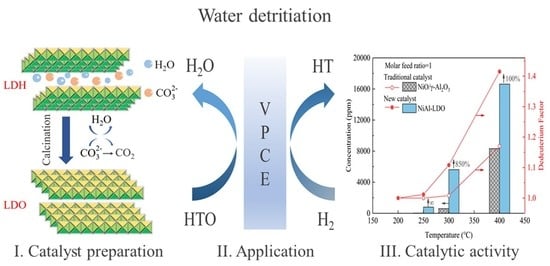
Ni-Based Catalyst Derived from NiAl Layered Double Hydroxide for Vapor Phase Catalytic Exchange between Hydrogen and Water
Abstract
1. Introduction
2. Experimental
2.1. Material
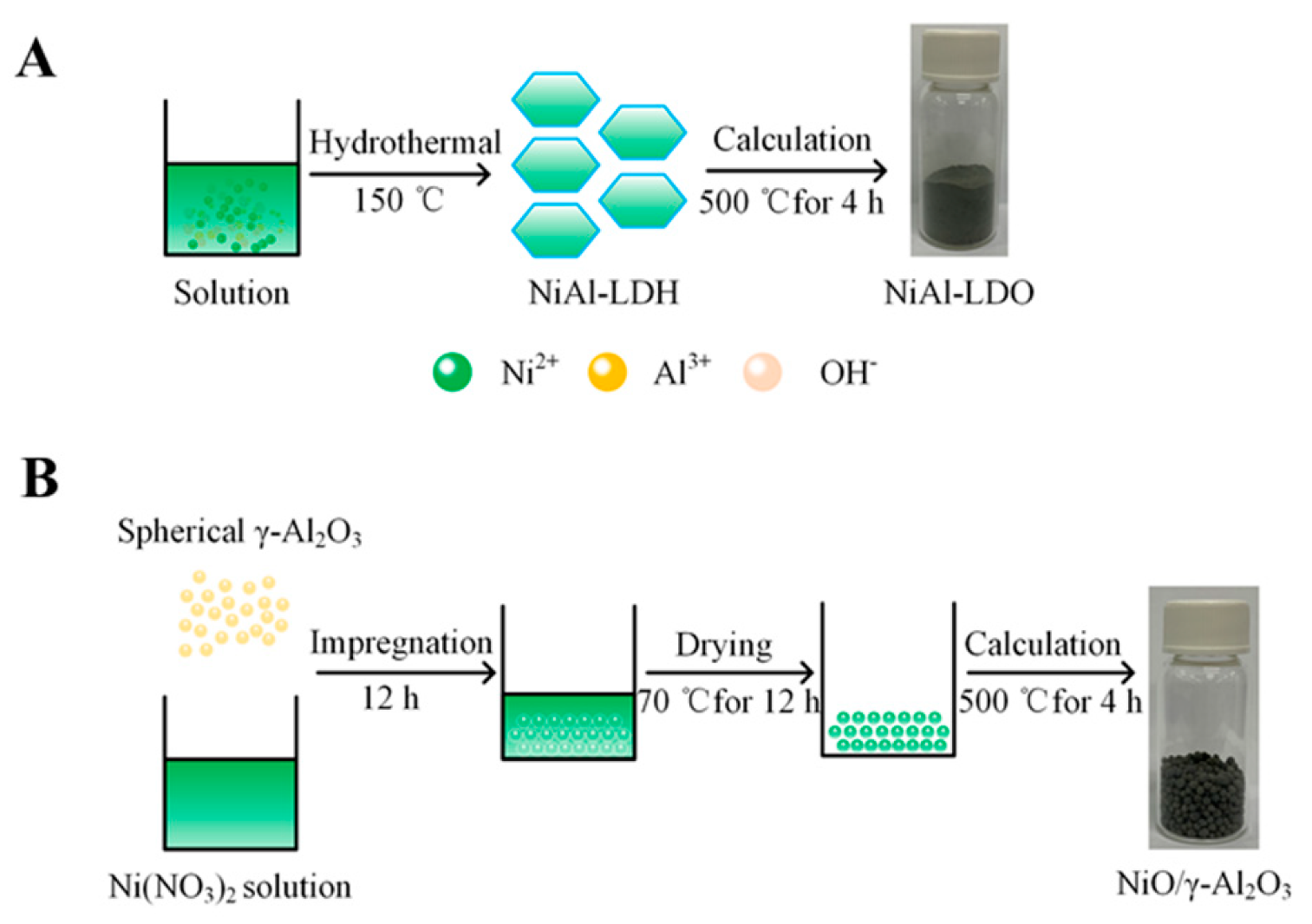
2.2. Synthesis of Petal-Like NiAl-LDH
2.3. Preparation of Spherical Ni(NO3)2/γ-Al2O3
2.4. Preparations of NiAl-LDO and NiO/γ-Al2O3
2.5. Characterization
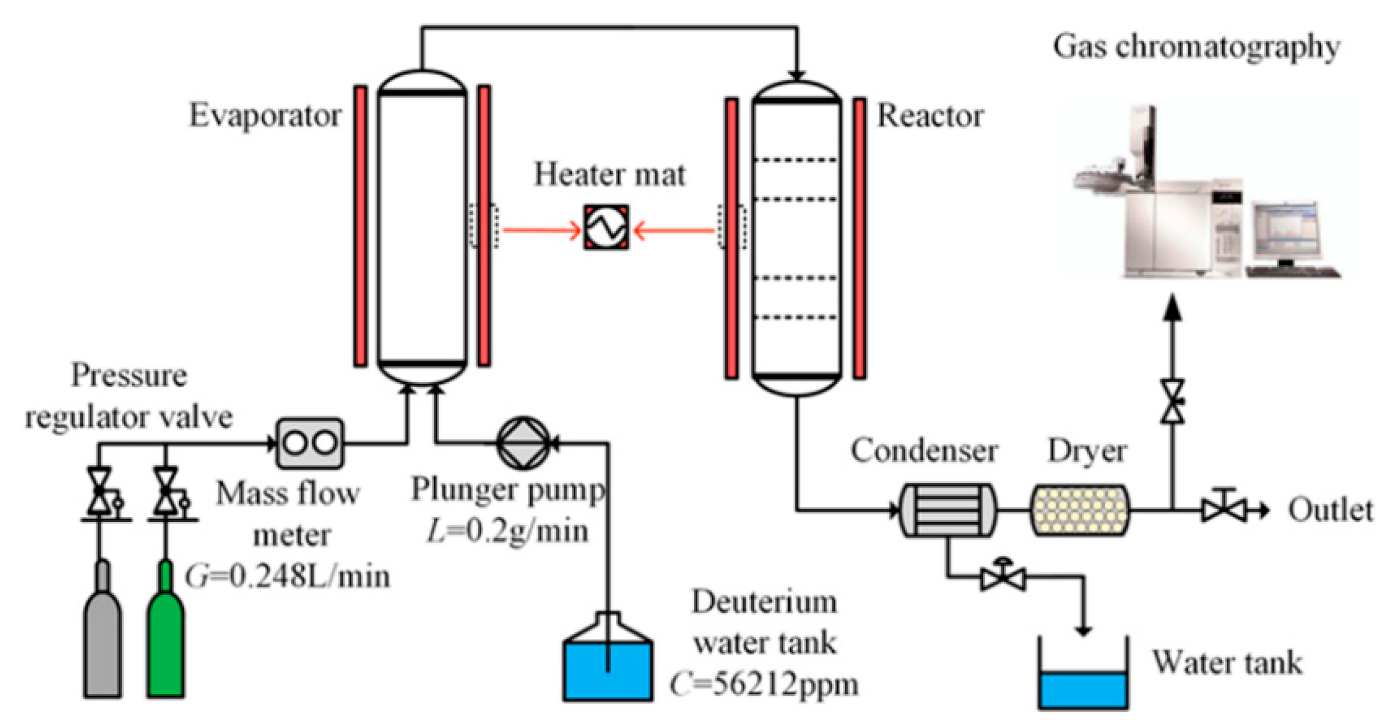
2.6. Catalytic Activity Evaluation
3. Results and Discussion
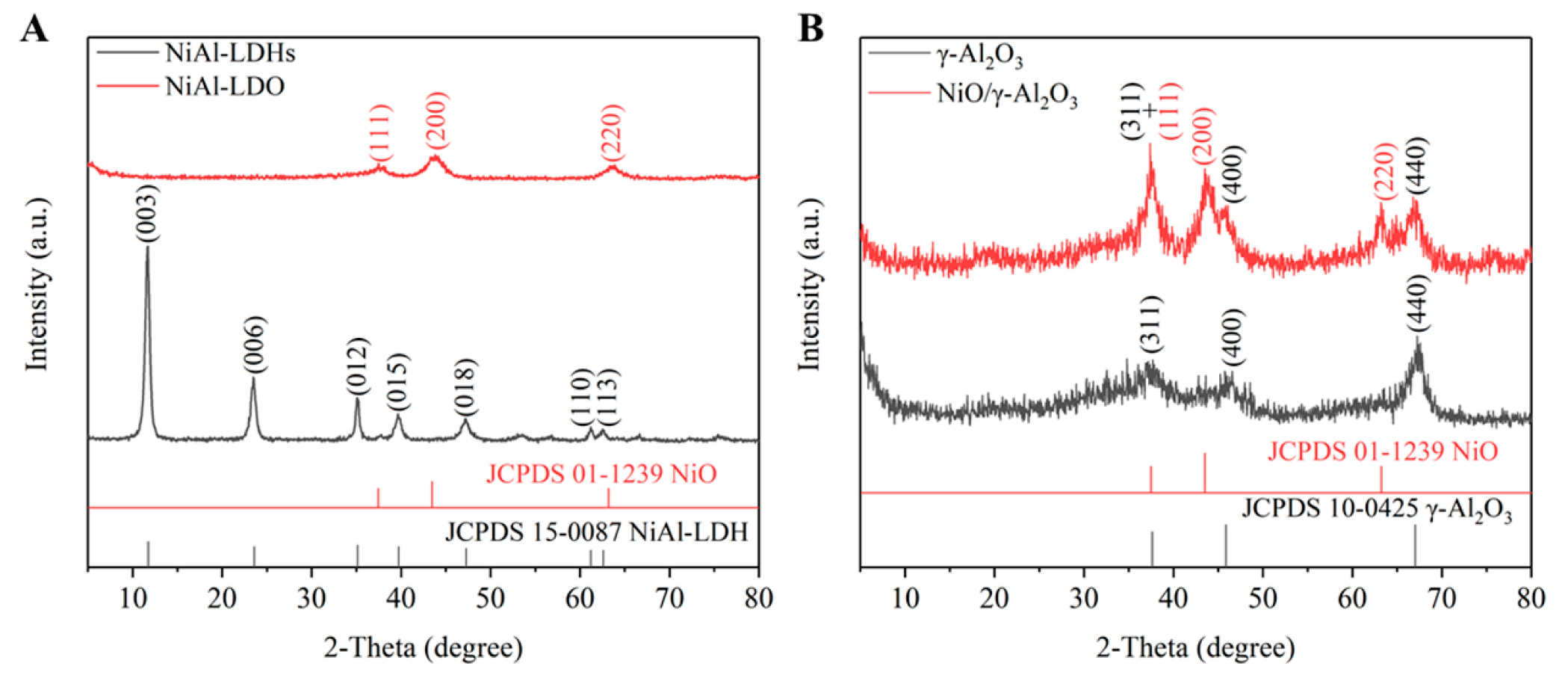
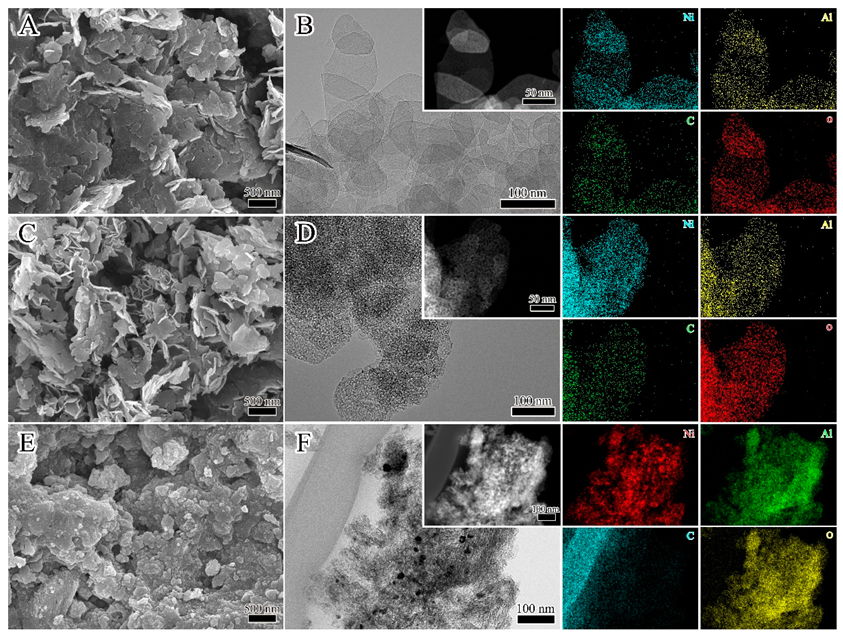
3.1. Material Characterization
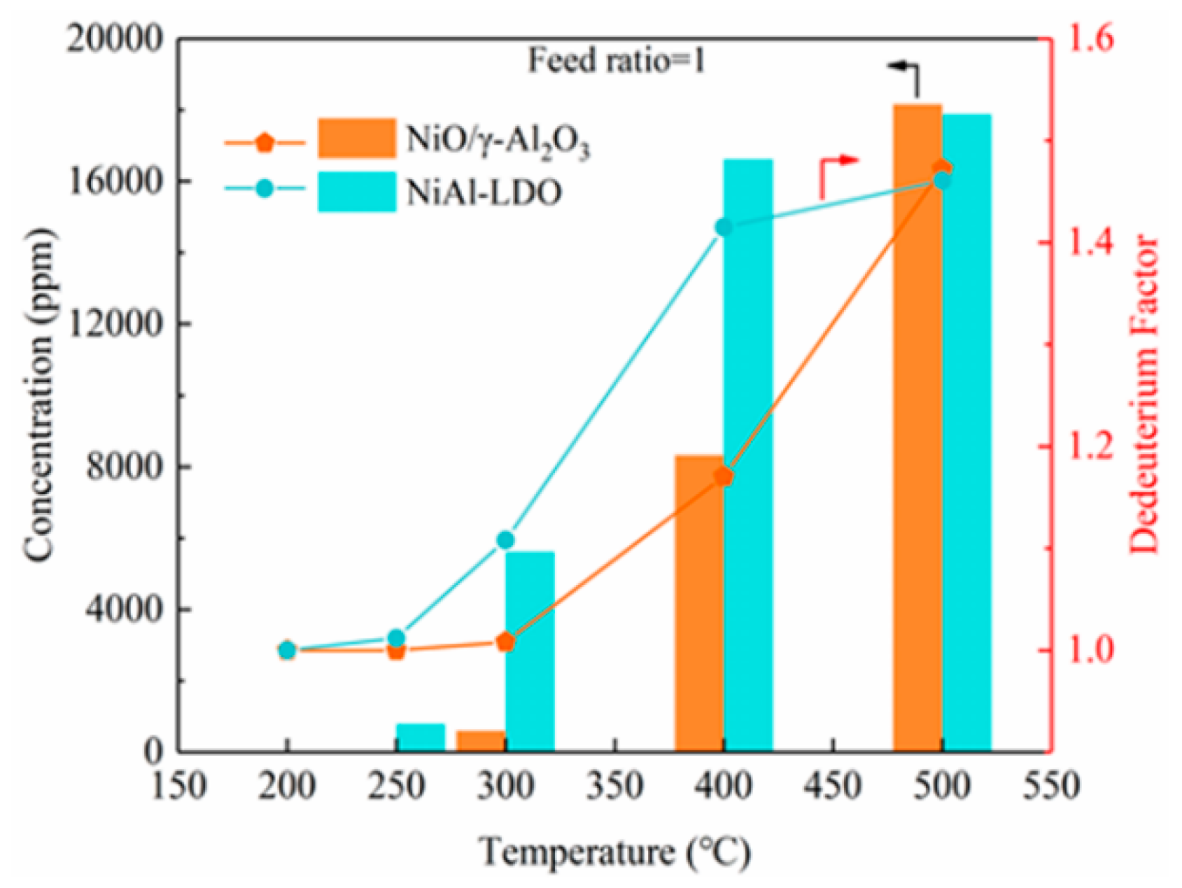
3.2. Catalytic Performance on Hydrogen Isotope Exchange
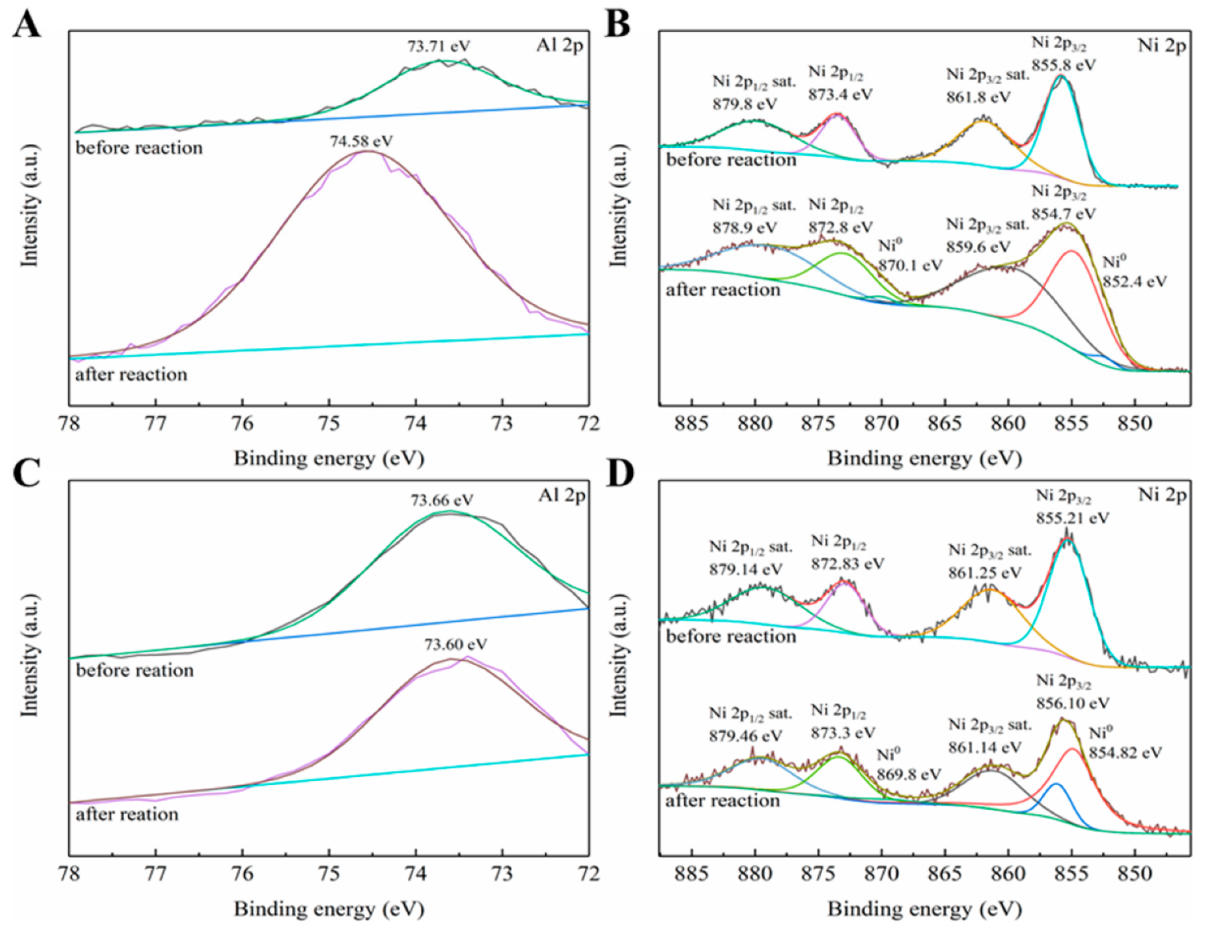
3.3. Relationship between Ni0 Species and Catalytic Activity
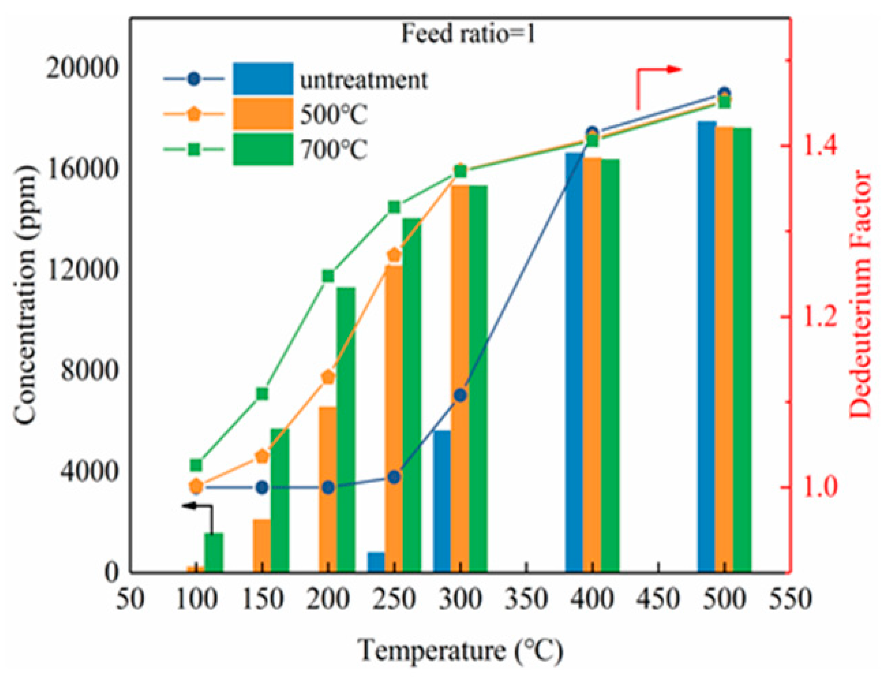
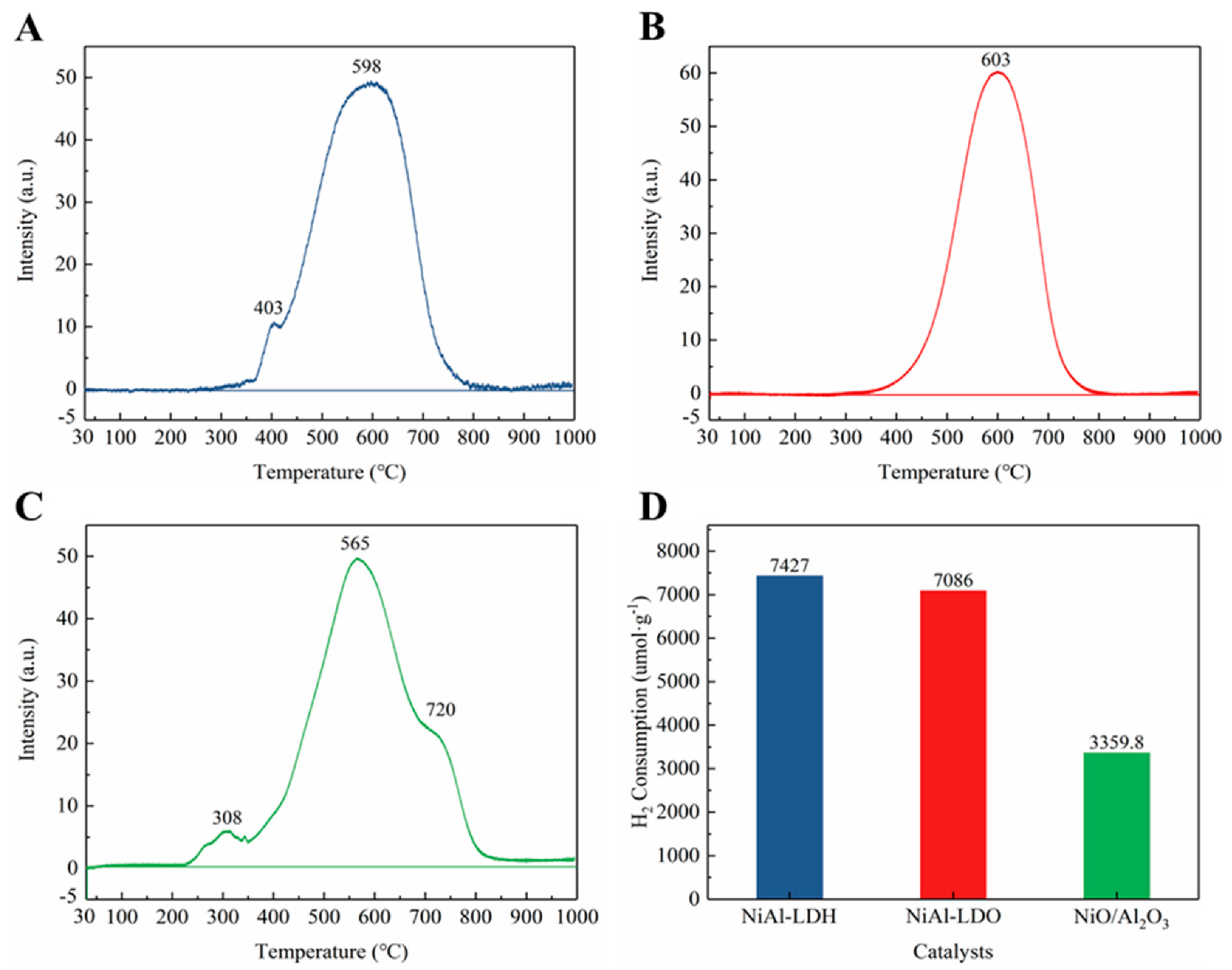
3.4. Reduction Property for Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Perevezentsev, A.N.; Bell, A.C.; Williams, J.; Brennan, P.D. Detritiation studies for JET decommissioning. Fusion Eng. Des. 2008, 83, 1364–1367. [Google Scholar] [CrossRef]
- Zhou, J.B.; Liu, Y.; Wang, C. Dynamic equations of tritium concentration during wastewater (light water) containing tritium electrolysis. Int. J. Hydrogen Energy 2016, 41, 16014–16018. [Google Scholar] [CrossRef]
- Bornea, A.; Petrutiu, C.; Zamfirache, M. Complex software dedicated for design and simulation of LPCE process for heavy water detritiation. Fusion Sci. Technol. 2015, 67, 270–273. [Google Scholar] [CrossRef]
