Single-Walled Carbon Nanotube-Assisted Antibiotic Delivery and Imaging in S. epidermidis Strains Addressing Antibiotic Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Dispersion of SWCNT in Antibiotic Solutions
2.2. Characterization of SWCNT-Antibiotic Dispersions
2.3. Disk Diffusion Assay
2.4. Colony Count Assay
2.5. MIC (Minimal Inhibitory Concentration) Turbidity Assay
2.6. Cytotoxicity Assay
2.7. Microscopy
3. Results and Discussion
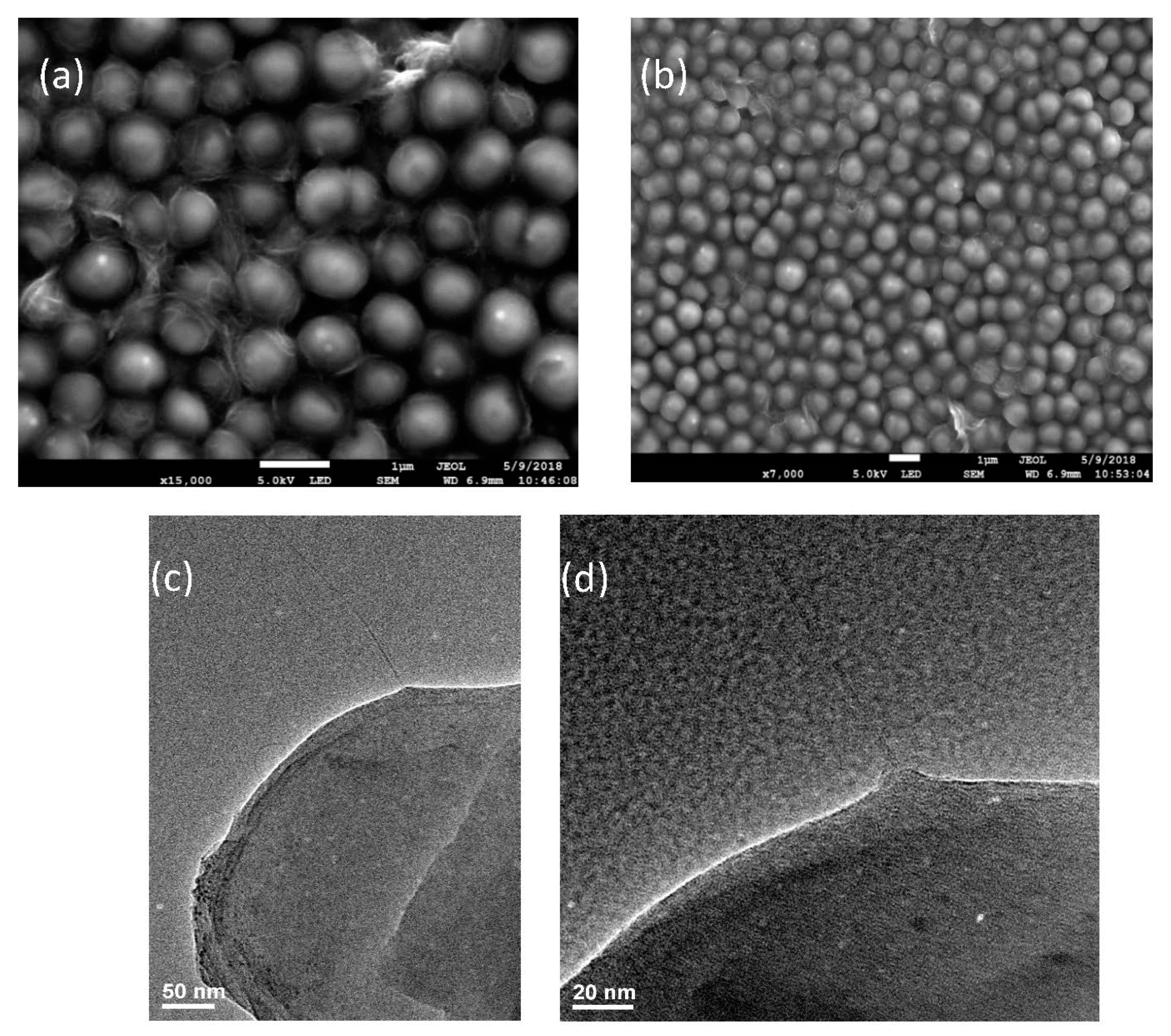
3.1. Characterization
3.2. Antibacterial Performance of SWCNT-Antibiotic Dispersions
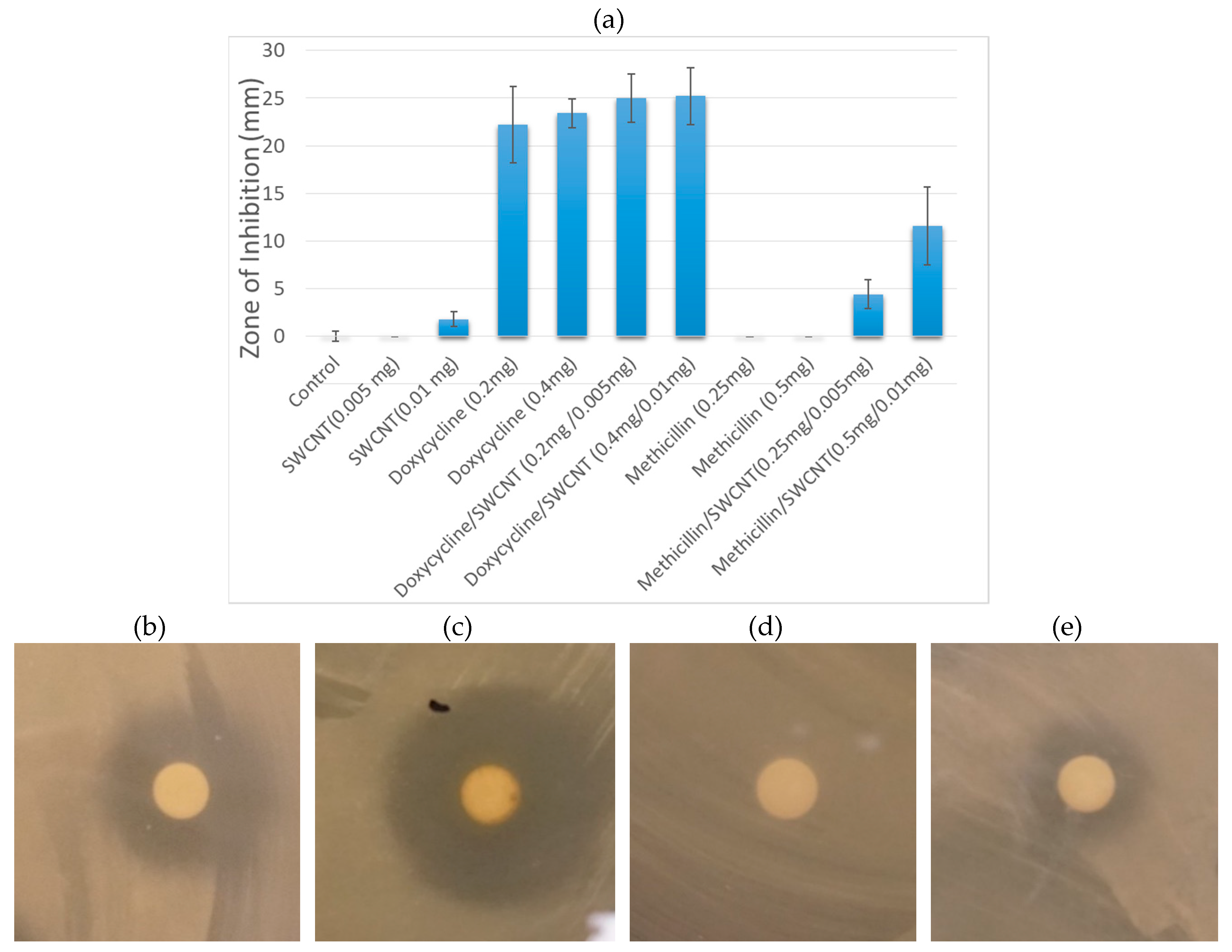
3.2.1. Zone Inhibition Assay
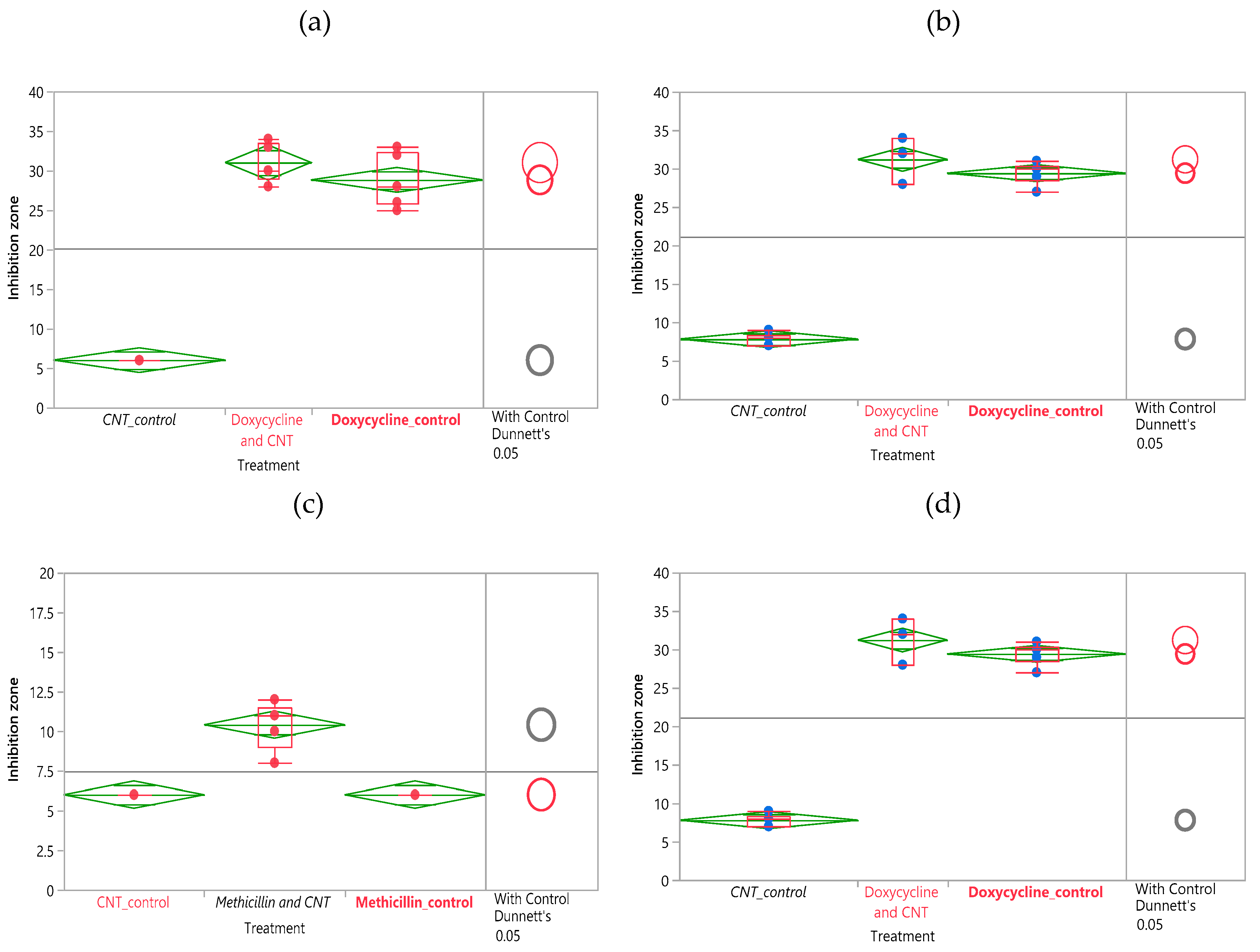
3.2.2. Statistical Analysis
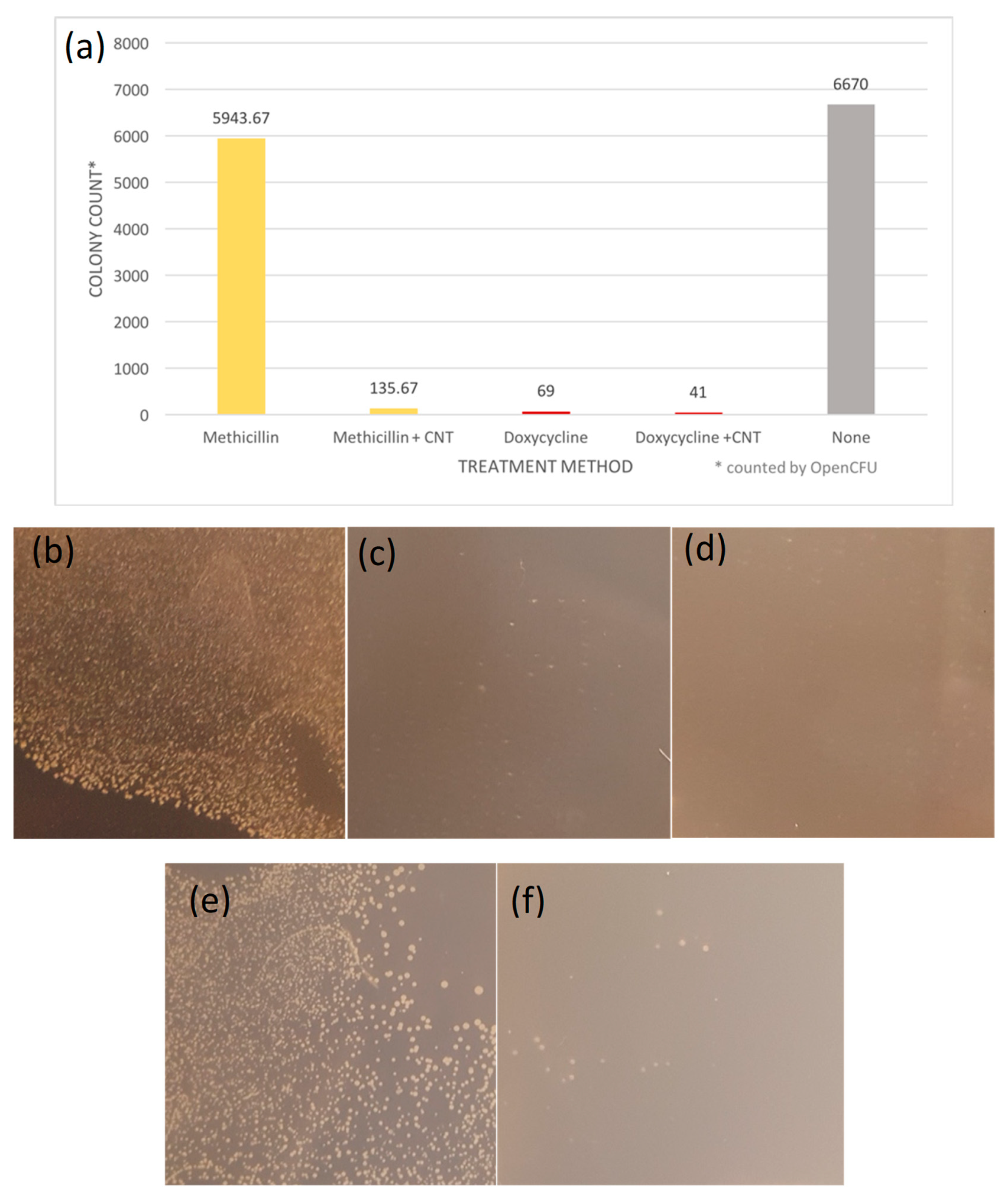
3.2.3. Colony Formation Assay
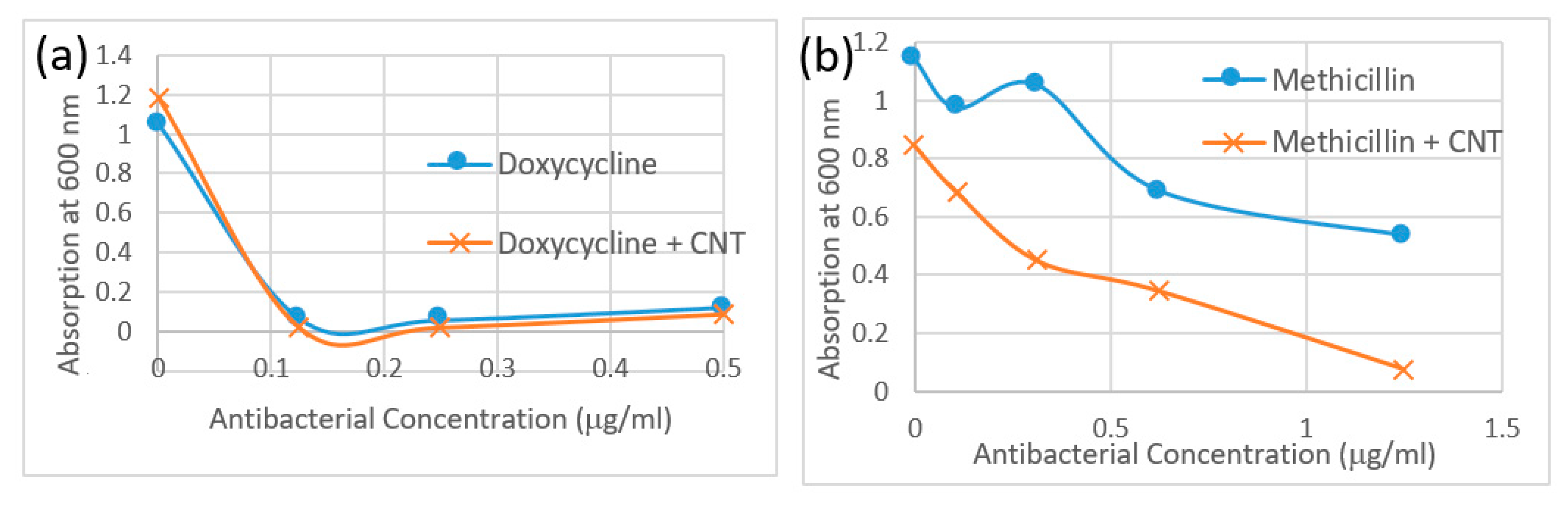
3.2.4. MIC turbidity Assay
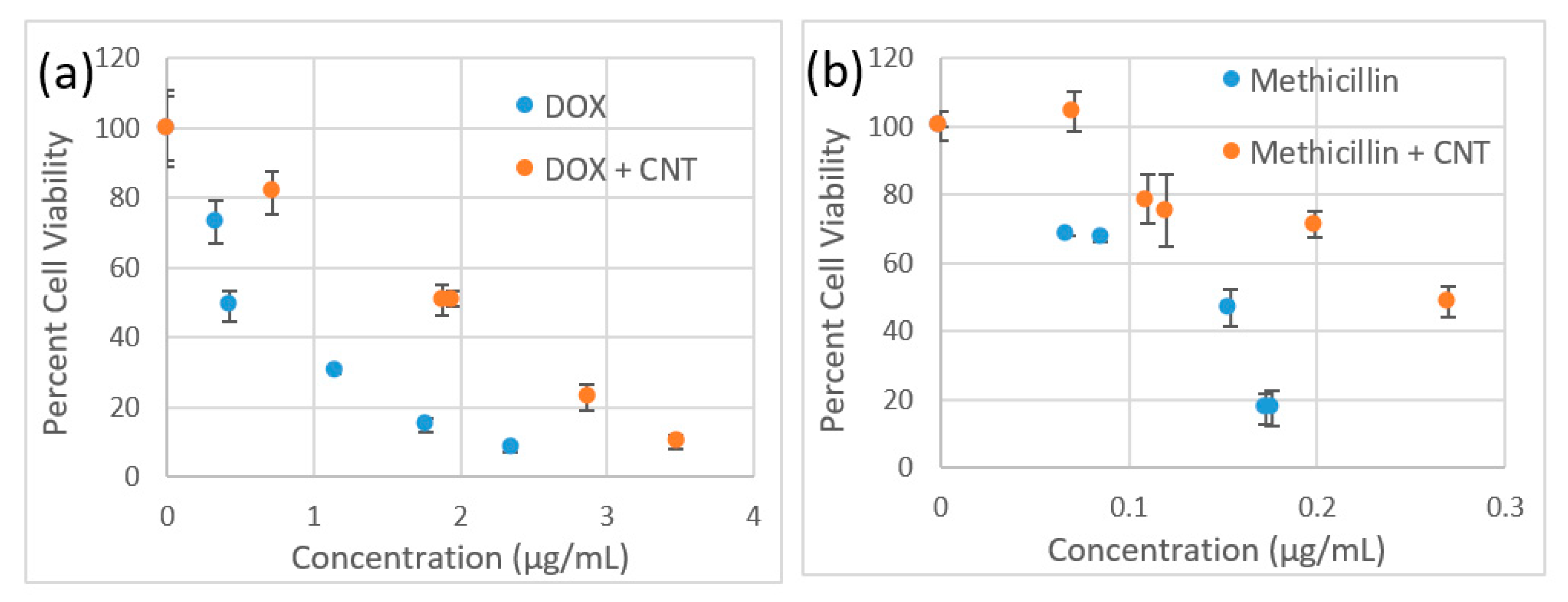
3.3. Cytotoxicity
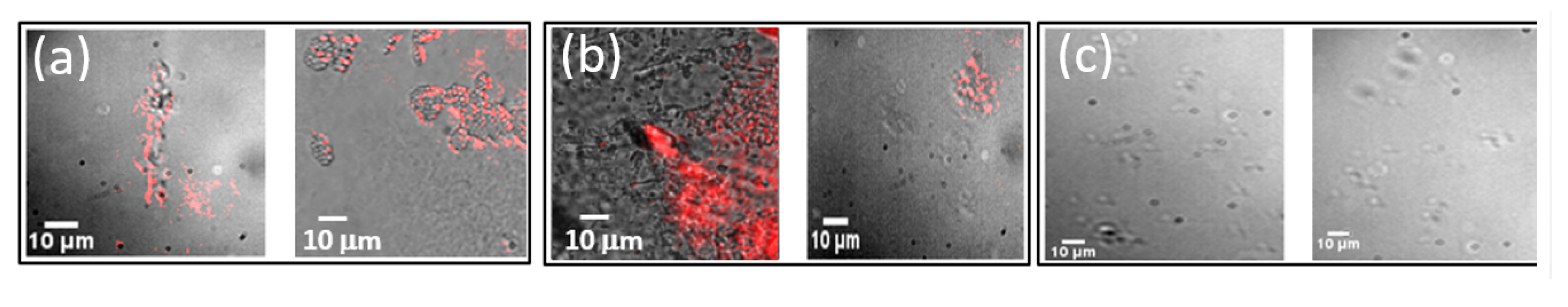
3.4. Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tom Frieden Antibiotic Resistance Threats in the United States; Centers for Diseases Control and Prevention: Atlanta, GA, USA, 2013; p. 114.
- Roberts, J.C.; Krueger, R.L.; Peak, K.K.; Veguilla, W.; Cannons, A.C.; Amuso, P.T.; Cattani, J. Community-Associated Methicillin-Resistant Staphylococcus Aureus Epidemic Clone Usa300 in Isolates from Florida and Washington. J. Clin. Microbiol. 2006, 44, 225–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, J.Y.; Nahm, M.H.; Moseley, M.A. Clinical Implications of Pneumococcal Serotypes: Invasive Disease Potential, Clinical Presentations, and Antibiotic Resistance. J. Korean Med. Sci. 2013, 28, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Hong, Z.; Gong, C.; Yan, D.; Liang, Z. Surgical Treatment Efficacy in 172 Cases of Tuberculosis-Destroyed Lungs. Eur. J. Cardio-Thorac. Surg. 2012, 41, 335–340. [Google Scholar] [CrossRef] [PubMed]
- O′Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Rev. Antimicrob. Resist 2014, 20, 1–16. [Google Scholar]
- Silver, L.L.; Bostian, K.A. Discovery and Development of New Antibiotics: The Problem of Antibiotic Resistance. Antimicrob. Agents Chemother. 1993, 37, 377–383. [Google Scholar] [CrossRef]
