Facile Synthesis of Antimony Tungstate Nanosheets as Anodes for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Sb2WO6
2.2. Material Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
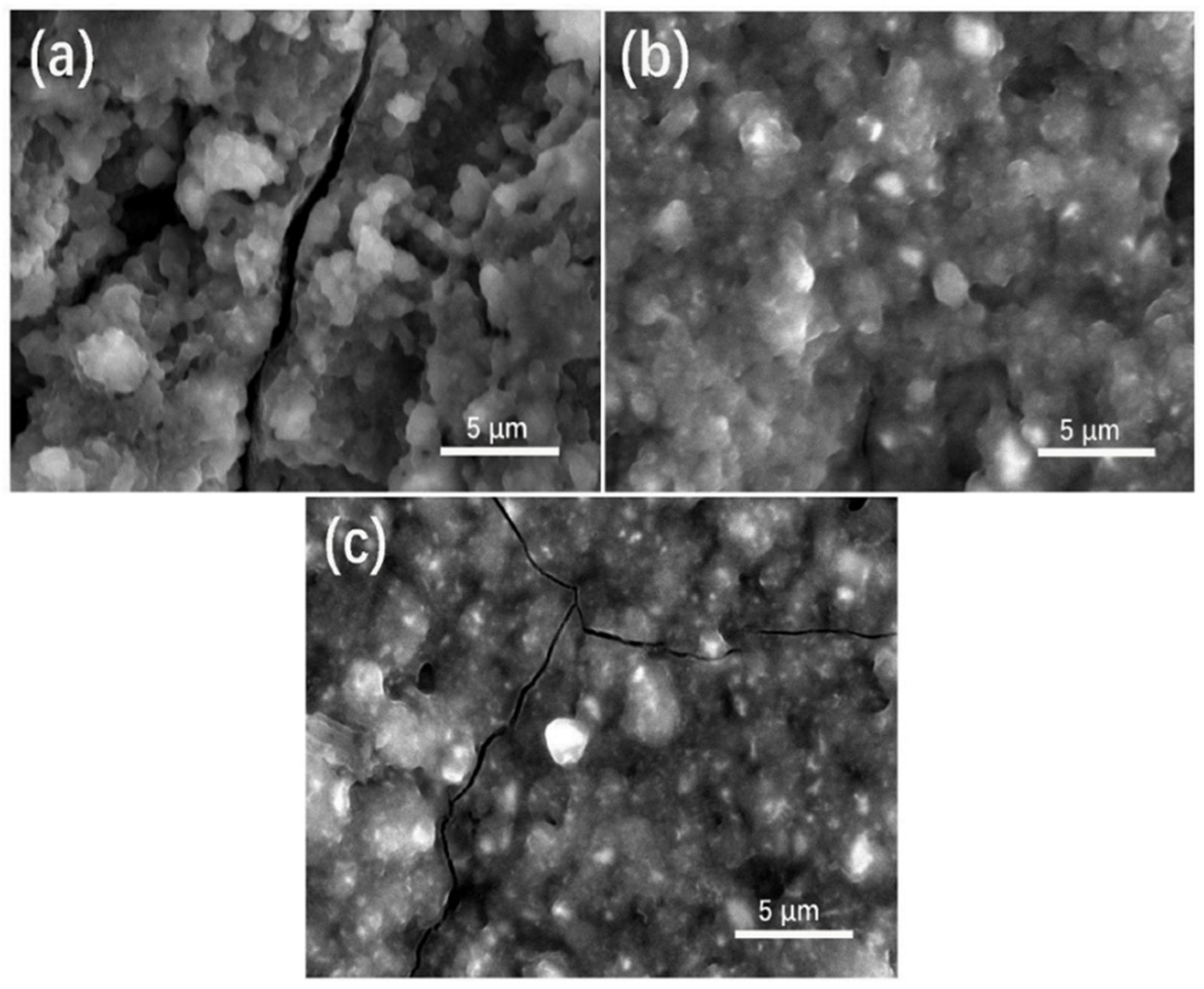
3.1. Structure and Morphology
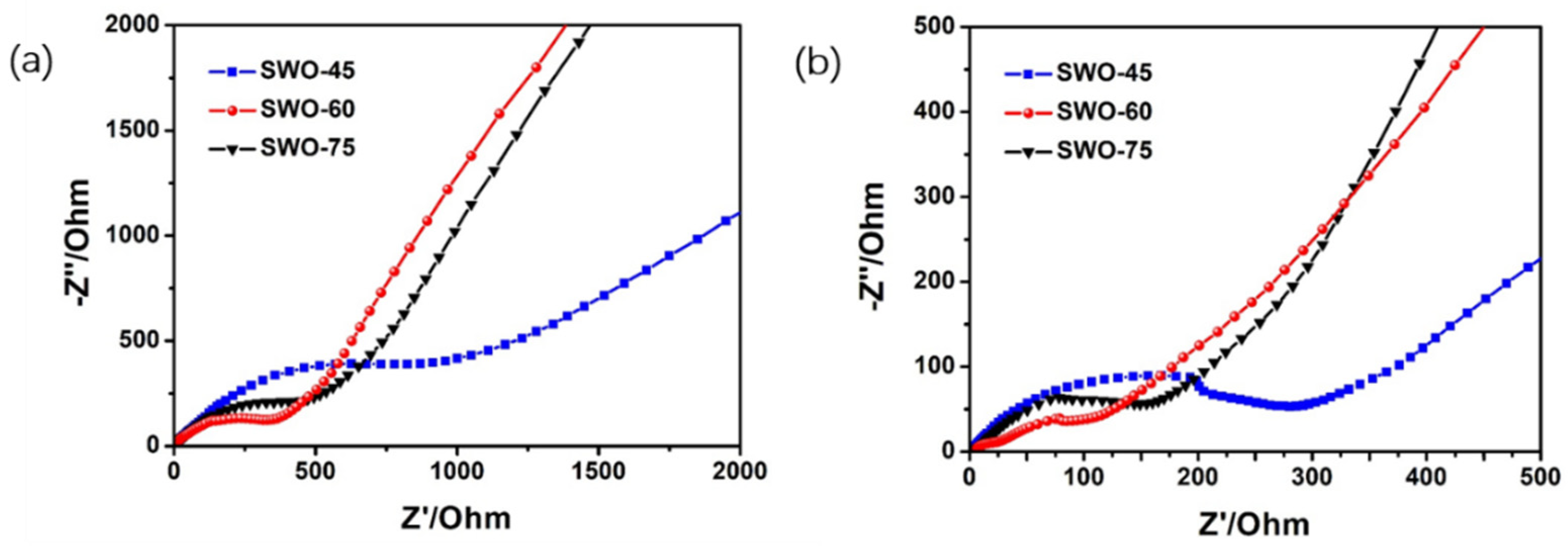
3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Geng, T.; Du, C.; Zuo, P.; Cheng, X.; Ma, Y.; Yin, G. Oxygen vacancies in SnO2 surface coating to enhance the activation of layered Li-Rich Li1.2 Mn0.54 Ni0.13 Co0.13 O2 cathode material for Li-ion batteries. J. Power Sources 2016, 331, 91–99. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lei, D.; Lv, W.; Zhao, Q.; Ni, B.; Liu, Y.; Li, B.; Kang, F.; He, Y.-B. Spherical Li Deposited inside 3D Cu Skeleton as Anode with Ultrastable Performance. ACS Appl. Mater. Interfaces 2018, 10, 20244–20249. [Google Scholar] [CrossRef]
- Zheng, J.-C.; Yang, Z.; He, Z.-J.; Tong, H.; Yu, W.-J.; Zhang, J.-F. In situ formed LiNi0.8Co0.15Al0.05O2@Li4SiO4 composite cathode material with high rate capability and long cycling stability for lithium-ion batteries. Nano Energy 2018, 53, 613–621. [Google Scholar] [CrossRef]