- Zhang, D.H.; Zhou, L.; Su, W.; Sun, Y. Equilibrium modeling for hydrogen isotope separation by cryogenic adsorption. Chin. J. Chem. Eng. 2006, 14, 526–531. [Google Scholar] [CrossRef]
- Elnour, F. Reactivation of promoted nickel-catalysts for deuterium-exchange between hydrogen and water vapor-phase. Isotopes Environ. Health Stud. 1980, 16, 370–372. [Google Scholar] [CrossRef]
- Abouelnour, F.H.; Belacy, A. Binary supported nickel catalysts for the deuterium exchange reaction between hydrogen and water vapour. Isotopes Environ. Health. Stud. 1980, 18, 131–132. [Google Scholar] [CrossRef]
- Sagert, N.H.; Shawwood, P.E.; Pouteau, R.M.L. Hydrogen–Water Deuterium Exchange Over Metal Oxide Promoted Nickel Catalysts. Can. J. Chem. 1975, 53, 3257–3262. [Google Scholar] [CrossRef]
- Kalyanam, K.M.; Sood, S.K. A Comparison of Process Characteristics for the Recovery of Tritium from Heavy Water and Light Water Systems. Fusion Sci. Technol. 2017, 14, 524–528. [Google Scholar] [CrossRef]
- Ovcharov, A.V. More Precise Values of Separation Factors in Water-Hydrogen Isotopic Exchange for Modeling of Combined Electrolysis and Catalytic Exchange Process. Fusion Sci. Technol. 2017, 71, 333–338. [Google Scholar] [CrossRef]
- Hu, S.; Hou, J.W.; Xiong, L.P.; Weng, K.P.; Ren, X.B.; Luo, Y.M. Preparation and characterization of hydrophobic Pt-Fe catalysts with enhanced catalytic activities for interface hydrogen isotope separation. J. Hazard. Mater. 2012, 209, 478–483. [Google Scholar] [CrossRef]