- Baquero, F. Gram-Positive Resistance: Challenge for the Development of New Antibiotics. J. Antimicrob. Chemother. 1997, 39 (Suppl. S1), 1–6. [Google Scholar] [CrossRef]
- Loomba, P.S.; Taneja, J.; Mishra, B. Methicillin and Vancomycin Resistant S. Aureus in Hospitalized Patients. J. Glob. Infect. Dis. 2010, 2, 275–283. [Google Scholar] [CrossRef]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Cirz, R.T.; Chin, J.K.; Andes, D.R.; de Crécy-Lagard, V.; Craig, W.A.; Romesberg, F.E. Inhibition of Mutation and Combating the Evolution of Antibiotic Resistance. PLoS Biol. 2005, 3, e176. [Google Scholar] [CrossRef]
- Tally, F.P.; DeBruin, M.F. Development of Daptomycin for Gram-Positive Infections. J. Antimicrob. Chemother. 2000, 46, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.D.; Brooks, A.E. Therapeutic Strategies to Combat Antibiotic Resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Abeylath, S.C.; Turos, E. Drug Delivery Approaches to Overcome Bacterial Resistance to Β-Lactam Antibiotics. Expert Opin. Drug Deliv. 2008, 5, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.W. Biofilms and Antibiotic Therapy: Is There a Role for Combating Bacterial Resistance by the Use of Novel Drug Delivery Systems? Adv. Drug Deliv. Rev. 2005, 57, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A New Antibiotic Kills Pathogens without Detectable Resistance. Nature 2015, 517, 455. [Google Scholar] [CrossRef]
- Finlay, A.C.; Hobby, G.L.; P′An, S.Y.; Regna, P.P.; Routien, J.B.; Seeley, D.B.; Shull, G.M.; Sobin, B.A.; Solomons, I.A.; Vinson, J.W.; et al. Terramycin, a New Antibiotic. Am. Assoc. Adv. Sci. 1950, 111, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Ruger, M.; Reagan, M.A.; Wolf, F.J.; Peck, R.L.; Wallick, H.; Woodruff, H.B. Discovery, Development, and Antimicrobial, Properties of D-4-Amino-3-Isoxazolidone (Oxamycin), a New Antibiotic Produced by Streptornyces Garyphalus N.sp. Antibiot. Chemother. 1955, 5, 183–190. [Google Scholar]
- Alekshun, M.N. New Advances in Antibiotic Development and Discovery. Expert Opin. Investig. Drugs 2005, 14, 117–134. [Google Scholar] [CrossRef]