- Liu, Y.; Zhai, X.; Yang, K.; Wang, F.; Wei, H.; Zhang, W.; Ren, F.; Pang, H. Mesoporous NH4NiPO4 center dot H2O for High-Performance Flexible All-Solid-State Asymmetric Supercapacitors. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Guo, X.T.; Zhang, Y.Z.; Zhang, F.; Li, Q.; Anjum, D.H.; Liang, H.F.; Liu, Y.; Liu, C.S.; Alshareef, H.N.; Pang, H. A novel strategy for the synthesis of highly stable ternary SiOx composites for Li-ion-battery anodes. J. Mater. Chem. A 2019, 7, 15969–15974. [Google Scholar] [CrossRef]
- Peng, T.; Liu, C.; Hou, X.Y.; Zhang, Z.W.; Wang, C.L.; Yan, H.L.; Lu, Y.; Liu, X.M.; Luo, Y.S. Control Growth of Mesoporous Nickel Tungstate Nanofiber and Its Application as Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2017, 224, 460–467. [Google Scholar] [CrossRef]
- Hao, X.G.; Zhao, Q.; Su, S.M.; Zhang, S.Q.; Ma, J.B.; Shen, L.; Yu, Q.P.; Zhao, L.; Liu, Y.; Kang, F.Y.; et al. Constructing Multifunctional Interphase between Li1.4Al0.4Ti1.6(PO4)3 and Li Metal by Magnetron Sputtering for Highly Stable Solid-State Lithium Metal Batteries. Adv. Energy Mater. 2019, 9, 1901604. [Google Scholar] [CrossRef]
- Peng, T.; Hou, X.Y.; Liu, C.; Yu, Q.H.; Luo, R.J.; Yan, H.L.; Lu, Y.; Liu, X.M.; Luo, Y.S. Controlled synthesis of hierarchical CoMn2O4 nanostructures for flexible all-solid-state battery-type electrodes. J. Solid State Electrochem. 2017, 21, 1579–1587. [Google Scholar] [CrossRef]