- Hu, S.; Xiong, L.P.; Ren, X.B.; Wang, C.B.; Luo, Y.M. Pt-Ir binary hydrophobic catalysts: Effects of Ir content and particle size on catalytic performance for liquid phase catalytic exchange. Int. J. Hydrogen Energy 2009, 34, 8723–8732. [Google Scholar] [CrossRef]
- Hui, C.L.; Li, X.G.; Hsing, I.M. Well-dispersed surfactant-stabilized Pt/C nanocatalysts for fuel cell application: Dispersion control and surfactant removal. Electrochim. Acta 2005, 51, 711–719. [Google Scholar] [CrossRef]
- Ye, L.S.; Luo, D.L.; Yang, W.; Guo, W.S.; Xu, Q.Y.; Jiang, C.L. Improved catalysts for hydrogen/deuterium exchange reactions. Int. J. Hydrogen Energy 2013, 38, 13596–13603. [Google Scholar] [CrossRef]
- He, J.C.; Wang, H.Y.; Xiao, C.J.; Li, J.M.; Chen, P.; Hou, J.W. Preparation and performance of Pt/PTFE/Foam SiC as a hydrophobic catalyst for LPCE. Fusion Eng. Des. 2016, 113, 269–274. [Google Scholar] [CrossRef]
- Ye, L.S.; Luo, D.L.; Yang, W.; Guo, W.S.; Xu, Q.Y.; Luo, L.Z. Preparation and characterization of hydrophobic carbon-supported Pt3M (M = Fe, Co, Ni and Cr) bimetals for H/D isotope separation between hydrogen and water. Int. J. Hydrogen Energy 2014, 39, 13793–13799. [Google Scholar] [CrossRef]
- Davidson, R.B.; Vonhatten, P.; Schaub, M.; Ulrich, D. Commissioning and 1st operating experience at Darlington tritium removal facility. Fusion Technol. 2017, 14, 472–479. [Google Scholar] [CrossRef]
- Pautrot, G.P. The tritium extraction facility at the institute-laue-langevin experience of operation with tritium. Fusion Sci. Technol. 2017, 14, 480–483. [Google Scholar] [CrossRef]