- Olivi, M.; Zanni, E.; De Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D.; et al. Inhibition of Microbial Growth by Carbon Nanotube Networks. Nanoscale 2013, 5, 9023–9029. [Google Scholar] [CrossRef]
- Gill, S.R.; Fouts, D.E.; Archer, G.L.; Mongodin, E.F.; DeBoy, R.T.; Ravel, J.; Paulsen, I.T.; Kolonay, J.F.; Brinkac, L.; Beanan, M.; et al. Insights on Evolution of Virulence and Resistance from the Complete Genome Analysis of an Early Methicillin-Resistant Staphylococcus Aureus Strain and a Biofilm-Producing Methicillin-Resistant Staphylococcus Epidermidis Strain. J. Bacteriol. 2005, 187, 2426–2438. [Google Scholar] [CrossRef]
- Kamel Chaieb, T.Z. Olfa Chehab, Ons Bouchami, Assia Ben Hasen, Kacem Mahdouani and Amina Bakhrouf, Antibiotic Resistance Genes Detected by Multiplex Pcr Assays in Staphylococcus Epidermidis Strains Isolated from Dialysis Fluid and Needles in a Dialysis Service. Jpn. J. Infect. Dis. 2007, 60, 183–187. [Google Scholar]
- Parisi, J.T. Coagulase-Negative Staphylococci and the Epidemiological Typing of Staphylococcus Epidermidis. Microbiol. Rev. 1985, 49, 126–139. [Google Scholar] [PubMed]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S. Biocompatible Mesoporous and Soft Nanoarchitectures. J. Inorg. Organomet. Polym. Mater. 2015, 25, 214–232. [Google Scholar] [CrossRef]
- Angelov, B.; Garamus, V.M.; Drechsler, M.; Angelova, A. Structural Analysis of Nanoparticulate Carriers for Encapsulation of Macromolecular Drugs. J. Mol. Liq. 2017, 235, 83–89. [Google Scholar] [CrossRef]
- Angelova, A.; Fajolles, C.; Hocquelet, C.; Djedaïni-Pilard, F.; Lesieur, S.; Bonnet, V.; Perly, B.; Lebas, G.; Mauclaire, L. Physico-Chemical Investigation of Asymmetrical Peptidolipidyl-Cyclodextrins. J. Colloid Interface Sci. 2008, 322, 304–314. [Google Scholar] [CrossRef]
- Guerzoni, L.P.B.; Nicolas, V.; Angelova, A. In Vitro Modulation of Trkb Receptor Signaling Upon Sequential Delivery of Curcumin-Dha Loaded Carriers Towards Promoting Neuronal Survival. Pharm. Res. 2017, 34, 492–505. [Google Scholar] [CrossRef]
- Rakotoarisoa, M.; Angelova, A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines 2018, 5, 126. [Google Scholar] [CrossRef]