- Wu, N.T.; Shen, J.K.; Sun, L.; Yuan, M.Y.; Shao, Y.Y.; Ma, J.M.; Liu, G.L.; Guo, D.L.; Liu, X.M.; He, Y.B. Hierarchical N-doped graphene coated 1D cobalt oxide microrods for robust and fast lithium storage at elevated temperature. Electrochim. Acta 2019, 310, 70–77. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.B.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 309–314. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Kwasi-Effah, C.C.; Rabczuk, T. Dimensional analysis and modelling of energy density of lithium-ion battery. J. Energy Storage 2018, 18, 308–315. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Wang, C.; Wang, F.; Wang, H.; Zhang, W.; Wang, X.; Yan, C.; Kim, B.H.; Ren, F. Nitrogen-Doped Carbon Coated WS2 Nanosheets as Anode for High-Performance Sodium-Ion Batteries. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Zhao, Y.; Wang, Y.; Wang, Z.; Zhang, W.; Ren, F. Facile Synthesis of Two-Dimensional Porous MgCo2O4 Nanosheets as Anode for Lithium-Ion Batteries. Appl. Sci. 2018, 8, 22. [Google Scholar] [CrossRef]
- Liu, G.; Cui, J.; Luo, R.; Liu, Y.; Huang, X.; Wu, N.; Jin, X.; Chen, H.; Tang, S.; Kim, J.-K.; et al. 2D MoS2 grown on biomass-based hollow carbon fibers for energy storage. Appl. Surf. Sci. 2019, 469, 854–863. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.C.; Yang, K.K.; Yang, Y.N.; Ma, J.Q.; Pan, K.M.; Wang, G.X.; Ren, F.Z.; Pang, H. Enhanced Electrochemical Performance of Sb2O3 as an Anode for Lithium-Ion Batteries by a Stable Cross-Linked Binder. Appl. Sci. 2019, 9, 2677. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Wagner, R.; Winter, M.; Cekic-Laskovic, I. Interfaces and Materials in Lithium Ion Batteries: Challenges for Theoretical Electrochemistry. Top. Curr. Chem. 2018, 376, 29. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Y.J.; Wang, J.T.; Long, D.H. Polymer-chelation synthesis of compositionally homogeneous LiNi1/3Co1/3Mn1/3O2 crystals for lithium-ion cathode. Electrochim. Acta 2018, 269, 724–732. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.-B.; Rogach, A.L.; Niu, C. Encapsulating Silica/Antimony into Porous Electrospun Carbon Nanofibers with Robust Structure Stability for High-Efficiency Lithium Storage. ACS Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef]
- Zeng, C.; Weng, W.; Lv, T.; Xiao, W. Low-Temperature Assembly of Ultrathin Amorphous MnO2 Nanosheets over Fe2O3 Spindles for Enhanced Lithium Storage. ACS Appl. Mater. Interfaces 2018, 10, 30470–30478. [Google Scholar] [CrossRef]
- Shim, H.W.; Cho, I.S.; Hong, K.S.; Lim, A.H.; Kim, D.W. Wolframite-type ZnWO4 Nanorods as New Anodes for Li-Ion Batteries. J. Phys. Chem. C 2011, 115, 16228–16233. [Google Scholar] [CrossRef]
- Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Electrochemical properties of carbon-coated CaWO4 versus Li. Electrochim. Acta 2005, 50, 5305–5312. [Google Scholar] [CrossRef]