- Sood, S.K.; Sissingh, R.A.P.; Kveton, O.K. Removal and Immobilization of Tritium from Ontario Hydro’s Nuclear Generating Stations. Fusion Technol. 2017, 8, 2478–2485. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Tian, Y.; Zhang, L.J.; Hu, S.; Xiang, J.; Wang, Y.; Xu, L.L.; Liu, Q.; Zhang, S.; Hu, X. Impacts of nickel loading on properties, catalytic behaviors of Ni/gamma-Al2O3 catalysts and the reaction intermediates formed in methanation of CO2. Int. J. Hydrogen Energy 2019, 44, 9291–9306. [Google Scholar] [CrossRef]
- Feng, J.T.; Lin, Y.J.; Evans, D.G.; Duan, X.; Li, D.Q. Enhanced metal dispersion and hydrodechlorination properties of a Ni/Al2O3 catalyst derived from layered double hydroxides. J. Catal. 2009, 266, 351–358. [Google Scholar] [CrossRef]
- Guo, Z.L.; Zheng, J.E.; Liu, Y.; Chu, W. Insight into the role of metal/oxide interaction and Ni availabilities on NiAl mixed metal oxide catalysts for methane decomposition. Appl. Catal. A-GEN. 2018, 555, 1–11. [Google Scholar] [CrossRef]
- David, G.E.; Robert, C.T.S. Structural Aspects of Layered Double Hydroxides. Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–87. [Google Scholar]
- Feng, J.T.; Ma, X.Y.; He, Y.F.; Evans, D.G.; Li, D.Q. Synthesis of hydrotalcite-supported shape-controlled Pd nanoparticles by a precipitation-reduction method. Appl. Catal. A-Gen. 2011, 413, 10–20. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.F.; Zhao, Y.X.; Shi, R.; Waterhouse, G.I.N.; Zhang, T.R. A Simple Synthetic Strategy toward Defect-Rich Porous Monolayer NiFe-Layered Double Hydroxide Nanosheets for Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2019, 9, 6. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.Y.; Qu, Z.P.; Zhao, Q.D.; Liu, S.M.; Hu, X.J. The NiAl mixed oxides: The relation between basicity and SO2 removal capacity. Sep. Purif. Technol. 2011, 80, 345–350. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Li, J.; Chen, F.P.; Jin, G.P.; Feng, X.S.; Li, X.X. Removals of aqueous sulfur dioxide and hydrogen sulfide using CeO2-NiAl-LDHs coating activated carbon and its mix with carbon nano-tubes. Colloid Surf. A 2015, 476, 90–97. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Wang, D.L.; Yang, X.Y.; Liu, S.; Liu, D.; Liu, J.; Xiao, H.D.; Hao, X.T.; Liu, J.Q. Investigation of the dye-sensitized solar cell designed by a series of mixed metal oxides based on ZnAl-layered double hydroxide. Appl. Phys. A 2017, 123, 10. [Google Scholar] [CrossRef]
- Li, P.L.; Guo, L.; Xiong, R.J.; Wen, M.; Yao, Y.; Zhang, Z.; Song, J.F.; Shi, Y.; Tang, T. Separation process study of liquid phase catalytic exchange reaction based on the Pt/C/PTFE catalysts. Chin. J. Chem. Eng. 2019, 27, 1837–1845. [Google Scholar] [CrossRef]
- Kovanda, F.; Rojka, T.; Bezdicka, P.; Jiratova, K.; Obalova, L.; Pacultova, K.; Bastl, Z.; Grygar, T. Effect of hydrothermal treatment on properties of Ni-Al layered double hydroxides and related mixed oxides. J. Solid State Chem. 2008, 182, 27–36. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhang, F.Z.; Fu, S.S.; Duan, X. In situ microstructure control of oriented layered double hydroxide monolayer films with curved hexagonal crystals as superhydrophobic materials. Adv. Mater. 2006, 18, 23. [Google Scholar] [CrossRef]
