Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Silver Nanosuspensions
2.2. Preparation of Different Media
2.3. pH and EC Measurement
2.4. Evaluation of the Stability
2.4.1. UV-VIS Spectroscopy
2.4.2. Transmission Electron Microscopy (TEM)
2.4.3. Dynamic Light Scattering (DLS)
3. Results
3.1. pH and EC
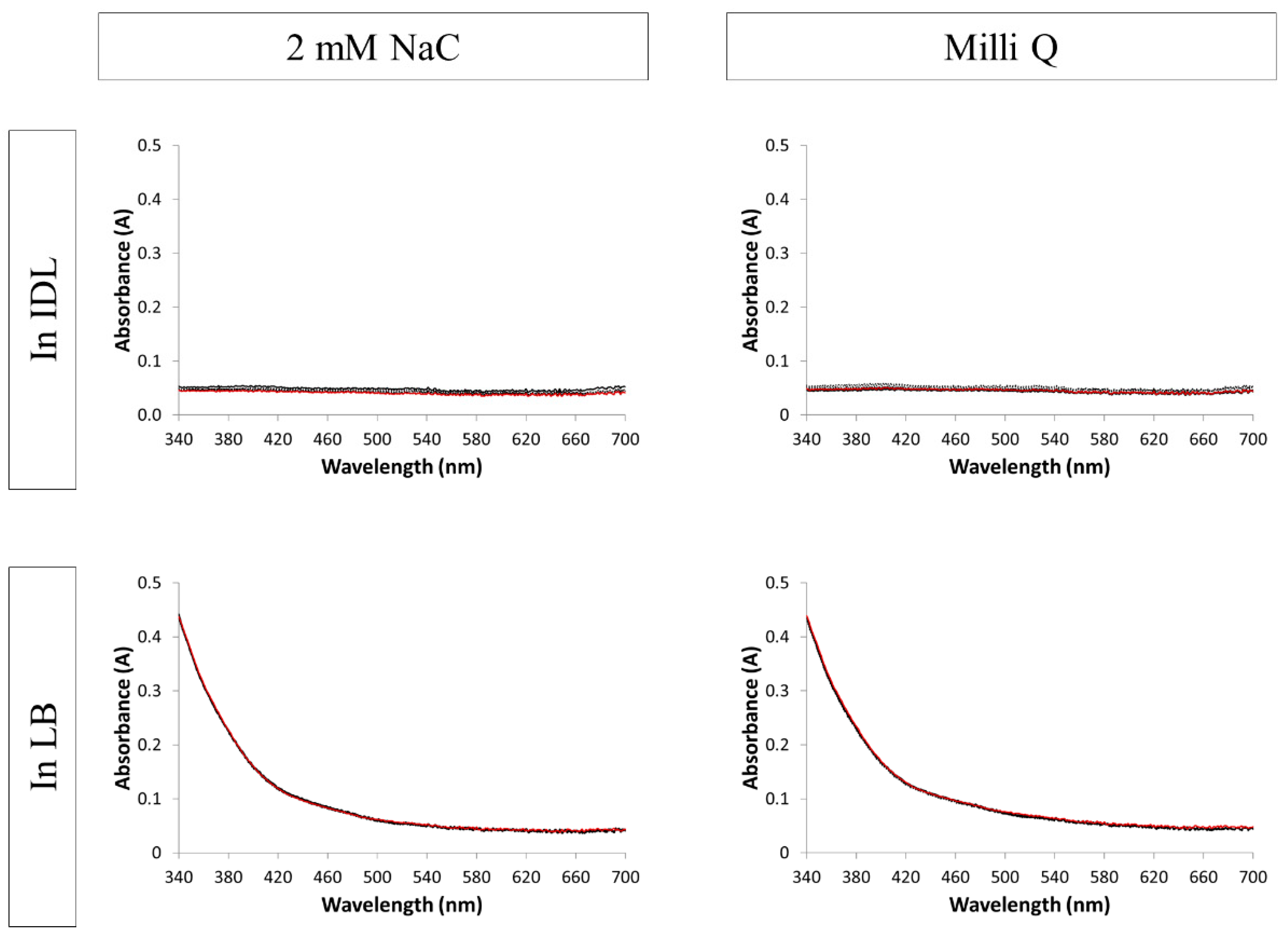
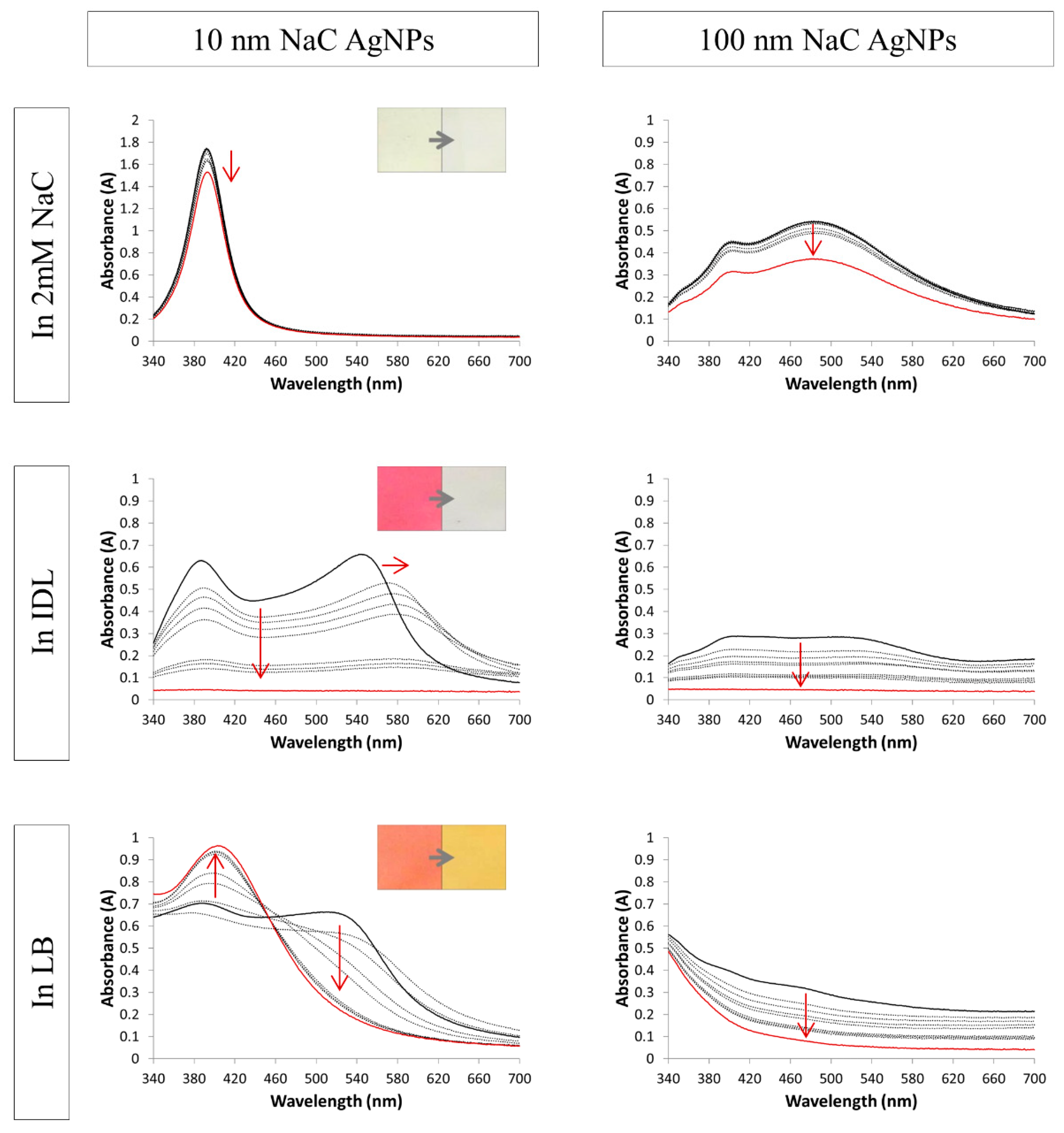
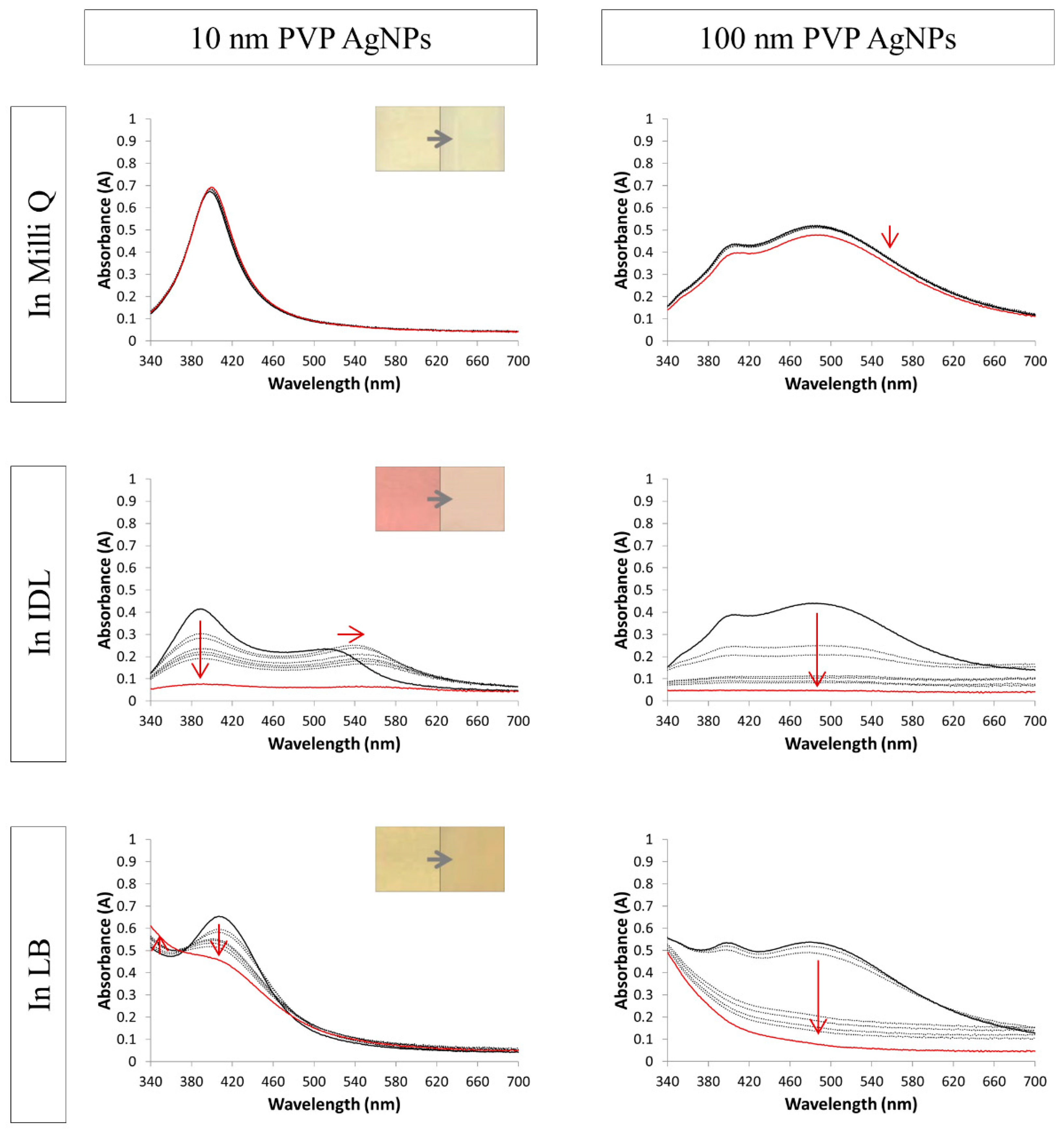
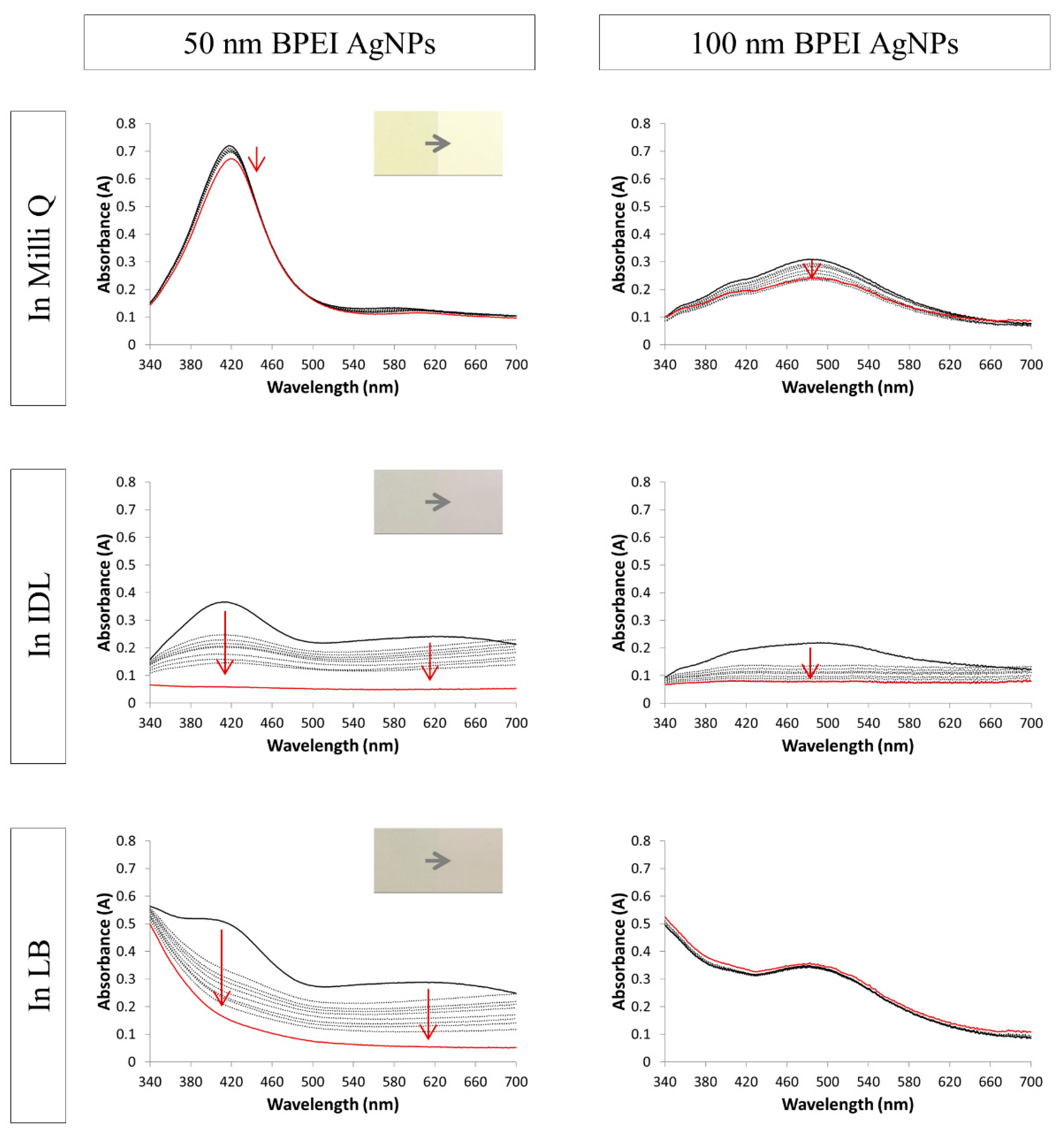
3.2. Evaluation of the Stability by UV-VIS Spectroscopy
3.3. Evaluation of the Stability by TEM
3.4. Evaluation of the Stability by DLS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calderón-Jiménez, B.; Johnson, M.E.; Bustos, A.R.M.; Murphy, K.E.; Winchester, M.R.; Baudrit, J.R.V. Silver nanoparticles: Technological advances, societal impacts, and metrological challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Stokłosa, K.; Banach, M. Nanosilver products and toxicity. Environ. Chem. Lett. 2015, 13, 59–68. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold nanoparticle-based colorimetric biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef]
- Instruments, M. Zeta potential: An Introduction in 30 minutes. Zetasizer Nano Serles Tech. Note Mrk654 2011, 1, 1–6. [Google Scholar]
- Van Vaerenbergh, B.; Lauwaert, J.; Thybaut, J.W.; Vermeir, P.; De Clercq, J. Pd nanoparticle and molecular Pd2+ leaching pathways for a strongly acid versus strongly basic resin supported Pd nanoparticle catalyst in Suzuki coupling. Chem. Eng. J. 2019, 374, 576–588. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Huang, H.; Lai, W.; Cui, M.; Liang, L.; Lin, Y.; Fang, Q.; Liu, Y.; Xie, L. An evaluation of blood compatibility of silver nanoparticles. Sci. Rep. 2016, 6, 25518. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Ault, A.P.; Stark, D.I.; Axson, J.L.; Keeney, J.N.; Maynard, A.D.; Bergin, I.L.; Philbert, M.A. Protein corona-induced modification of silver nanoparticle aggregation in simulated gastric fluid. Environ. Sci. Nano 2016, 3, 1510–1520. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 106–117. [Google Scholar] [CrossRef]
- Lacerda, S.H.D.P.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2009, 4, 365–379. [Google Scholar] [CrossRef]
- Szakal, C.; Roberts, S.M.; Westerhoff, P.; Bartholomaeus, A.; Buck, N.; Illuminato, I.; Canady, R.; Rogers, M. Measurement of nanomaterials in foods: Integrative consideration of challenges and future prospects. ACS Nano 2014, 8, 3128–3135. [Google Scholar] [CrossRef]
- Qiu, K.; Durham, P.G.; Anselmo, A.C. Inorganic nanoparticles and the microbiome. Nano Res. 2018, 11, 4936–4954. [Google Scholar] [CrossRef]
- Cross, R.K.; Tyler, C.; Galloway, T.S. Transformations that affect fate, form and bioavailability of inorganic nanoparticles in aquatic sediments. Environ. Chem. 2015, 12, 627–642. [Google Scholar] [CrossRef]
- Tran, Q.H.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.; Peijnenburg, W.J.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Influence of growth media components on the antibacterial effect of silver ions on Bacillus subtilis in a liquid growth medium. Sci. Rep. 2018, 8, 9325. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the anti-bacterial activity on the nanosilver shapes: Nanoparticles, nanorods and nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Hernandez-Moreno, D.; Catalan, J.; Cross, R.; Stockmann-Juvala, H.; Cabellos, J.; Lopes, V.R.; Matzke, M.; Ferraz, N.; Izquierdo, J.J. Quality evaluation of human and environmental toxicity studies performed with nanomaterials—The GUIDEnano approach. Environ. Sci. Nano 2018, 5. [Google Scholar] [CrossRef]