- Hyun-Woo, S.; In-Sun, C.; Kug Sun, H.; Won Il, C.; Dong-Wan, K. Li electroactivity of iron (II) tungstate nanorods. Nanotechnology 2010, 21, 465602. [Google Scholar]
- Zhang, L.S.; Bai, Q.L.; Jin, K.; Wang, L.Z.; Zhang, Y.; Song, Y.H. Synthesis and electrochemical performance of Bi2WO6/graphene composite as anode material for lithium-ion batteries. Mater. Lett. 2015, 141, 88–91. [Google Scholar] [CrossRef]
- Zhang, L.S.; Zhao, Z.J.; Xia, T.C.; Zhang, S.L.; Li, X.F.; Zhang, A.Q. Anchoring Bi2WO6 nanoparticles on 3D graphene frameworks for enhanced lithium storage. Mater. Lett. 2018, 210, 345–349. [Google Scholar] [CrossRef]
- Singh, A.; Dutta, D.P.; Roy, M.; Tyagi, A.K.; Fulekar, M.H. Sonochemical synthesis, characterization, and photocatalytic properties of Bi2−xSbxWO6 nanorods. J. Mater. Sci. 2014, 49, 2085–2097. [Google Scholar] [CrossRef]
- Yang, C.Y.; Yang, X.J.; Li, F.; Li, T.H.; Cao, W. Controlled synthesis of hierarchical flower-like Sb2WO6 microspheres: Photocatalytic and superhydrophobic property. J. Ind. Eng. Chem. 2016, 39, 93–100. [Google Scholar] [CrossRef]
- Hu, S.P.; Xu, C.Y.; Ma, F.X.; Cao, L.; Zhen, L. Solvothermal synthesis of orthorhombic Sb2WO6 hierarchical structures and their visible-light-driven photocatalytic activity. Dalton Trans. 2014, 43, 8439–8445. [Google Scholar] [CrossRef]
