Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Ag Dendrites Structural and Optical Characterizations
2.3. Raman and SERS Analyses
3. Results and Discussions
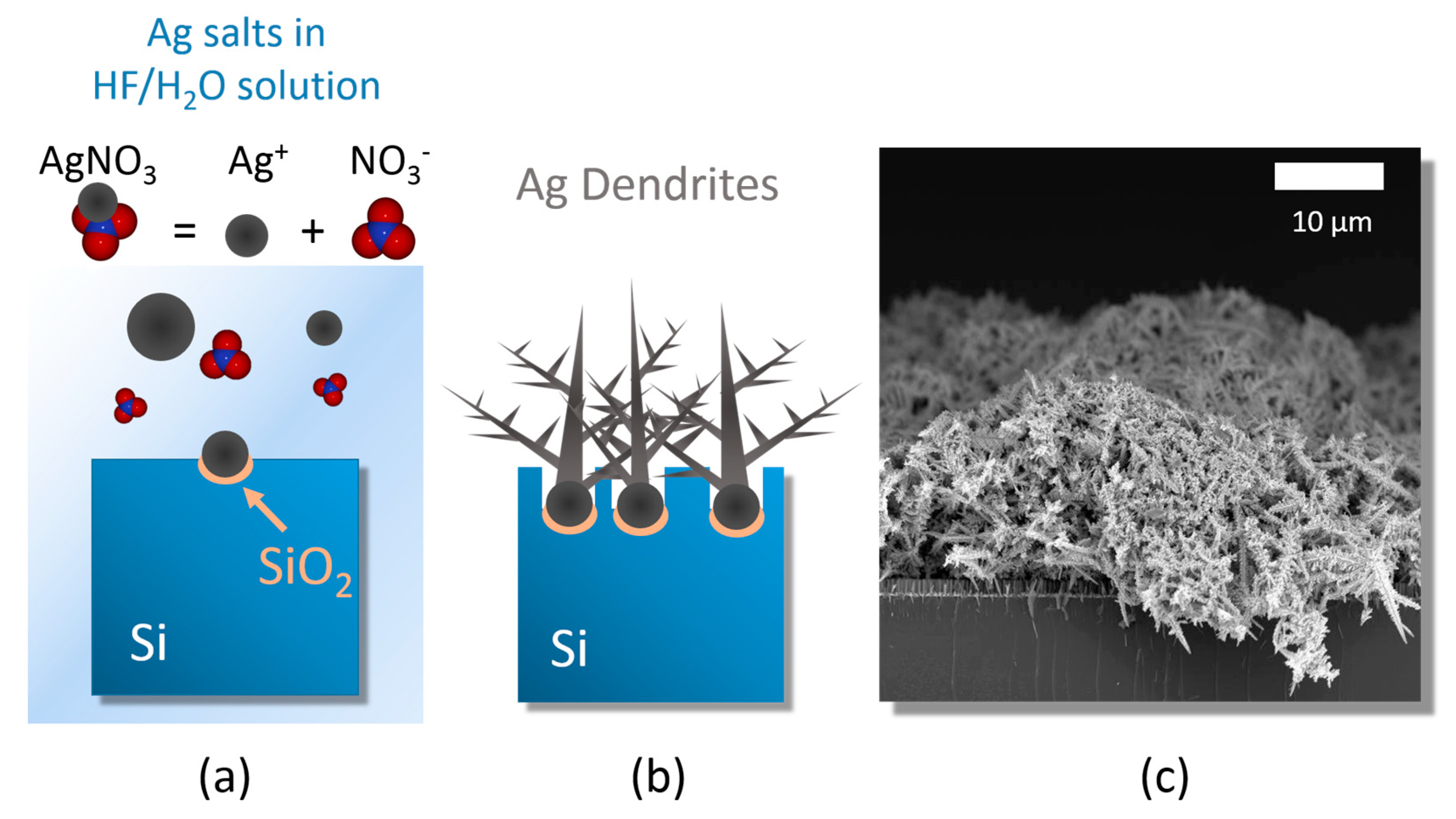
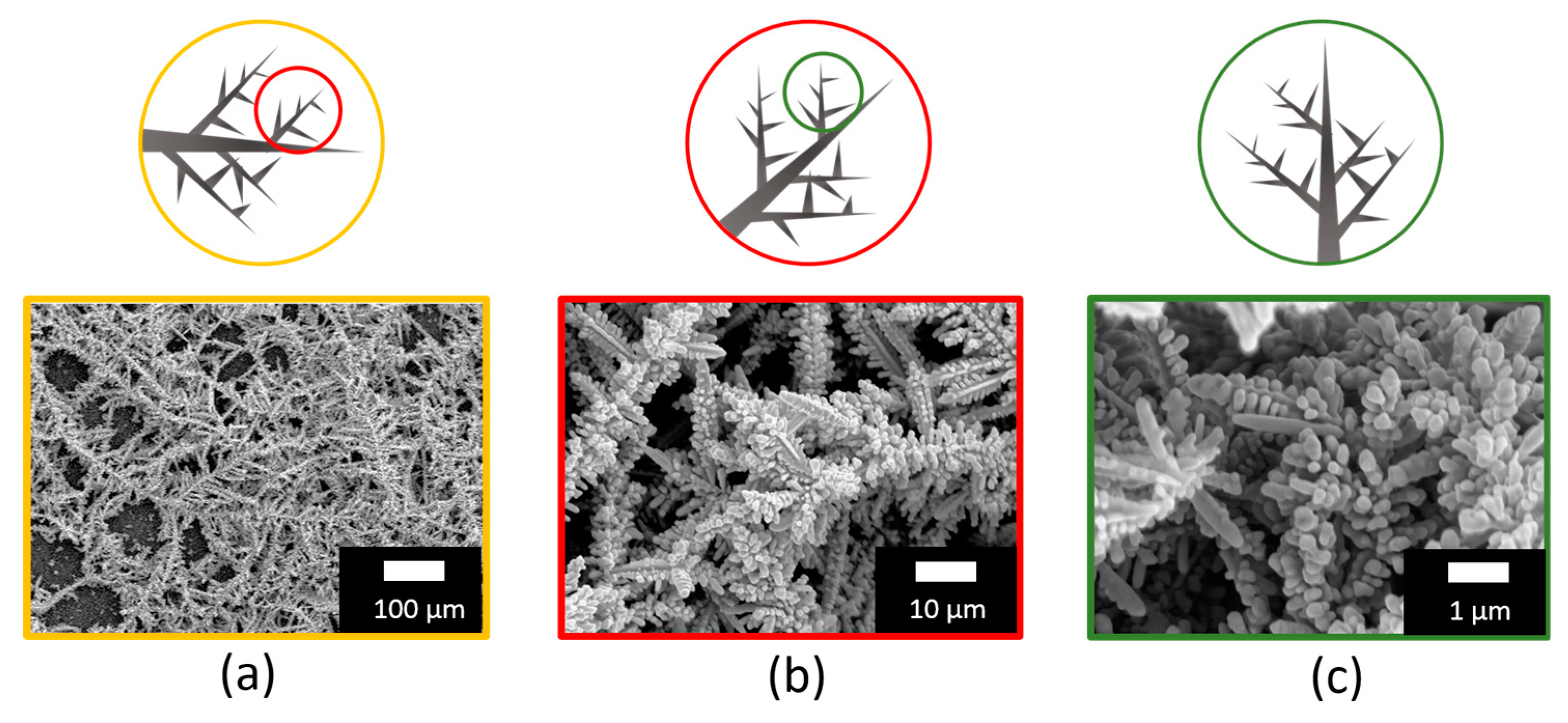
3.1. Ag Dendrite Synthesis and Characterization
- (i)
- silver salt dissolution by Ag reduction;
- (ii)
- random Ag seed aggregation and deposition;
- (iii)
- stem growth onto the Ag nucleation sites [45].