- Behra, R.; Sigg, L.; Clift, M.J.; Herzog, F.; Minghetti, M.; Johnston, B.; Petri-Fink, A.; Rothen-Rutishauser, B. Bioavailability of silver nanoparticles and ions: From a chemical and biochemical perspective. J. R. Soc. Interface 2013, 10, 20130396. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Deng, B. Silver nanoparticles in aquatic environments: Physiochemical behavior and antimicrobial mechanisms. Water Res. 2016, 88, 403–427. [Google Scholar] [CrossRef]
- Lodeiro, P.; Achterberg, E.P.; Pampín, J.; Affatati, A.; El-Shahawi, M.S. Silver nanoparticles coated with natural polysaccharides as models to study AgNP aggregation kinetics using UV-Visible spectrophotometry upon discharge in complex environments. Sci. Total Environ. 2016, 539, 7–16. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Grillo, R.; Rosa, A.H.; Fraceto, L.F. Engineered nanoparticles and organic matter: A review of the state-of-the-art. Chemosphere 2015, 119, 608–619. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Mousavi, M.P.; Chen, L.D.; Bühlmann, P.; Haynes, C.L. Characterization of silver ion dissolution from silver nanoparticles using fluorous-phase ion-selective electrodes and assessment of resultant toxicity to Shewanella oneidensis. Chem. Sci. 2013, 4, 2564–2572. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular cloning A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Desai, R.; Mankad, V.; Gupta, S.K.; Jha, P.K. Size distribution of silver nanoparticles: UV-visible spectroscopic assessment. Nanosci. Nanotechnol. Lett. 2012, 4, 30–34. [Google Scholar] [CrossRef]
- Hsu, C.W.; Zhen, B.; Qiu, W.; Shapira, O.; DeLacy, B.G.; Joannopoulos, J.D.; Soljačić, M. Transparent displays enabled by resonant nanoparticle scattering. Nat. Commun. 2014, 5, 3152. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Gold and silver nanoparticles: A class of chromophores with colors tunable in the range from 400 to 750 nm. Analyst 2003, 128, 686–691. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Nanometals: Formation and color. Mater. Today 2004, 7, 26–31. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Bastús, N.G.; Piella, J.; Puntes, V. Quantifying the sensitivity of multipolar (dipolar, quadrupolar, and octapolar) surface plasmon resonances in silver nanoparticles: The effect of size, composition, and surface coating. Langmuir 2015, 32, 290–300. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. Proc. Natl. Acad. Sci. USA 2010, 107, 14530–14534. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Size-controlled synthesis of nanoparticles. 2. Measurement of extinction, scattering, and absorption cross sections. J. Phys. Chem. B 2004, 108, 13957–13962. [Google Scholar] [CrossRef]
- Novak, J.P.; Feldheim, D.L. Assembly of phenylacetylene-bridged silver and gold nanoparticle arrays. J. Am. Chem. Soc. 2000, 122, 3979–3980. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Singh, P.; Bodycomb, J.; Travers, B.; Tatarkiewicz, K.; Travers, S.; Matyas, G.R.; Beck, Z. Particle size analyses of polydisperse liposome formulations with a novel multispectral advanced nanoparticle tracking technology. Int. J. Pharm. 2019, 566, 680–686. [Google Scholar] [CrossRef]
- The European Commission. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial (2011/696/EU). Off. J. Eur. Communities Legis 2011, 275, 38. Available online: https://ec.europa.eu/research/industrial_technologies/pdf/policy/commission-recommendation-on-the-definition-of-nanomater-18102011_en.pdf (accessed on 1 October 2019).
- ISO. ISO/TS 80004-1:2015 (Nanotechnologies—Vocabulary—Part 1: Core Terms). Available online: https://www.iso.org/obp/ui/#iso:std:iso:ts:80004:-1:ed-2:v1:en (accessed on 1 October 2019).