- Wang, P.; Xie, S.M.; She, Y.Y.; Fan, W.G.; Leung, M.K.H.; Wane, H.K. Microwave-Hydrothermal Synthesis of Hierarchical Sb2WO6 Nanostructures as a New Anode Material for Sodium Storage. Chemistryselect 2019, 4, 1078–1083. [Google Scholar] [CrossRef]
- Chen, S.Q.; Zhou, M.Y.; Li, T.H.; Cao, W. Synthesis of Ag-loaded Sb2WO6 microsphere with enhanced photocatalytic ability for organic dyes degradations under different light irradiations. J. Mol. Liq. 2018, 272, 27–36. [Google Scholar] [CrossRef]
- Liu, Y.; Hori, A.; Kusaka, S.; Hosono, N.; Li, M.; Guo, A.; Du, D.; Li, Y.; Yang, W.; Ma, Y.; et al. Microwave-Assisted Hydrothermal Synthesis of Al(OH)(1,4-NDC) Membranes with Superior Separation Performances. Chem. Asian J. 2019. [Google Scholar] [CrossRef]
- Park, H.C.; Lee, K.H.; Lee, Y.W.; Kim, S.J.; Kim, D.M.; Kim, M.C.; Park, K.W. Mesoporous molybdenum nitride nanobelts as an anode with improved electrochemical properties in lithium ion batteries. J. Power Sources 2014, 269, 534–541. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z.Y.; Lu, L.; Yang, G.; Niu, C.M.; Wang, H.K. Synthesis of Hierarchical Sb2MoO6 Architectures and Their Electrochemical Behaviors as Anode Materials for Li-Ion Batteries. Inorg. Chem. 2016, 55, 7012–7019. [Google Scholar] [CrossRef]