- Rudolf, C.; Dragoi, B.; Ungureanu, A.; Chirieac, A.; Royer, S.; Nastro, A.; Dumitriu, E. NiAl and CoAl materials derived from takovite-like LDHs and related structures as efficient chemoselective hydrogenation catalysts. Catal. Sci. Technol. 2014, 4, 179–189. [Google Scholar] [CrossRef]
- Li, L.; Hui, K.S.; Hui, K.N.; Xia, Q.X.; Fu, J.J.; Cho, Y.R. Facile synthesis of NiAl layered double hydroxide nanoplates for high-performance asymmetric supercapacitor. J. Alloy Compd. 2017, 721, 803–812. [Google Scholar] [CrossRef]
- Parida, K.M.; Pradhan, A.C.; Das, J.; Sahu, N. Synthesis and characterization of nano-sized porous gamma-alumina by control precipitation method. Mater. Chem. Phys. 2008, 113, 244–248. [Google Scholar] [CrossRef]
- Wang, Z.C.; Fang, P.F.; Kumar, P.; Wang, W.W.; Liu, B.; Li, J. Controlled Growth of LDH Films with Enhanced Photocatalytic Activity in a Mixed Wastewater Treatment. Nanomaterials 2019, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hui, K.S.; Hui, K.N.; Cho, Y.R. Ultrathin petal-like NiAl layered double oxide/sulfide composites as an advanced electrode for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 19687–19696. [Google Scholar] [CrossRef]
- Abdelsadek, Z.; Sehailia, M.; Halliche, D.; Gonzalez-Delacruz, V.M.; Holgado, J.P.; Bachari, K.; Caballero, A.; Cherifi, O. In-situ hydrogasification/regeneration of NiAl-hydrotalcite derived catalyst in the reaction of CO2 reforming of methane: A versatile approach to catalyst recycling. J. CO2 Util. 2016, 14, 98–105. [Google Scholar] [CrossRef]