- Speed, D. Environmental aspects of planarization processes. In Advances in Chemical Mechanical Planarization (CMP); Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–269. [Google Scholar]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanoparticle Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Albanese, A.; Chan, W.C. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef]
- Shih, Y.H.; Liu, W.S.; Su, Y.F. Aggregation of stabilized TiO2 nanoparticle suspensions in the presence of inorganic ions. Environ. Toxicol. Chem. 2012, 31, 1693–1698. [Google Scholar] [CrossRef]
- Zahrim, A.; Azreen, I.; Jie, S.; Yoiying, C.; Felijia, J.; Hasmilah, H.; Gloriana, C. Nanoparticles Enhanced Coagulation of Biologically Digested Leachate. In Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–241. [Google Scholar]
- Dan, A.; Ghosh, S.; Moulik, S.P. The solution behavior of poly (vinylpyrrolidone): Its clouding in salt solution, solvation by water and isopropanol, and interaction with sodium dodecyl sulfate. J. Phys. Chem. B 2008, 112, 3617–3624. [Google Scholar] [CrossRef]
- Güner, A. Effects of inorganic salts on the spectral behavior of poly (N-vinyl-2-pyrrolidone) in aqueous solutions. J. Appl. Polym. Sci. 2000, 75, 1434–1439. [Google Scholar] [CrossRef]
- Güner, A. Properties of aqueous salt solutions of polyvinylpyrrolidone. I. Viscosity characteristics. J. Appl. Polym. Sci. 1996, 62, 785–788. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Huynh, K.A.; Chen, K.L. Aggregation kinetics of citrate and polyvinylpyrrolidone coated silver nanoparticles in monovalent and divalent electrolyte solutions. Environ. Sci. Technol. 2011, 45, 5564–5571. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Tanguler, H.; Erten, H. Utilisation of spent brewer’s yeast for yeast extract production by autolysis: The effect of temperature. Food Bioprod. Process. 2008, 86, 317–321. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Holmes, C.; Demain, A.L. Applications of protein hydrolysates in biotechnology. In Protein Hydrolysates in Biotechnology; Springer: Berlin, Germany, 2008; pp. 1–9. [Google Scholar]