- Jin, H.; Heller, D.A.; Sharma, R.; Strano, M.S. Size-Dependent Cellular Uptake and Expulsion of Single-Walled Carbon Nanotubes: Single Particle Tracking and a Generic Uptake Model for Nanoparticles. ACS Nano 2009, 3, 149–158. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of Nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial Activity Ofcarbon-Based Nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar]
- Durham, E. Using Carbon Nanotubes for Drug Delivery. Nanomaterials News. 2016. Available online: https://phys.org/news/2016-05-carbon-nanotubes-drug-delivery.html (accessed on 9 September 2019).
- Hasan, T.M.; Campbell, E.; Sizova, O.; Lyle, V.; Akkaraju, G.; Kirkpatrick, L.D.; Naumov, V.A. Multi-Drug/Gene Nash Therapy Delivery and Selective Hyperspectral Nir Imaging Using Chirality-Sorted Single-Walled Carbon Nanotubes. Cancers 2019, 11, 1175. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, D.L.; Weiss, M.; Naumov, A.; Bartholomeusz, G.; Weisman, R.B.; Gliko, O. Carbon Nanotubes: Solution for the Therapeutic Delivery of Sirna? Materials 2012, 5, 278–301. [Google Scholar] [CrossRef] [PubMed]
- Podesta, J.E.; Al-Jamal, K.T.; Herrero, M.A.; Tian, B.; Ali-Boucetta, H.; Hegde, V.; Bianco, A.; Prato, M.; Kostarelos, K. Antitumor Activity and Prolonged Survival by Carbon-Nanotube-Mediated Therapeutic Sirna Silencing in a Human Lung Xenograft Model. Small 2009, 5, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Hanene Ali-Boucetta, K.T.A.-J.; McCarthy, D.; Prato, M.; Bianco, A.; Kostarelos, K. Multiwalled Carbon Nanotube—Doxorubicin Supramolecular Complexes for Cancer Therapeutics. Chem. Commun. 2008, 4, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Cristian Samorì, H.A.-B.; Sainz, R.; Guo, C.; Toma, F.M.; Fabbro, C.; da Ros, T.; Prato, M.; Kostarelos, K.; Bianco, A. Enhanced Anticancer Activity of Multi-Walled Carbon Nanotube—Methotrexate Conjugates Using Cleavable Linkers. Chem. Commun. 2010, 46, 1494–1496. [Google Scholar] [CrossRef]
- Feazell, R.P.; Nakayama-Ratchford, N.; Dai, H.; Lippard, S.J. Soluble Single-Walled Carbon Nanotubes as Longboat Delivery Systems for Platinum(Iv) Anticancer Drug Design. J. Am. Chem. Soc. 2007, 129, 8438–8439. [Google Scholar] [CrossRef]