- Wu, X.; Yao, S.Y. Flexible electrode materials based on WO3 nanotube bundles for high performance energy storage devices. Nano Energy 2017, 42, 143–150. [Google Scholar] [CrossRef]
- Su, Y.B.; Zhang, H.J.; Liang, P.; Liu, K.; Cai, M.Y.; Huang, Z.Y.; Wang, C.A.; Zhong, M.L. A new binder-free and conductive-additive-free TiO2/WO3-W integrative anode material produced by laser ablation. J. Power Sources 2018, 378, 362–368. [Google Scholar] [CrossRef]
- Lu, X.; Wang, P.; Liu, K.; Niu, C.M.; Wang, H.K. Encapsulating nanoparticulate Sb/MoOx into porous carbon nanofibers via electrospinning for efficient lithium storage. Chem. Eng. J. 2018, 336, 701–709. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, B.C.; Zhou, J.F.; Fang, Z.T.; Li, K.Z.; Ma, H.Y.; Dai, J.M.; Zhu, X.B.; Sun, Y.P. Improved Electrochemical Performance of Ultrathin MoS2 Nanosheet/Co Composites for Lithium-Ion Battery Anodes. Chemelectrochem 2019, 6, 1930–1938. [Google Scholar] [CrossRef]
- Jin, J.; Huang, S.-Z.; Shu, J.; Wang, H.-E.; Li, Y.; Yu, Y.; Chen, L.-H.; Wang, B.-J.; Su, B.-L. Highly porous TiO2 hollow microspheres constructed by radially oriented nanorods chains for high capacity, high rate and long cycle capability lithium battery. Nano Energy 2015, 16, 339–349. [Google Scholar] [CrossRef]
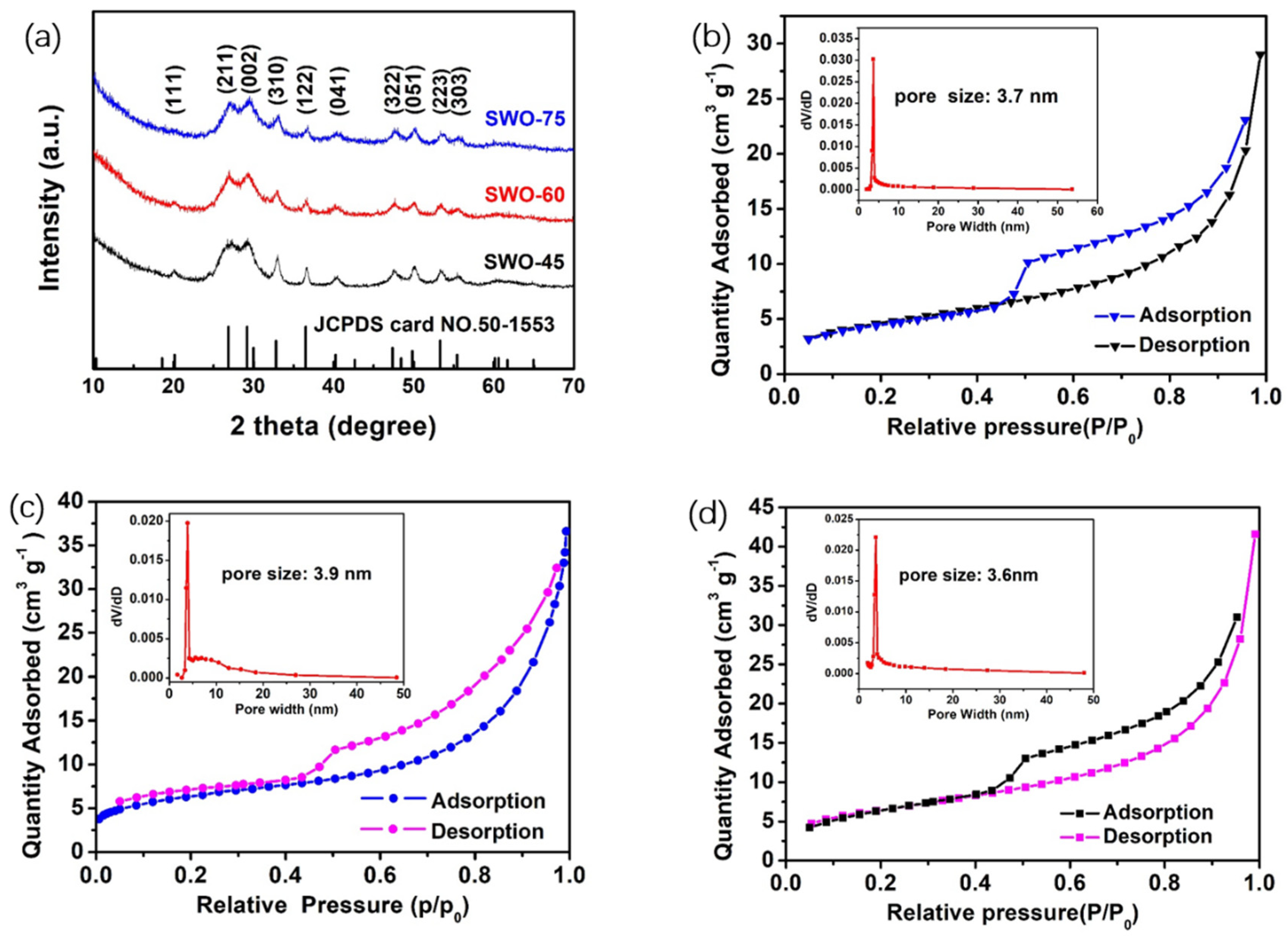
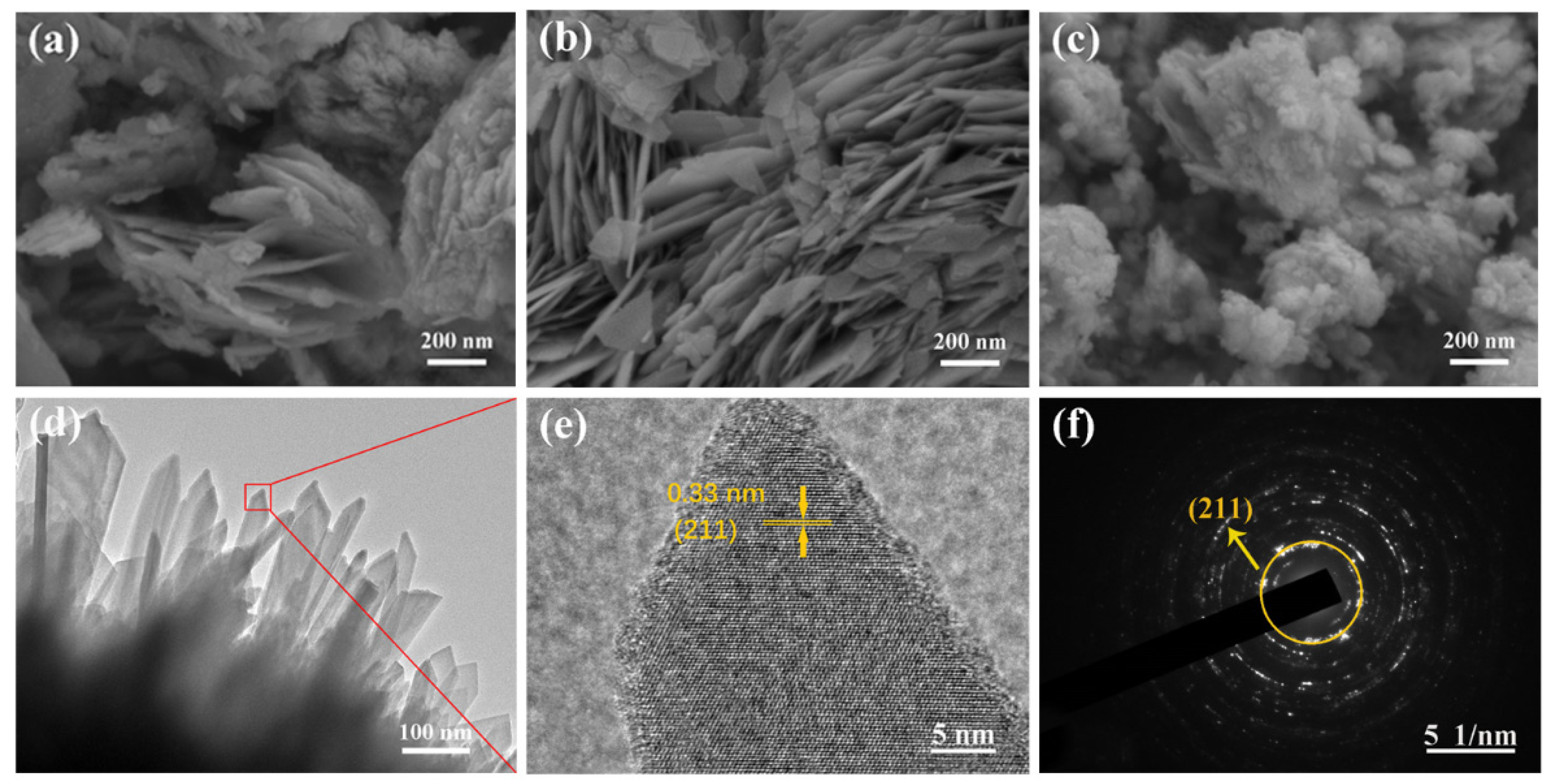
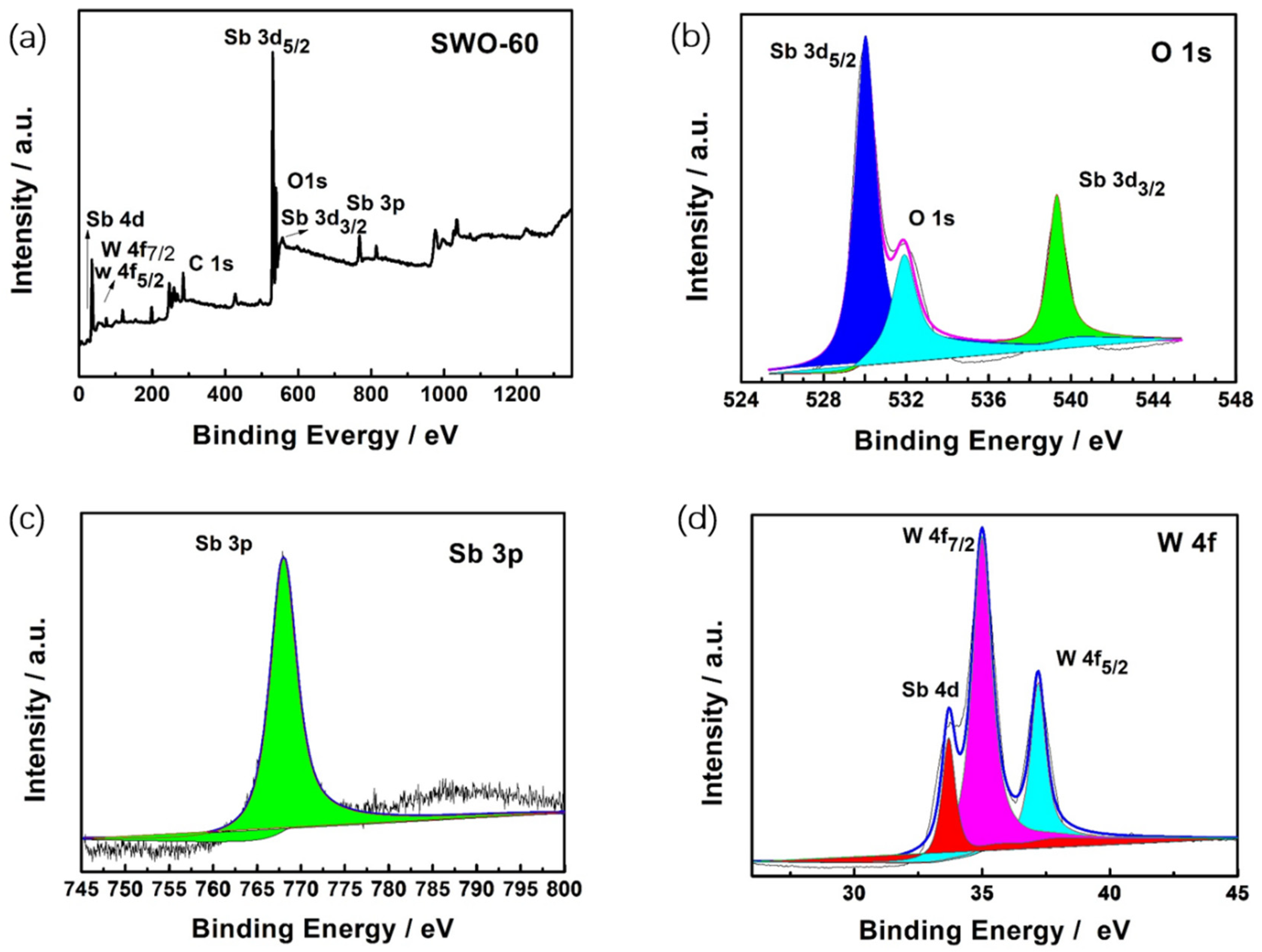