- Baalousha, M.; Nur, Y.; Römer, I.; Tejamaya, M.; Lead, J. Effect of monovalent and divalent cations, anions and fulvic acid on aggregation of citrate-coated silver nanoparticles. Sci. Total Environ. 2013, 454, 119–131. [Google Scholar] [CrossRef]
- Delay, M.; Dolt, T.; Woellhaf, A.; Sembritzki, R.; Frimmel, F.H. Interactions and stability of silver nanoparticles in the aqueous phase: Influence of natural organic matter (NOM) and ionic strength. J. Chromatogr. A 2011, 1218, 4206–4212. [Google Scholar] [CrossRef]
- Cumberland, S.A.; Lead, J.R. Particle size distributions of silver nanoparticles at environmentally relevant conditions. J. Chromatogr. A 2009, 1216, 9099–9105. [Google Scholar] [CrossRef]
- Chinnapongse, S.L.; MacCuspie, R.I.; Hackley, V.A. Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci. Total Environ. 2011, 409, 2443–2450. [Google Scholar] [CrossRef]
- Quik, J.T.; Lynch, I.; Van Hoecke, K.; Miermans, C.J.; De Schamphelaere, K.A.; Janssen, C.R.; Dawson, K.A.; Stuart, M.A.C.; Van De Meent, D. Effect of natural organic matter on cerium dioxide nanoparticles settling in model fresh water. Chemosphere 2010, 81, 711–715. [Google Scholar] [CrossRef]
- Louie, S.M.; Tilton, R.D.; Lowry, G.V. Critical review: Impacts of macromolecular coatings on critical physicochemical processes controlling environmental fate of nanomaterials. Environ. Sci. Nano 2016, 3, 283–310. [Google Scholar] [CrossRef]
- Raza, G.; Amjad, M.; Kaur, I.; Wen, D. Stability and aggregation kinetics of titania nanomaterials under environmentally realistic conditions. Environ. Sci. Technol. 2016, 50, 8462–8472. [Google Scholar] [CrossRef]