- Liu, Z.; Winters, M.; Holodniy, M.; Dai, H. Sirna Delivery into Human T-Cells and Primary Cells with Carbon-Nanotube Transporters. Angew. Chem. Int. Ed. 2007, 46, 2023–2027. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Han, S. Adsorption of Tetracycline from Aqueous Solutions onto Multi-Walled Carbon Nanotubes with Different Oxygen Contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of Antibiotic Ciprofloxacin on Carbon Nanotubes: Ph Dependence and Thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef]
- Cong, Q.; Yuan, X.; Qu, J. A Review on the Removal of Antibiotics by Carbon Nanotubes. Water Sci. Technol. 2013, 68, 1679. [Google Scholar] [CrossRef]
- Assali, M.; Zaid, A.N.; Abdallah, F.; Almasri, M.; Khayyat, R. Single-Walled Carbon Nanotubes-Ciprofloxacin Nanoantibiotic: Strategy to Improve Ciprofloxacin Antibacterial Activity. Int. J. Nanomed. 2017, 12, 6647–6659. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Nguyen, F.T.; Barone, P.W.; Heller, D.A.; Moll, A.E.; Patel, D.; Boppart, S.A.; Strano, M.S. Multimodal Biomedical Imaging with Asymmetric Single-Walled Carbon Nanotube/Iron Oxide Nanoparticle Complexes. Nano Lett. 2007, 7, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Robinson, J.T.; Yaghi, O.K.; Hong, G.; Diao, S.; Luong, R.; Dai, H. Ultra-Low Doses of Chirality Sorted (6,5) Carbon Nanotubes for Simultaneous Tumor Imaging and Photothermal Therapy. ACS Nano 2013, 7, 3644–3652. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Dai, H. In Vivo Fluorescence Imaging in the Second near-Infrared Window Using Carbon Nanotubes. In In Vivo Fluorescence Imaging: Methods and Protocols; Bai, M., Ed.; Springer: New York, NY, USA, 2016; pp. 167–181. [Google Scholar]
- Giorgia Pastorin, W.W.; SWieckowski, é.; Briand, J.; Kostarelos, K.; Prato, M.; Bianco, A. Double Functionalisation of Carbon Nanotubes for Multimodal Drug Delivery. Chem. Commun. 2006, 11, 1182–1184. [Google Scholar] [CrossRef]
