtration | Removal of toxic dyes | Methylene blue, methylene orange, rhodamine | [105] |
| Hemodialysis membranes | Nanofibrillated cellulose with polypyrrole | [106] | |
| Removal of viruses | Swine influenza virus | [83] | |
| Murine leukemia virus | [107] | ||
| Bacteriophages | [87] | ||
| Packaging | Food, sensitive devices | Self-standing nanocellulose films from birch pulp | [33] |
| Paper sheets modified with nanocellulose and chitosan | [108] | ||
| Conservation | Historical papers, cotton canvas | Cellulose nanofibrils, carboxymethylated cellulose nanofibrils, cellulose nanocrystals | [109] |
| Thermal applications | Thermal insulators | Wood-derived nanofibrils with extremely low thermal conductivity | [110] |
| Fire retardants | Wood-derived cellulose nanofibrils with silica nanoparticles | [111] | |
| Wood-derived nanocellulose with montmorillonite clay | [67] | ||
| Energy extraction and storage | Lithium batteries | Nanocellulose/polypyrrole | [112] |
| Nanocellulose/polyethylene | [113] | ||
| Graphene/nanocellulose/silicon | [114] | ||
| Solar cells/panels | Nanofibers from sisal with graphene oxide | [81] | |
| (Super)capacitors | Bacterial nanocellulose/carbon nanotubes/triblock-copolymer ion gels | [115] | |
| Nanocellulose with polyaniline | [116] | ||
| Acoustics | Membranes for loudspeakers | Cellulose nanofibers with Fe3O4 nanoparticles | [79] |
| (Bio)sensors | Optical SERS-based | Detection of pesticides, dyes, bacteria | [117,118] |
| Optical fluorescence-based | Detection of heavy metals | [119] | |
| Detection of thiols | [120] | ||
| Detection of elastase | [121] | ||
| Chemical | Detection of vapors (NH3.H2O, H2O, HCl, acetic acid) | [19] | |
| Electrochemical | Detection of cations in biological fluids (Na+, K+, Ca2+) | [46] | |
| Detection of cholesterol | [122] | ||
| Detection of avian leukosis virus | [123] | ||
| Piezoelectric | Based on bacterial cellulose | [124] | |
| Based on plant-derived cellulose nanofibrils | [125] | ||
| Based on nanocellulose with chitosan | [126] | ||
| Tactile sensor (simultaneous sensing of temperature and pressure) | [127] | ||
| Strain-sensing protonated aerogels from cellulose nanofibrils | [128] | ||
| Drug delivery | Peroral | Paracetamol | [57] |
| Ibuprofen (colonic release) | [78] | ||
| Methotrexate (colonic release) | [129] | ||
| Transdermal | Analgesics, antiphlogistics, corticoids, antihypertensives | [58,59] | |
| Diclophenac | [130] | ||
| Propranolol | [131] | ||
| Topical | Local anesthetics | [132] | |
| Antiseptics | [133,134] | ||
| Antibiotics (gentamycin, ceftriaxone) | [41,51] | ||
| Antibacterial peptides | [135] | ||
| Other antimicrobial, anti-inflammatory and antitumor drugs | [136,137] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. https://doi.org/10.3390/nano9020164
Bacakova L, Pajorova J, Bacakova M, Skogberg A, Kallio P, Kolarova K, Svorcik V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials. 2019; 9(2):164. https://doi.org/10.3390/nano9020164
Chicago/Turabian StyleBacakova, Lucie, Julia Pajorova, Marketa Bacakova, Anne Skogberg, Pasi Kallio, Katerina Kolarova, and Vaclav Svorcik. 2019. "Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing" Nanomaterials 9, no. 2: 164. https://doi.org/10.3390/nano9020164
APA StyleBacakova, L., Pajorova, J., Bacakova, M., Skogberg, A., Kallio, P., Kolarova, K., & Svorcik, V. (2019). Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials, 9(2), 164. https://doi.org/10.3390/nano9020164