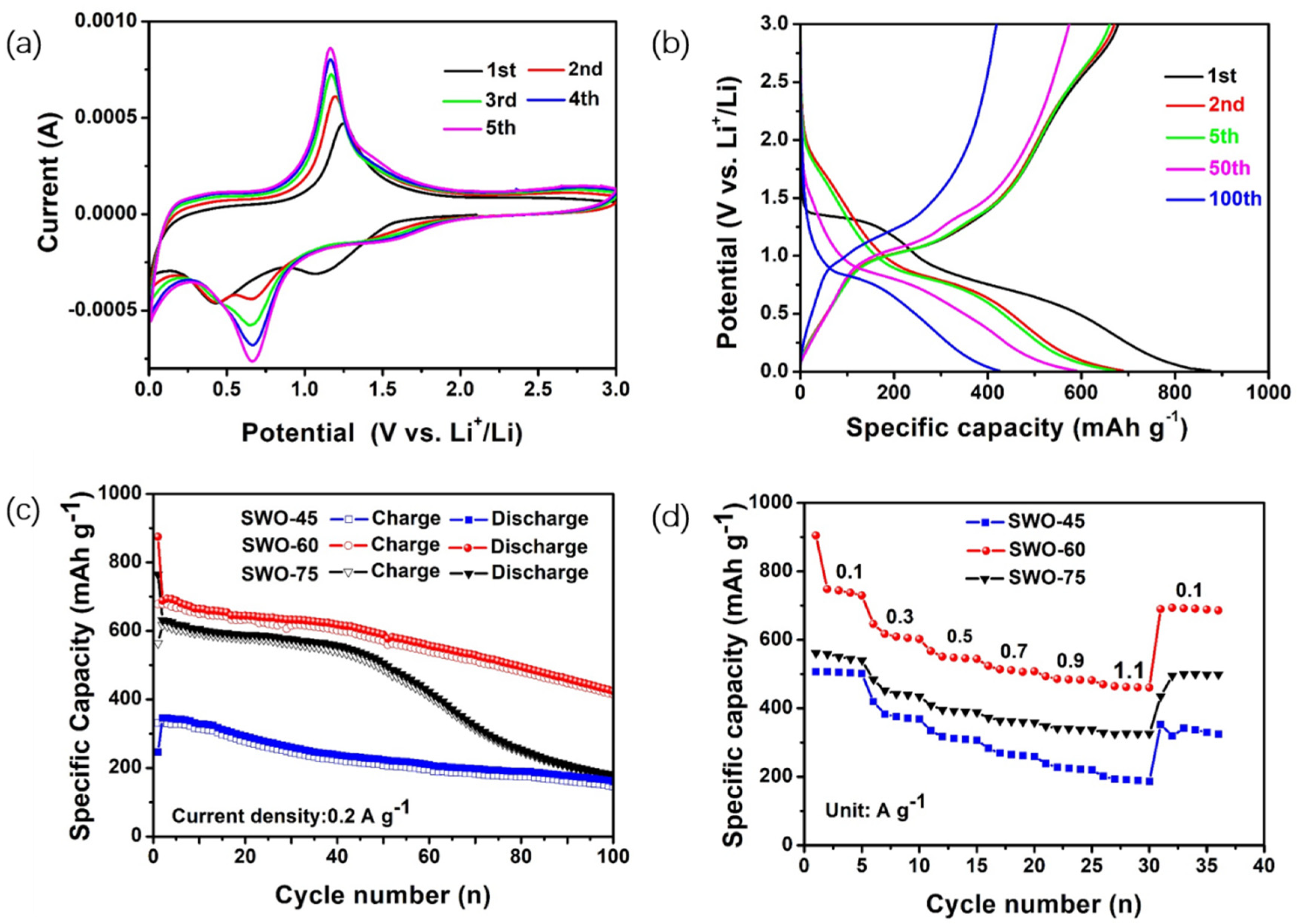
| Materials | Specific Surface Area (m2/g) | Mean Pore Size (nm) | Mean Crystallite Size (nm) |
|---|---|---|---|
| SWO-45 | 16.79 | 3.7 | 15.37 |
| SWO-60 | 22.07 | 3.9 | 13.82 |
| SWO-75 | 22.98 | 3.6 | 13.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Y.; Wang, F.; Lei, Z.; Zhang, W.; Pan, K.; Liu, J.; Chen, M.; Wang, G.; Ren, F.; et al. Facile Synthesis of Antimony Tungstate Nanosheets as Anodes for Lithium-Ion Batteries. Nanomaterials 2019, 9, 1689. https://doi.org/10.3390/nano9121689
Liu Y, Wang Y, Wang F, Lei Z, Zhang W, Pan K, Liu J, Chen M, Wang G, Ren F, et al. Facile Synthesis of Antimony Tungstate Nanosheets as Anodes for Lithium-Ion Batteries. Nanomaterials. 2019; 9(12):1689. https://doi.org/10.3390/nano9121689
Chicago/Turabian StyleLiu, Yong, Yue Wang, Fei Wang, Zhenxiao Lei, Wanhong Zhang, Kunming Pan, Jing Liu, Min Chen, Guangxin Wang, Fengzhang Ren, and et al. 2019. "Facile Synthesis of Antimony Tungstate Nanosheets as Anodes for Lithium-Ion Batteries" Nanomaterials 9, no. 12: 1689. https://doi.org/10.3390/nano9121689
APA StyleLiu, Y., Wang, Y., Wang, F., Lei, Z., Zhang, W., Pan, K., Liu, J., Chen, M., Wang, G., Ren, F., & Wei, S. (2019). Facile Synthesis of Antimony Tungstate Nanosheets as Anodes for Lithium-Ion Batteries. Nanomaterials, 9(12), 1689. https://doi.org/10.3390/nano9121689