- Dong, L.; Henderson, A.; Field, C. Antimicrobial Activity of Single-Walled Carbon Nanotubes Suspended in Different Surfactants. J. Nanotechnol. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Hombach, M.; Maurer, F.P.; Pfiffner, T.; Böttger, E.C.; Furrer, R. Standardization of Operator-Dependent Variables Affecting Precision and Accuracy of the Disk Diffusion Method for Antibiotic Susceptibility Testing. J. Clin. Microbiol. 2015, 53, 3864–3869. [Google Scholar] [CrossRef]
- Hallander, H.O.; Laurell, G. Identification of Cephalosporin-Resistant Staphylococcus Aureus with the Disc Diffusion Method. Antimicrob. Agents Chemother. 1972, 1, 422–426. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Taylor, P.W. Methicillin Resistance in Staphylococcus Aureus: Mechanisms and Modulation. Sci. Prog. 2002, 85 Pt 1, 57–72. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Otto, M. Improved Understanding of Factors Driving Methicillin-Resistant Staphylococcus Aureus Epidemic Waves. Clin. Epidemiol. 2013, 5, 205–217. [Google Scholar]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.-P.; Muller, S.; et al. Cellular Uptake of Functionalized Carbon Nanotubes Is Independent of Functional Group and Cell Type. Nat. Nanotechnol. 2007, 2, 108. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Liu, S.; Ng, A.K.; Xu, R.; Wei, J.; Tan, C.M.; Yang, Y.; Chen, Y. Antibacterial Action of Dispersed Single-Walled Carbon Nanotubes on Escherichia Coli and Bacillus Subtilis Investigated by Atomic Force Microscopy. Nanoscale 2010, 2, 2744–2750. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Pasquini, L.M.; Hashmi, S.M.; Sommer, T.J.; Elimelech, M.; Zimmerman, J.B. Impact of Surface Functionalization on Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. Environ. Sci. Technol. 2012, 46, 6297–6305. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon Nanotubes as Molecular Transporters for Walled Plant Cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef]
- Yang, C.; Mamouni, J.; Tang, Y.; Yang, L. Antimicrobial Activity of Single-Walled Carbon Nanotubes: Length Effect. Langmuir 2010, 26, 16013–16019. [Google Scholar] [CrossRef]
- Kim, H.-Y. Analysis of Variance (Anova) Comparing Means of More Than Two Groups. Restor. Dent. Endod. 2014, 39, 74–77. [Google Scholar] [CrossRef]
