Controllable Synthesis of All Inorganic Lead Halide Perovskite Nanocrystals with Various Appearances in Multiligand Reaction System
Abstract
:1. Introduction
2. Experiment Section
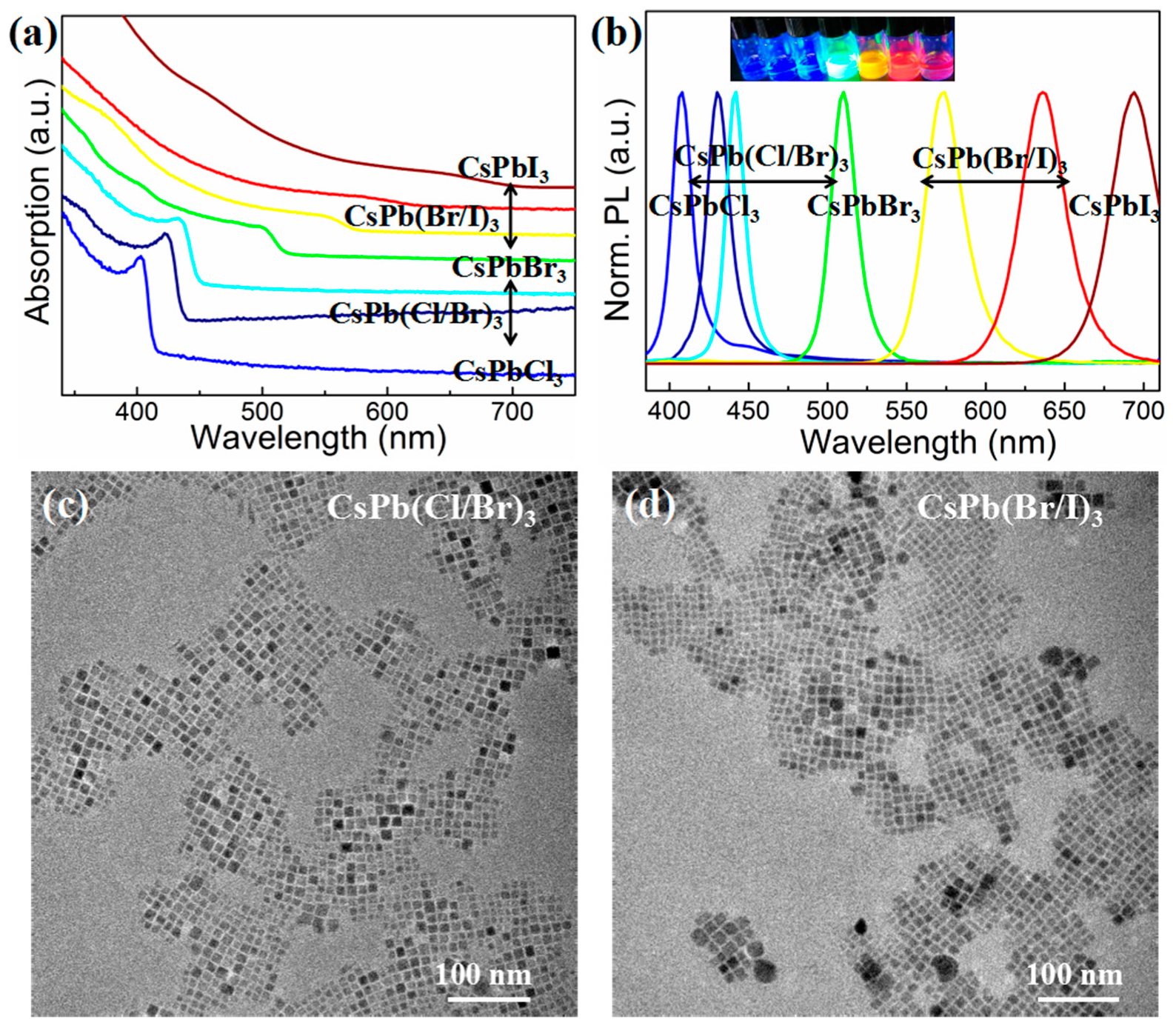
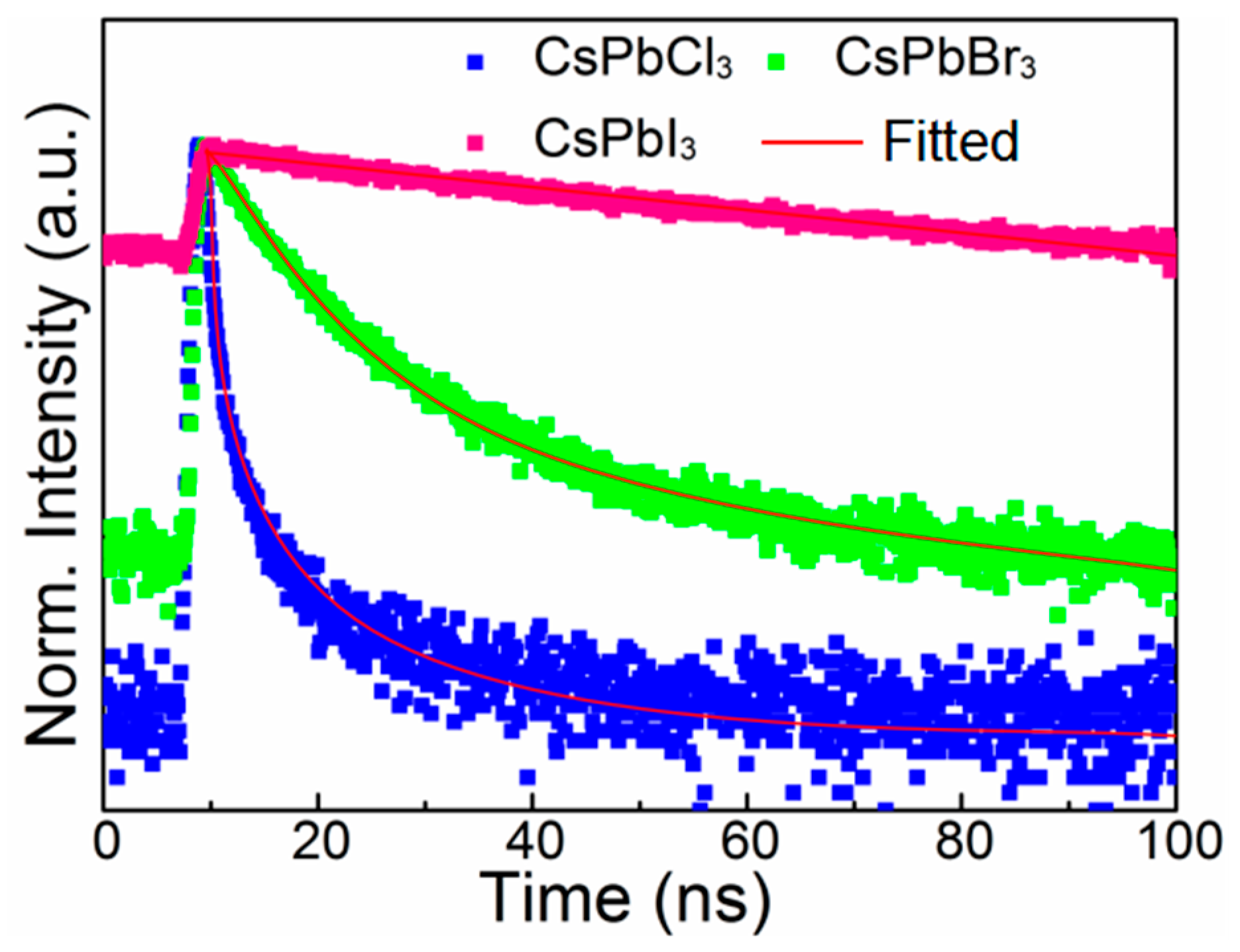
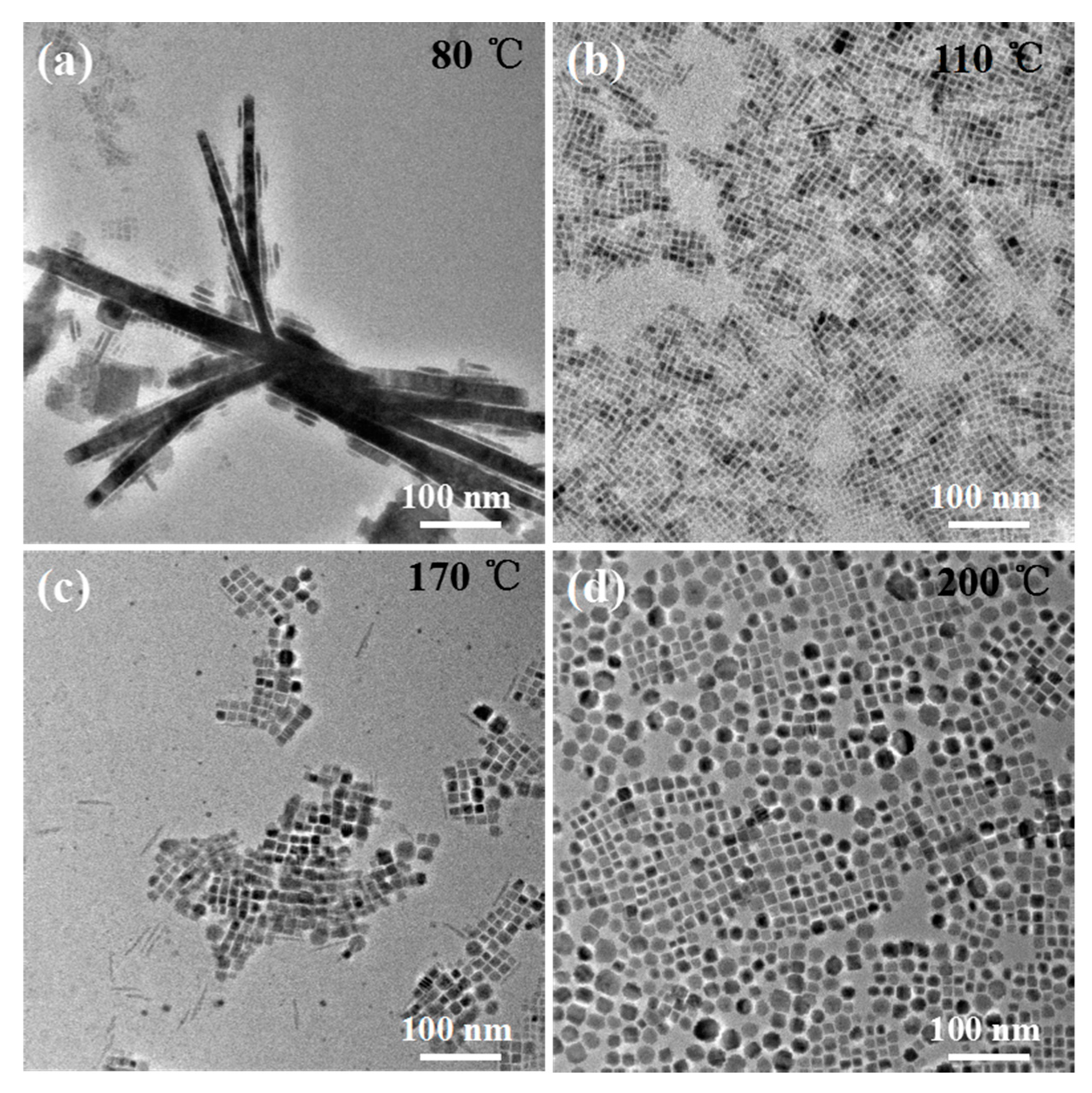
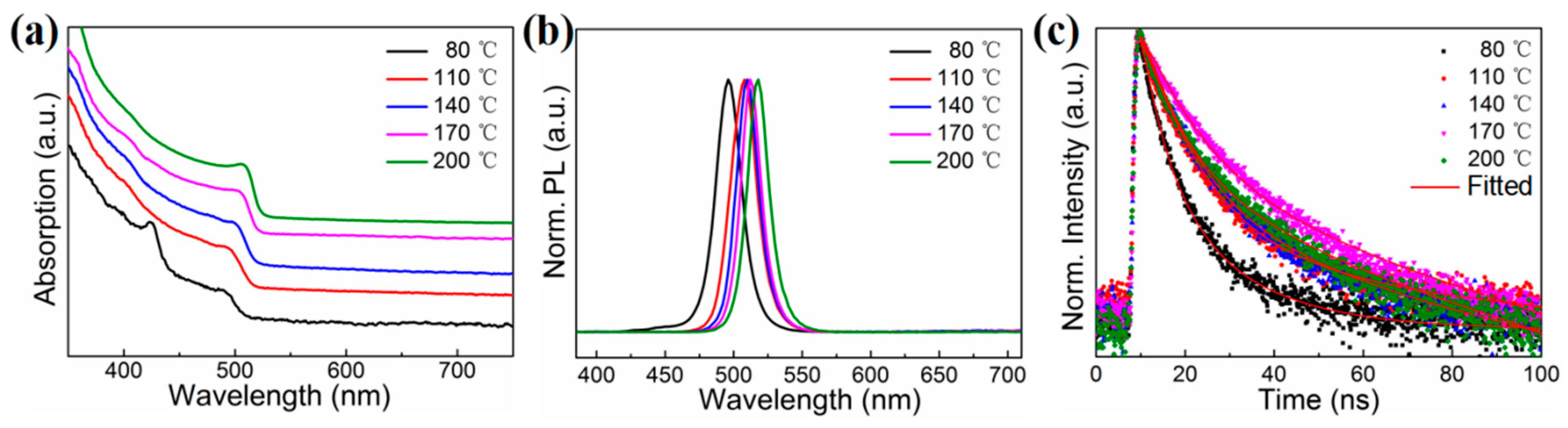
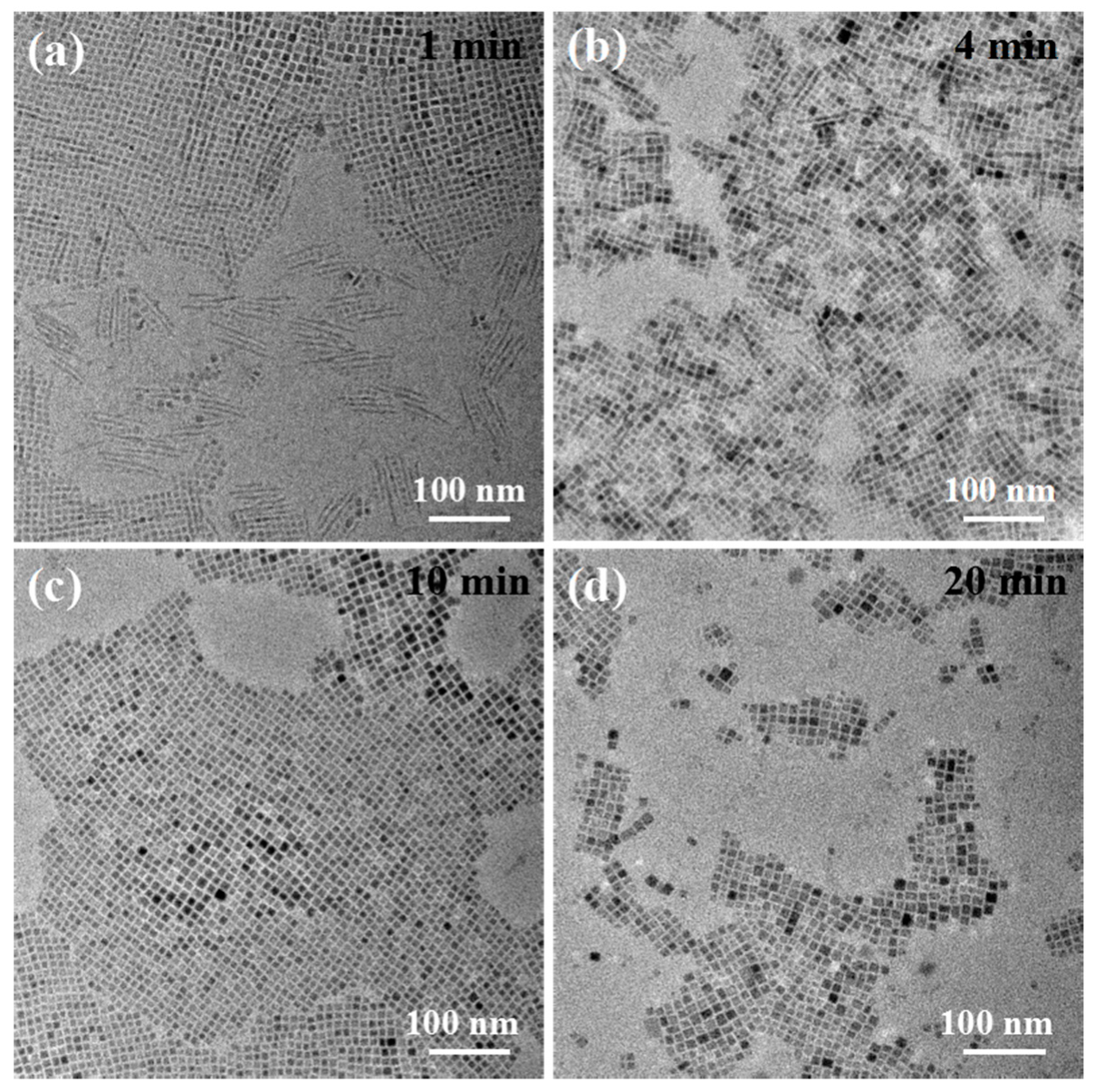
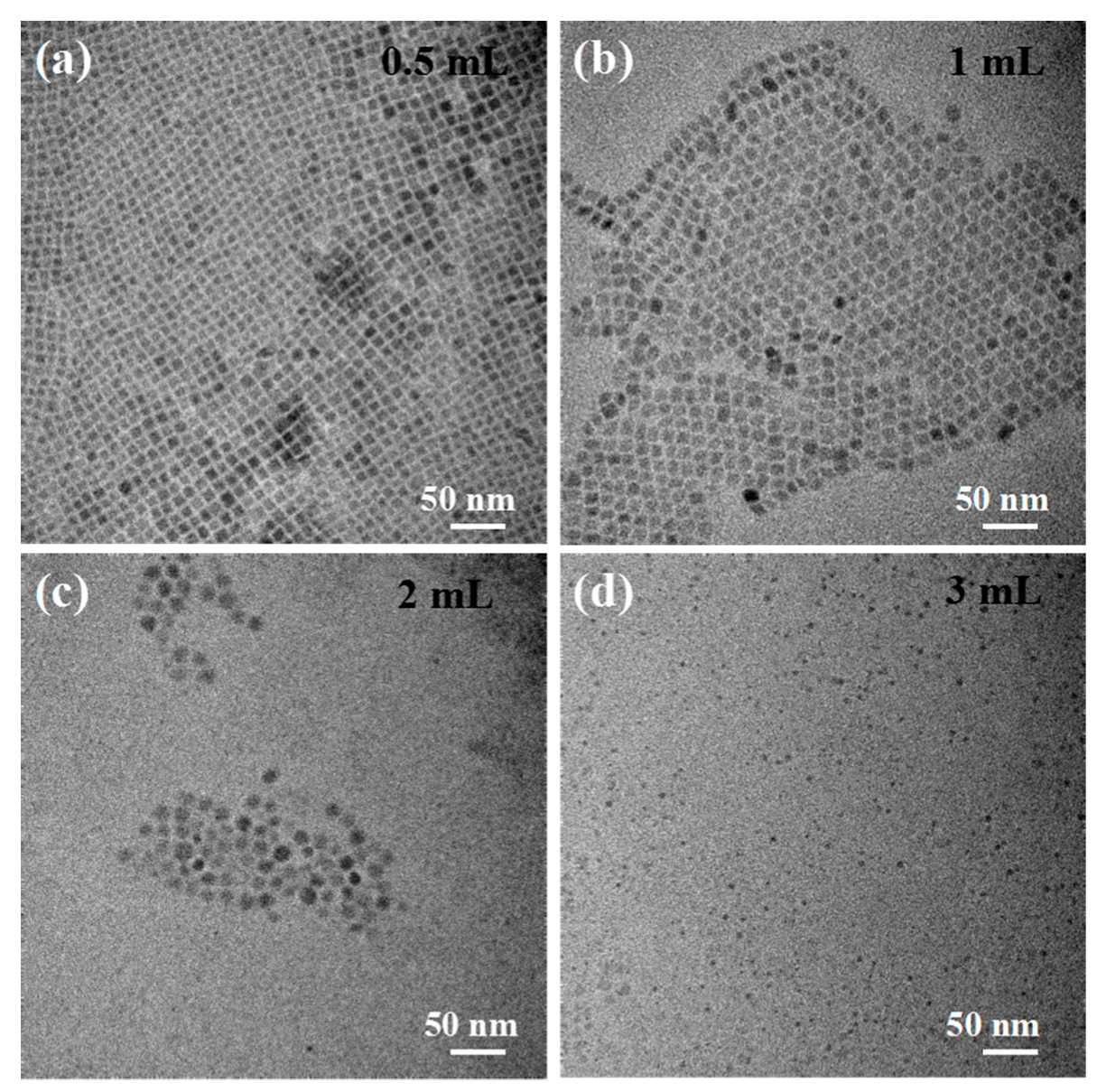
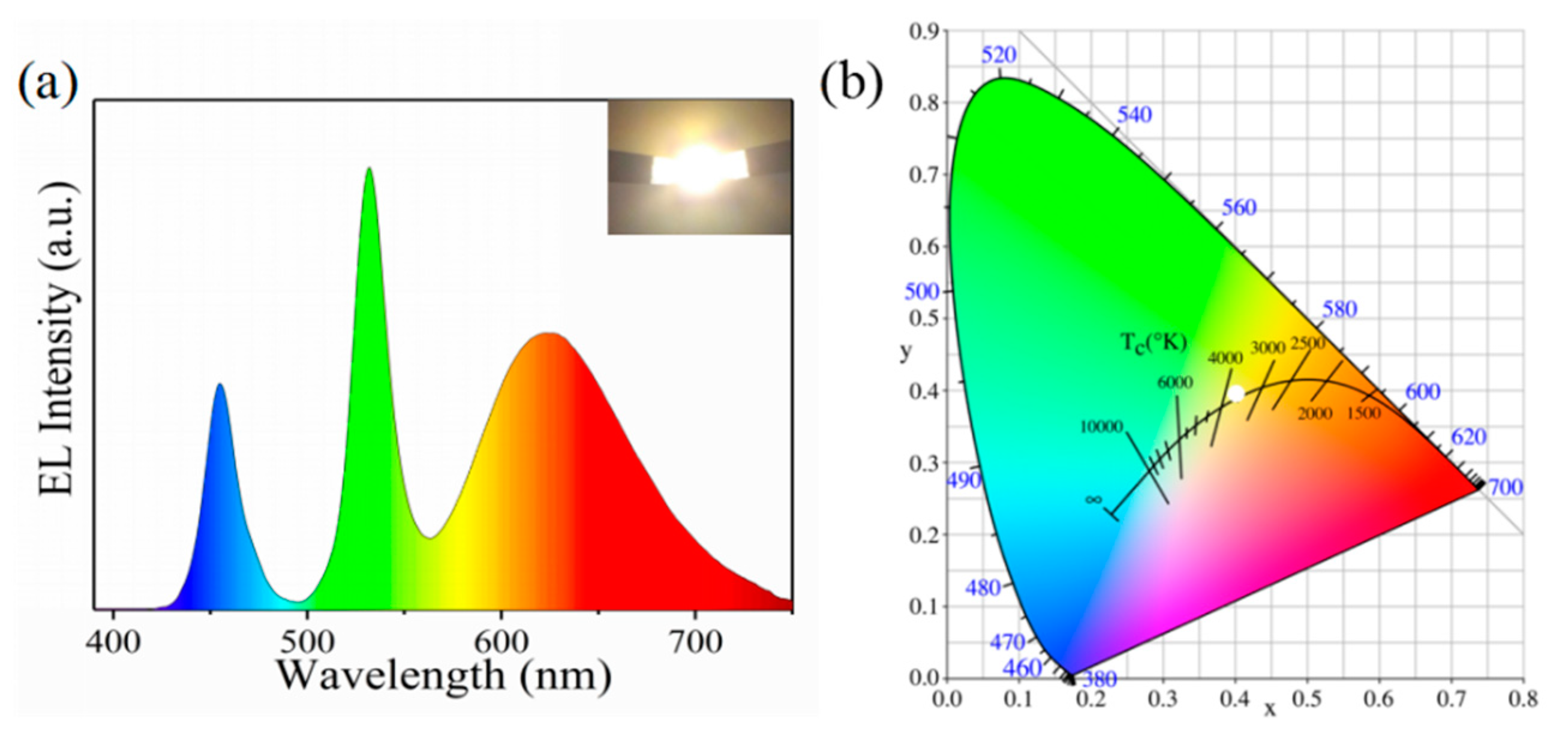
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.; Duan, H.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, S.; Li, M.; Li, L.; Zhang, J.; Fan, L.; Cheng, F.; Li, C.; Zhu, M.; Kang, Z.; et al. Strategies for air-stable and tunable monolayer MoS2-based hybrid photodetectors with high performance by regulating the fully inorganic trihalide perovskite nanocrystals. Adv. Opt. Mater. 2019, 7, 1801744. [Google Scholar] [CrossRef]