- Pang, H.; Wei, C.Z.; Li, X.X.; Li, G.C.; Ma, Y.H.; Li, S.J.; Chen, J.; Zhang, J.S. Microwave-assisted synthesis of NiS2 nanostructures for supercapacitors and cocatalytic enhancing photocatalytic H2 production. Sci. Rep. 2014. [Google Scholar] [CrossRef]
- Yang, L.J.; Zhao, X.C.; Yang, R.Z.; Zhao, P.X.; Li, Y.T.; Yang, P.; Wang, J.C.; Astruc, D. In-situ growth of carbon nanotubes on Ni/NiO nanofibers as efficient hydrogen evolution reaction catalysts in alkaline media. Appl. Surf. Sci. 2019, 491, 294–300. [Google Scholar] [CrossRef]
- Del Arco, M.; Malet, P.; Trujillano, R.; Rives, V. Synthesis and characterization of hydrotalcites containing Ni(II) and Fe(III) and their calcination products. Chem. Mater. 1999, 11, 624–633. [Google Scholar] [CrossRef]
- Cheng, D.D.; Wang, Z.L.; Xia, Y.Y.; Wang, Y.M.; Zhang, W.X.; Zhu, W.C. Catalytic amination of diethylene glycol with tertiarybutylamine over Ni-Al2O3 catalysts with different Ni/Al ratios. RSC Adv. 2016, 6, 102373–102380. [Google Scholar] [CrossRef]
- Heracleous, E.; Lee, A.F.; Wilson, K.; Lemonidou, A.A. Investigation of Ni-based alumina-supported catalysts for the oxidative dehydrogenation of ethane to ethylene: Structural characterization and reactivity studies. J. Catal. 2005, 231, 159–171. [Google Scholar] [CrossRef]
- Hundt, P.M.; Jiang, B.; van Reijzen, M.E.; Guo, H.; Beck, R.D. Vibrationally Promoted Dissociation of Water on Ni(111). Science 2014, 355, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Hayward, D.O.; Taylor, A.O. On the translationally activated dissociative chemisorption of hydrogen and deuterium at a nickel (111) surface. Chem. Phys. Lett. 1988, 146, 221–226. [Google Scholar] [CrossRef]