- Yehia, H.N.; Draper, R.K.; Mikoryak, C.; Walker, E.K.; Bajaj, P.; Musselman, I.H.; Daigrepont, M.C.; Dieckmann, G.R.; Pantano, P. Single-Walled Carbon Nanotube Interactions with Hela Cells. J. Nanobiotechnol. 2007, 5, 8. [Google Scholar] [CrossRef]
- Yang, K.; Qin, W.; Tang, H.; Tan, L.; Xie, Q.; Ma, M.; Zhang, Y.; Yao, S. Polyamidoamine Dendrimer-Functionalized Carbon Nanotubes-Mediated Gfp Gene Transfection for Hela Cells: Effects of Different Types of Carbon Nanotubes. J. Biomed. Mater. Res. Part A 2011, 99, 231–239. [Google Scholar] [CrossRef]
- Gu, Y.-J.; Cheng, J.; Jin, J.; Cheng, S.H.; Wong, W.-T. Development and Evaluation of Ph-Responsive Single-Walled Carbon Nanotube-Doxorubicin Complexes in Cancer Cells. Int.J. Nanomed. 2011, 6, 2889–2898. [Google Scholar]
- Gangupomu, V.K.; Capaldi, F.M. Interactions of Carbon Nanotube with Lipid Bilayer Membranes. J. Nanomater. 2011, 2011, 6. [Google Scholar] [CrossRef]
- Montanari, M.P.; Massidda, O.; Mingoia, M.; Varaldo, P.E. Borderline Susceptibility to Methicillin in Staphylococcus Aureus: A New Mechanism of Resistance? Microb. Drug Resist. 1996, 2, 257–260. [Google Scholar] [CrossRef]
- Gaisford, W.C.; Reynolds, P.E. Methicillin Resistance in Staphylococcus Epidermidis. Eur.J. Biochem. 1989, 185, 211–218. [Google Scholar] [CrossRef]
- Komatsuzawa, H.; Suzuki, J.; Sugai, M.; Miyake, Y.; Suginaka, H. The Effect of Triton X-100 on the in-Vitro Susceptibility of Methicillin-Resistant Staphylococcus Aureus to Oxacillin. J. Antimicrob. Chemother. 1994, 34, 885–897. [Google Scholar] [CrossRef]
- Kavosi, A.; Hosseini Ghale Noei, S.; Madani, S.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Mirzaei, M.; Kalhori, M.R.; Yavarian, M.; Alizadeh, A.M.; et al. The Toxicity and Therapeutic Effects of Single-and Multi-Wall Carbon Nanotubes on Mice Breast Cancer. Sci. Rep. 2018, 8, 8375. [Google Scholar] [CrossRef]
- Dong, L.; Joseph, K.L.; Witkowski, C.M.; Craig, M.M. Cytotoxicity of Single-Walled Carbon Nanotubes Suspended in Various Surfactants. Nanotechnology 2008, 19, 255702. [Google Scholar] [CrossRef]
- Tabet, L.; Bussy, C.; Setyan, A.; Simon-Deckers, A.; Rossi, M.J.; Boczkowski, J.; Lanone, S. Coating Carbon Nanotubes with a Polystyrene-Based Polymer Protects against Pulmonary Toxicity. Part. Fibre Toxicol. 2011, 8, 3. [Google Scholar] [CrossRef]