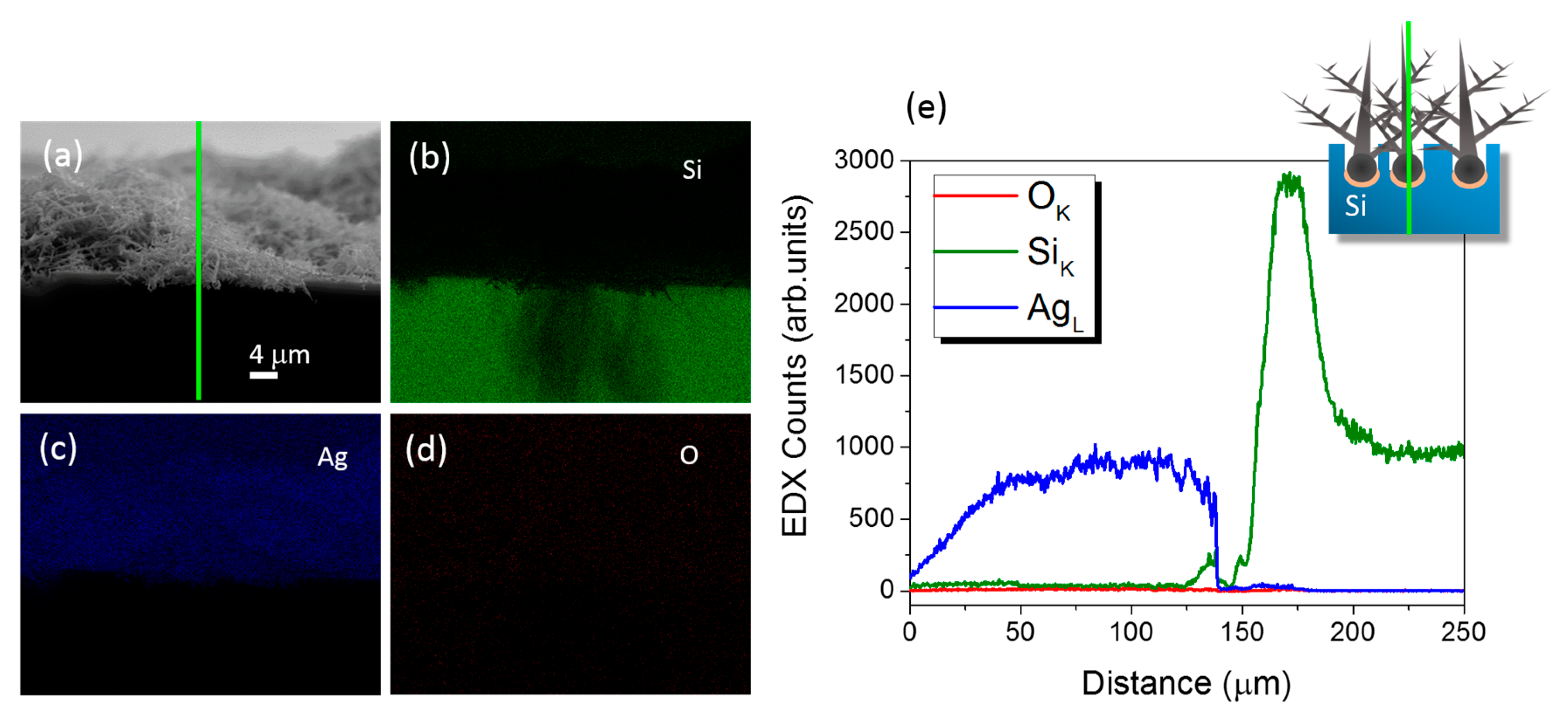
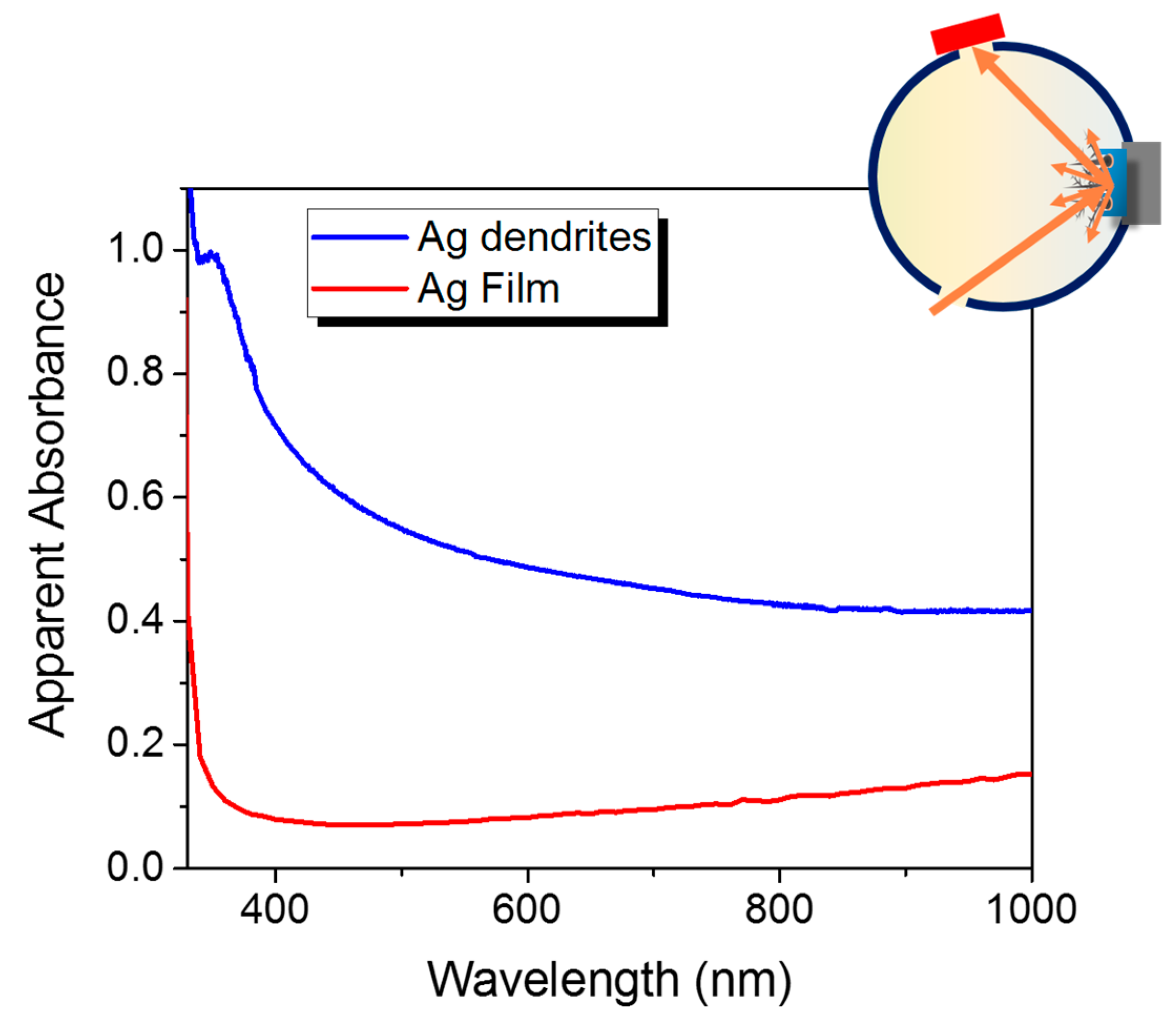
3.2. Structural and Optical Characterizations
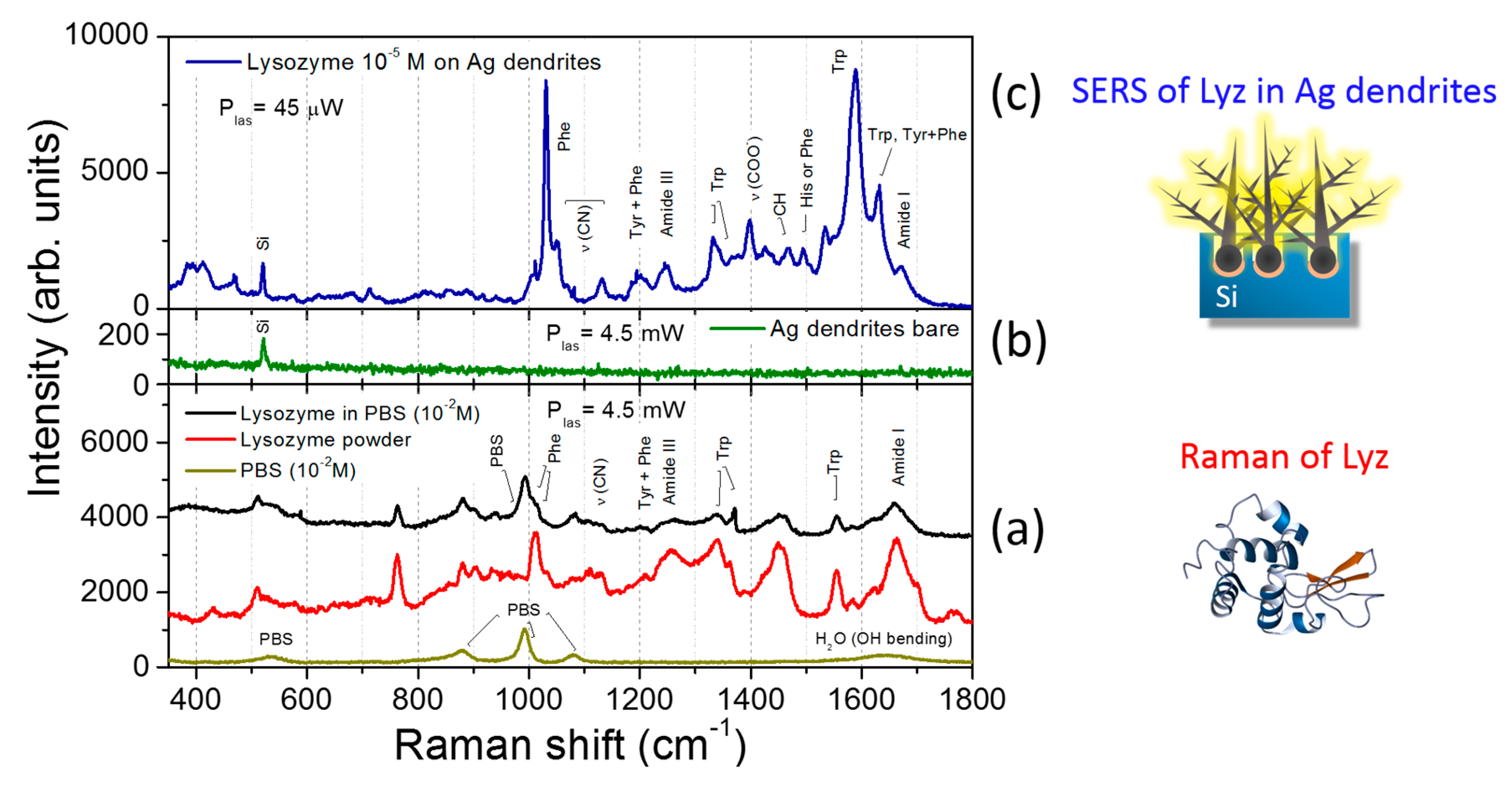
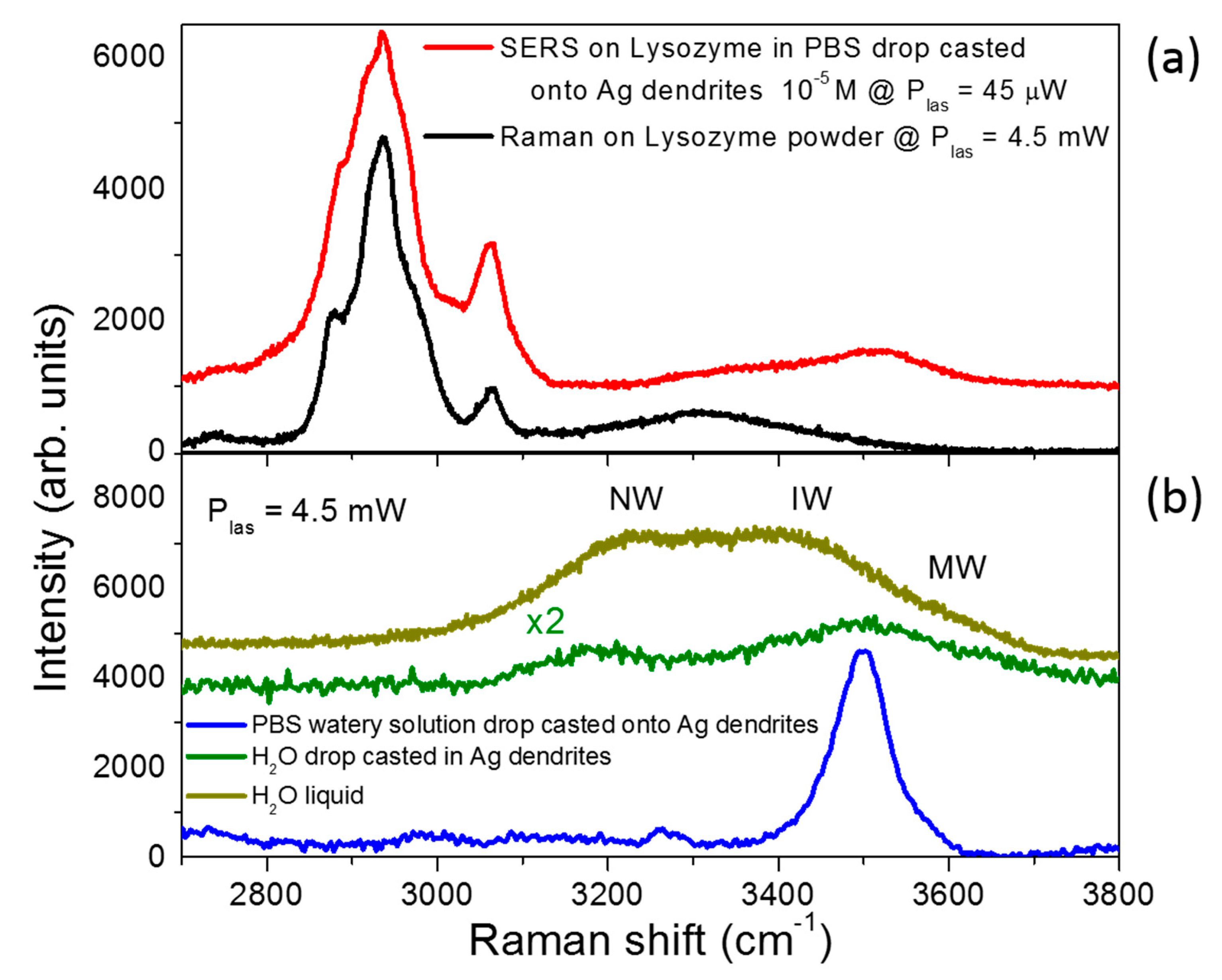
3.3. SERS Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radziuk, D.; Moehwald, H. Prospects for plasmonic hot spots in single molecule SERS