- Amgar, D.; Aharon, S.; Etgar, L. Inorganic and hybrid organo-metal perovskite nanostructures: Synthesis, properties, and applications. Adv. Funct. Mater. 2016, 26, 8576–8593. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, S.; Li, L.; Zhang, Z.; Liu, N.; Gao, Y. Application and development of cesium lead halide perovskite based planar heterojunction LEDs. J. Inorg. Mater. 2019, 34, 37. [Google Scholar]
- Shan, Q.; Song, J.; Zou, Y.; Li, J.; Xu, L.; Xue, J.; Dong, Y.; Han, B.; Chen, J.; Zeng, H. High performance metal halide perovskite light-emitting diode: From material design to device optimization. Small 2017, 13, 1701770. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Zhu, M.; Li, C.; Li, L.; Su, J.; Gao, Y. Pink all-inorganic halide perovskite nanocrystals with adjustable characteristics: Fully reversible cation exchange, improving the stability of dopant emission and light-emitting diode application. J. Alloys Compd. 2019, 152913. [Google Scholar] [CrossRef]
- Palazon, F.; Di Stasio, F.; Akkerman, Q.A.; Krahne, R.; Prato, M.; Manna, L. Polymer-free films of inorganic halide perovskite nanocrystals as UV-to-white color-conversion layers in LEDs. Chem. Mater. 2016, 28, 2902–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, S.; Yu, M.; Zhao, M.; Song, J.; Qu, J. Low temperature synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals in open air and their upconversion luminescence. J. Alloys Compd. 2018, 730, 62–70. [Google Scholar] [CrossRef]
- Meyns, M.; Perálvarez, M.; Heuer-Jungemann, A.; Hertog, W.; Ibáñez, M.; Nafria, R.; Genç, A.; Arbiol, J.; Kovalenko, M.V.; Carreras, J.; et al. Polymer-enhanced stability of inorganic perovskite nanocrystals and their application in color conversion LEDs. ACS Appl. Mater. Interfaces 2016, 8, 19579–19586. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Guo, A.; Gu, X.; Feng, M. Highly luminescent CsPbX3 (X=Cl, Br, I) perovskite nanocrystals with tunable photoluminescence properties. J. Alloys Compd. 2019, 789, 392–399. [Google Scholar] [CrossRef]