- Liu, H.Y.; Li, K.; Zhang, R.G.; Ling, L.X.; Wang, B.J. Insight into the influence of addition of a second metal Fe and supports with different morphology on H2 dissociation over Ni/MgO catalysts. Appl. Surf. Sci. 2017, 426, 827–832. [Google Scholar] [CrossRef]
- Yang, H.; Whitten, J.L. Dissociative adsorption of H2 on Ni (111). J. Chem. Phys. 1993, 98, 5039–5049. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Li, P.; Zhang, X.; Yu, B.; Lv, C.; Zeng, N.; Luo, J.; Zhang, Z.; Song, J.; Liu, Y. Ni-Based Catalyst Derived from NiAl Layered Double Hydroxide for Vapor Phase Catalytic Exchange between Hydrogen and Water. Nanomaterials 2019, 9, 1688. https://doi.org/10.3390/nano9121688
Hu X, Li P, Zhang X, Yu B, Lv C, Zeng N, Luo J, Zhang Z, Song J, Liu Y. Ni-Based Catalyst Derived from NiAl Layered Double Hydroxide for Vapor Phase Catalytic Exchange between Hydrogen and Water. Nanomaterials. 2019; 9(12):1688. https://doi.org/10.3390/nano9121688
Chicago/Turabian StyleHu, Xiaoyu, Peilong Li, Xin Zhang, Bin Yu, Chao Lv, Ning Zeng, Junhong Luo, Zhi Zhang, Jiangfeng Song, and Yong Liu. 2019. "Ni-Based Catalyst Derived from NiAl Layered Double Hydroxide for Vapor Phase Catalytic Exchange between Hydrogen and Water" Nanomaterials 9, no. 12: 1688. https://doi.org/10.3390/nano9121688
APA StyleHu, X., Li, P., Zhang, X., Yu, B., Lv, C., Zeng, N., Luo, J., Zhang, Z., Song, J., & Liu, Y. (2019). Ni-Based Catalyst Derived from NiAl Layered Double Hydroxide for Vapor Phase Catalytic Exchange between Hydrogen and Water. Nanomaterials, 9(12), 1688. https://doi.org/10.3390/nano9121688