- Lundqvist, M.; Sethson, I.; Jonsson, B.-H. Protein adsorption onto silica nanoparticles: Conformational changes depend on the particles’ curvature and the protein stability. Langmuir 2004, 20, 10639–10647. [Google Scholar] [CrossRef]
- Dastafkan, K.; Khajeh, M.; Bohlooli, M.; Ghaffari-Moghaddam, M.; Sheibani, N. Mechanism and behavior of silver nanoparticles in aqueous medium as adsorbent. Talanta 2015, 144, 1377–1386. [Google Scholar] [CrossRef]
- Seitz, F.; Rosenfeldt, R.R.; Storm, K.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Bundschuh, M. Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna. Ecotoxicol. Environ. Saf. 2015, 111, 263–270. [Google Scholar] [CrossRef]
- Kaiser, J.-P.; Roesslein, M.; Diener, L.; Wichser, A.; Nowack, B.; Wick, P. Cytotoxic effects of nanosilver are highly dependent on the chloride concentration and the presence of organic compounds in the cell culture media. J. Nanobiotechnol. 2017, 15, 5. [Google Scholar] [CrossRef]
- Mu, H.; Chen, Y. Long-term effect of ZnO nanoparticles on waste activated sludge anaerobic digestion. Water Res. 2011, 45, 5612–5620. [Google Scholar] [CrossRef]
- Zhang, W.X.; Elliott, D.W. Applications of iron nanoparticles for groundwater remediation. Remediat. J. 2006, 16, 7–21. [Google Scholar] [CrossRef]
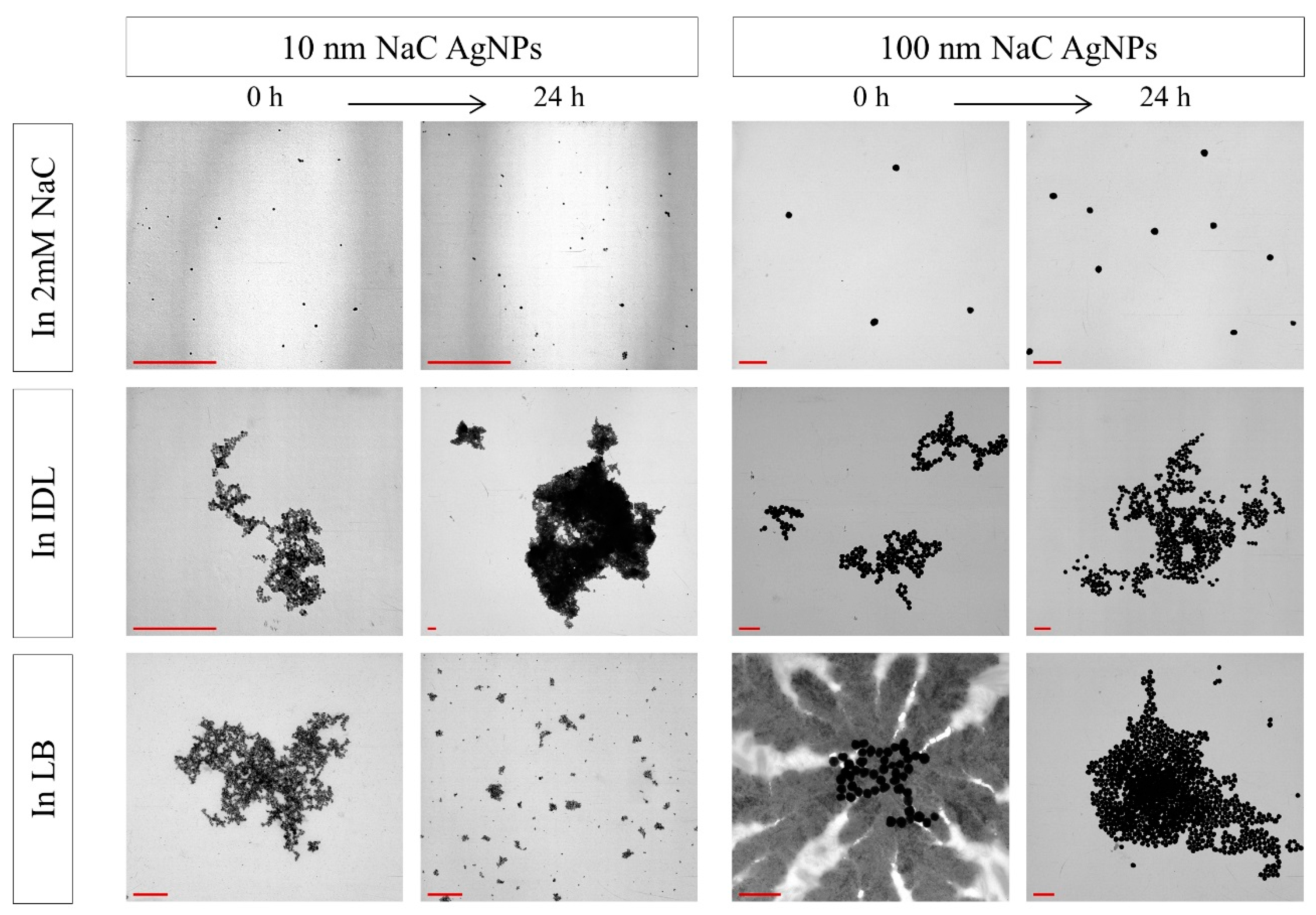
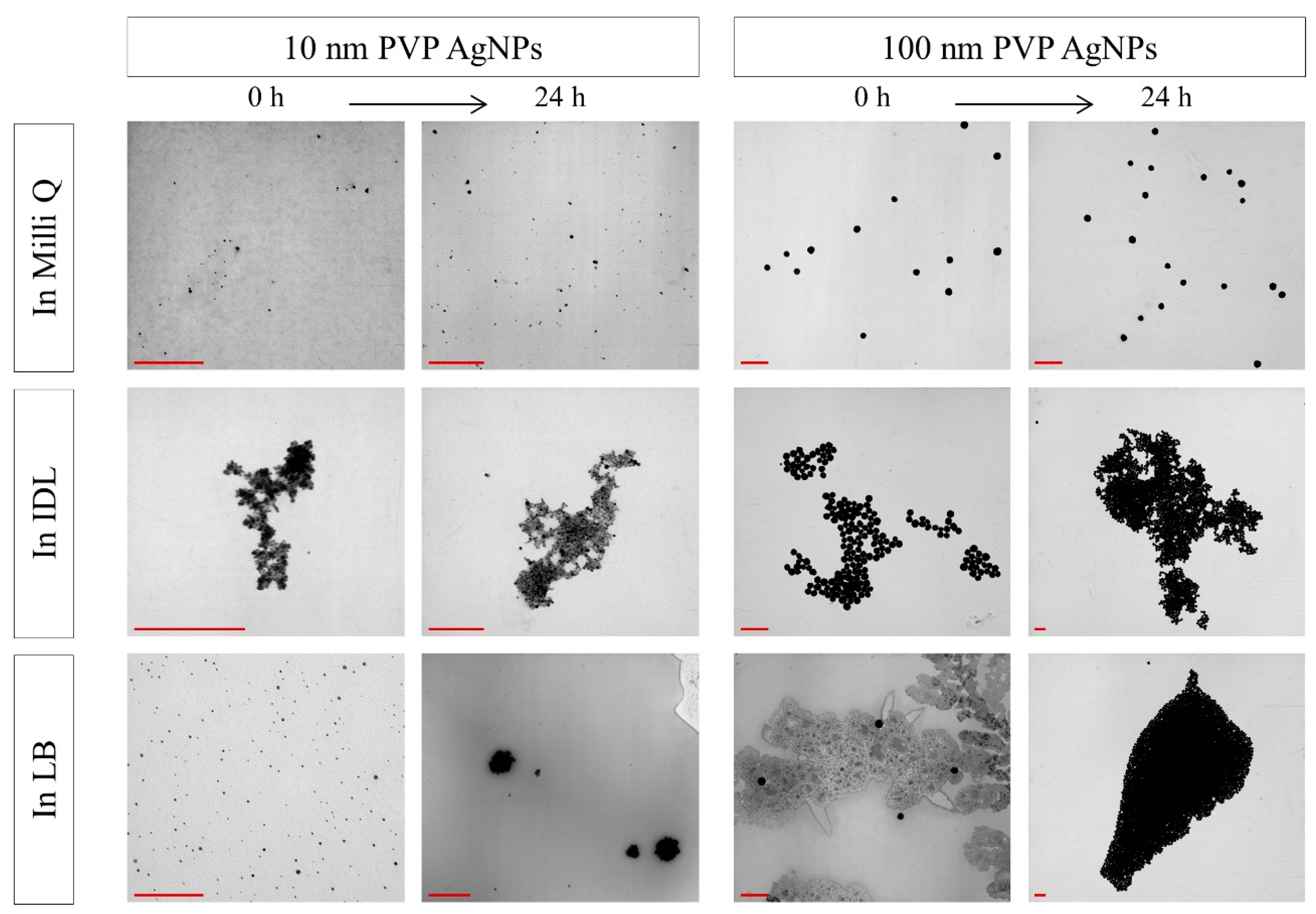

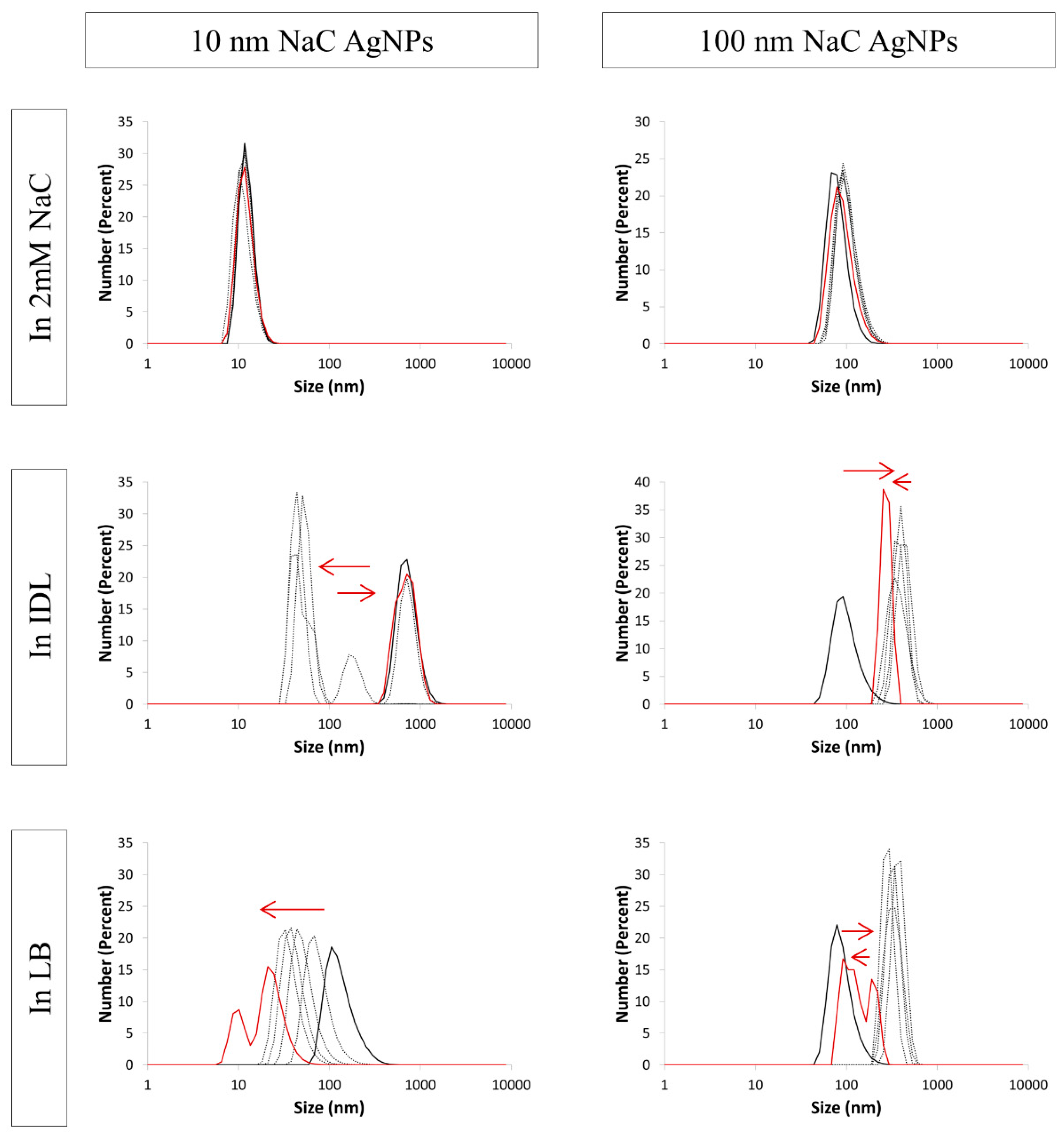

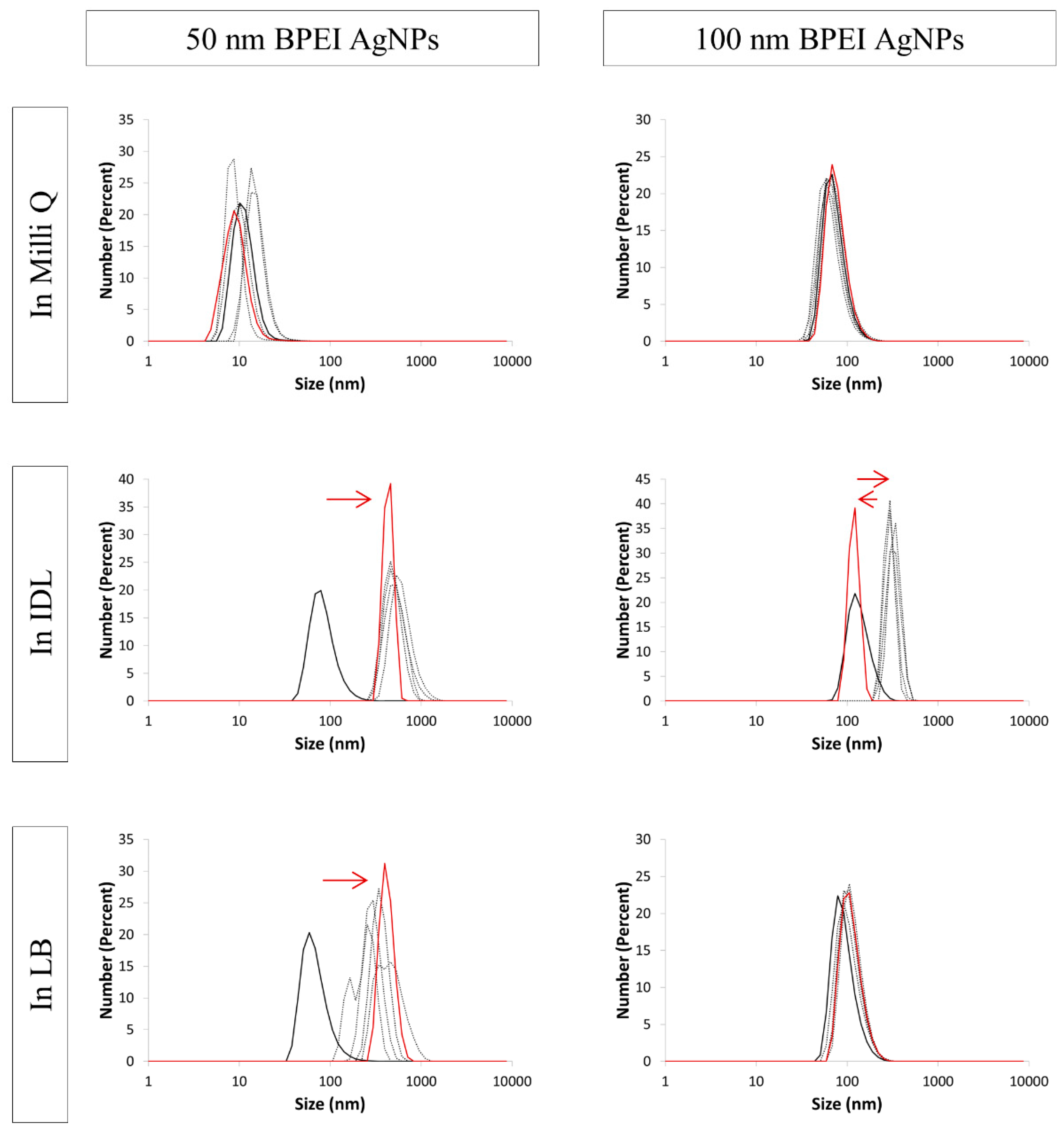
| NaC AgNPs | ||||||
| 10 nm | 100 nm | |||||
| In 2 mM NaC | In IDL | In LB | In 2 mM NaC | In IDL | In LB | |
| 0 h | 0.371 | 0.340 | 0.172 | 0.055 | 0.191 | 0.189 |
 | 0.316 | 0.686 | 0.155 | 0.067 | 0.379 | 0.564 |
| 0.312 | 0.552 | 0.174 | 0.077 | 0.645 | 0.648 | |
| 0.289 | 0.510 | 0.191 | 0.078 | 0.782 | 0.797 | |
| 0.423 | 0.698 | 0.215 | 0.056 | 0.700 | 0.965 | |
| 24 h | 0.200 | 0.948 | 0.233 | 0.119 | 1.000 | 1.000 |