- Loiudice, A.; Saris, S.; Oveisi, E.; Alexander, D.T.L.; Buonsanti, R. CsPbBr3 QD/AlOx inorganic nanocomposites with exceptional stability in water, light, and heat. Angew. Chem. Int. Ed. 2017, 56, 10696–10701. [Google Scholar] [CrossRef]
- Xuan, T.; Yang, X.; Lou, S.; Huang, J.; Liu, Y.; Yu, J.; Li, H.; Wong, K.L.; Wang, C.; Wang, J. Highly stable CsPbBr3 quantum dots coated with alkyl phosphate for white light-emitting diodes. Nanoscale 2017, 9, 15286–15290. [Google Scholar] [CrossRef]
- Li, Z.; Kong, L.; Huang, S.; Li, L. Highly luminescent and ultrastable CsPbBr3 perovskite quantum dots incorporated into a silica alumina monolith. Angew. Chem. 2017, 129, 8246–8250. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, H.; Zhang, J.; Wang, L.; An, M.; Wang, Y.; Zhang, X.; Liu, Y. Luminescent perovskite nanocrystal-epoxy resin composite with high stability against water and air. J. Alloys Compd. 2019, 789, 209–214. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Eaton, S.W.; Yu, Y.; Dou, L.; Yang, P. Solution-phase synthesis of cesium lead halide perovskite nanowires. J. Am. Chem. Soc. 2015, 137, 9230–9233. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Ayggler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Docampo, P.; Bals, S.; Urban, A.S.; Polavarapu, L.; et al. Highly luminescent cesium lead halide perovskite nanocrystals with tunable composition and thickness by ultrasonication. Angew. Chem. Int. Ed. 2016, 55, 13887–13892. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.; Huang, K.; Shi, Z.; Xie, R.; Yang, W. Non-injection gram-scale synthesis of cesium lead halide perovskite quantum dots with controllable size and composition. Nano Res. 2016, 9, 1994–2006. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Z.; Gao, H.; Shao, J.; Zou, H.; Yao, D.; Liu, Y.; Zhang, H.; Yang, B. One-step preparation of cesium lead halide CsPbX3 (X = Cl, Br, and I) perovskite nanocrystals by microwave irradiation. ACS Appl. Mater. Interfaces 2017, 9, 42919–42927. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wang, A.; Wang, Q.; Tang, H.; Shen, W.; Li, Z.; Deng, Z. Controlled synthesis of composition tunable formamidinium cesium double cation lead halide perovskite nanowires and nanosheets with improved stability. Chem. Mater. 2017, 29, 2157–2166. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Motti, S.G.; Kandada, A.R.S.; Mosconi, E.; D’Innocenzo, V.; Bertoni, G.; Marras, S.; Kamino, B.A.; Miranda, L.; De Angelis, F.; et al. Solution synthesis approach to colloidal cesium lead halide perovskite nanoplatelets with monolayer-level thickness control. J. Am. Chem. Soc. 2016, 138, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Tan, Y.; Zhong, Q.; Xu, Y.; Liu, H.; et al. Solvothermal synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals: From nanocube to ultrathin nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Aharon, S.; Etgar, L. Two dimensional organometal halide perovskite nanorods with tunable optical properties. Nano Lett. 2016, 16, 3230–3235. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Heo, J.H.; Kim, T.S.; Myoung, N.; Lee, C.L.; Im, S.H.; Lee, T.W. Multicolored organic/inorganic hybrid perovskite light-emitting diodes. Adv. Mater. 2015, 27, 1248–1254. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; et al. 50-Fold EQE improvement up to 6.27% of solution-processed all-inorganic perovskite CsPbBr3 QLEDs via surface ligand density control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef]
- Almeida, G.; Goldoni, L.; Akkerman, Q.; Dang, Z.; Khan, A.H.; Marras, S.; Moreels, I.; Manna, L. Role of acid-base equilibria in the size, shape, and phase control of cesium lead bromide nanocrystals. ACS Nano 2018, 12, 1704–1711. [Google Scholar] [CrossRef] [Green Version]
- Ghorai, A.; Midya, A.; Ray, S.K. Surfactant-induced anion exchange and morphological evolution for composition-controlled caesium lead halide perovskites with tunable optical properties. ACS Omega 2019, 4, 12948–12954. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhang, L.; Shi, T.; Liao, G.; Tang, Z. Controllable Synthesis of All Inorganic Lead Halide Perovskite Nanocrystals with Various Appearances in Multiligand Reaction System. Nanomaterials 2019, 9, 1751. https://doi.org/10.3390/nano9121751
Chen C, Zhang L, Shi T, Liao G, Tang Z. Controllable Synthesis of All Inorganic Lead Halide Perovskite Nanocrystals with Various Appearances in Multiligand Reaction System. Nanomaterials. 2019; 9(12):1751. https://doi.org/10.3390/nano9121751
Chicago/Turabian StyleChen, Chen, Louwen Zhang, Tielin Shi, Guanglan Liao, and Zirong Tang. 2019. "Controllable Synthesis of All Inorganic Lead Halide Perovskite Nanocrystals with Various Appearances in Multiligand Reaction System" Nanomaterials 9, no. 12: 1751. https://doi.org/10.3390/nano9121751
APA StyleChen, C., Zhang, L., Shi, T., Liao, G., & Tang, Z. (2019). Controllable Synthesis of All Inorganic Lead Halide Perovskite Nanocrystals with Various Appearances in Multiligand Reaction System. Nanomaterials, 9(12), 1751. https://doi.org/10.3390/nano9121751