- Doan, B.-T.; Seguin, J.; Breton, M.; Beherec, R.L.; Bessodes, M.; Rodríguez-Manzo, J.A.; Banhart, F.; Beloeil, J.-C.; Scherman, D.; Richard, C. Functionalized Single-Walled Carbon Nanotubes Containing Traces of Iron as New Negative Mri Contrast Agents for in Vivo Imaging. Contrast Media Mol. Imaging 2012, 7, 153–159. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of Toxicity Studies of Carbon Nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- Fujita, K.; Fukuda, M.; Endoh, S.; Maru, J.; Kato, H.; Nakamura, A.; Shinohara, N.; Uchino, K.; Honda, K. Size Effects of Single-Walled Carbon Nanotubes on in Vivo and in Vitro Pulmonary Toxicity. Inhal. Toxicol. 2015, 27, 207–223. [Google Scholar] [CrossRef]
- Mohanta, D.; Patnaik, S.; Sood, S.; Das, N. Carbon Nanotubes: Evaluation of Toxicity at Biointerfaces. J. Pharm. Anal. 2019, 9, 293–300. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazi-Syed, A.; Hasan, M.T.; Campbell, E.; Gonzalez-Rodriguez, R.; Naumov, A.V. Single-Walled Carbon Nanotube-Assisted Antibiotic Delivery and Imaging in S. epidermidis Strains Addressing Antibiotic Resistance. Nanomaterials 2019, 9, 1685. https://doi.org/10.3390/nano9121685
Khazi-Syed A, Hasan MT, Campbell E, Gonzalez-Rodriguez R, Naumov AV. Single-Walled Carbon Nanotube-Assisted Antibiotic Delivery and Imaging in S. epidermidis Strains Addressing Antibiotic Resistance. Nanomaterials. 2019; 9(12):1685. https://doi.org/10.3390/nano9121685
Chicago/Turabian StyleKhazi-Syed, Afeefah, Md Tanvir Hasan, Elizabeth Campbell, Roberto Gonzalez-Rodriguez, and Anton V. Naumov. 2019. "Single-Walled Carbon Nanotube-Assisted Antibiotic Delivery and Imaging in S. epidermidis Strains Addressing Antibiotic Resistance" Nanomaterials 9, no. 12: 1685. https://doi.org/10.3390/nano9121685
APA StyleKhazi-Syed, A., Hasan, M. T., Campbell, E., Gonzalez-Rodriguez, R., & Naumov, A. V. (2019). Single-Walled Carbon Nanotube-Assisted Antibiotic Delivery and Imaging in S. epidermidis Strains Addressing Antibiotic Resistance. Nanomaterials, 9(12), 1685. https://doi.org/10.3390/nano9121685