| PVP AgNPs | ||||||
| 10 nm | 100 nm | |||||
| In Milli Q | In IDL | In LB | In Milli Q | In IDL | In LB | |
| 0 h | 0.200 | 0.327 | 0.240 | 0.058 | 0.076 | 0.029 |
 | 0.205 | 0.693 | 0.188 | 0.048 | 0.461 | 0.449 |
| 0.195 | 0.757 | 0.319 | 0.071 | 0.669 | 0.682 | |
| 0.196 | 0.575 | 0.245 | 0.065 | 0.780 | 0.704 | |
| 0.168 | 0.620 | 0.213 | 0.046 | 0.882 | 0.906 | |
| 24 h | 0.384 | 0.844 | 0.487 | 0.050 | 1.000 | 1.000 |
| BPEI AgNPs | ||||||
| 50 nm | 100 nm | |||||
| In Milli Q | In IDL | In LB | In Milli Q | In IDL | In LB | |
| 0 h | 0.324 | 0.217 | 0.226 | 0.105 | 0.262 | 0.084 |
 | 0.352 | 0.460 | 0.459 | 0.126 | 0.578 | 0.107 |
| 0.335 | 0.504 | 0.599 | 0.172 | 0.946 | 0.090 | |
| 0.360 | 0.856 | 0.795 | 0.156 | 0.976 | 0.035 | |
| 0.333 | 0.627 | 0.656 | 0.174 | 1.000 | 0.010 | |
| 24 h | 0.319 | 1.000 | 0.917 | 0.215 | 1.000 | 0.092 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leersnyder, I.; De Gelder, L.; Van Driessche, I.; Vermeir, P. Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium. Nanomaterials 2019, 9, 1684. https://doi.org/10.3390/nano9121684
De Leersnyder I, De Gelder L, Van Driessche I, Vermeir P. Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium. Nanomaterials. 2019; 9(12):1684. https://doi.org/10.3390/nano9121684
Chicago/Turabian StyleDe Leersnyder, Ilse, Leen De Gelder, Isabel Van Driessche, and Pieter Vermeir. 2019. "Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium" Nanomaterials 9, no. 12: 1684. https://doi.org/10.3390/nano9121684
APA StyleDe Leersnyder, I., De Gelder, L., Van Driessche, I., & Vermeir, P. (2019). Revealing the Importance of Aging, Environment, Size and Stabilization Mechanisms on the Stability of Metal Nanoparticles: A Case Study for Silver Nanoparticles in a Minimally Defined and Complex Undefined Bacterial Growth Medium. Nanomaterials, 9(12), 1684. https://doi.org/10.3390/nano9121684