towards the chemical imaging of live cells. Phys. Chem. Chem. Phys. 2015, 17, 21072–21093. [Google Scholar] [CrossRef] [PubMed]
- Lequin, R.M. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed]
- Grinyte, R.; Barroso, J.; Möller, M.; Saa, L.; Pavlov, V. Microbead QD-ELISA: Microbead ELISA using biocatalytic formation of quantum dots for ultra high sensitive optical and electrochemical detection. ACS Appl. Mater. Interfaces 2016, 8, 29252–29260. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, Q.; Duan, Y. Fluorescent labels in biosensors for pathogen detection. Crit. Rev. Biotechnol. 2015, 35, 82–93. [Google Scholar] [CrossRef]
- Fazio, B.; Irrera, A.; Pirotta, S.; D’Andrea, C.; Del Sorbo, S.; Josè Lo Faro, M.; Gucciardi, P.G.; Iatì, M.A.; Saija, R.; Patrini, M.; et al. Coherent backscattering of raman light. Nat. Photonics 2017, 11, 170–176. [Google Scholar] [CrossRef]
- Chan, S.; Fauchet, P.M. Tunable, narrow, and directional luminescence from porous silicon light emitting devices. Appl. Phys. Lett. 1999, 75, 274–276. [Google Scholar] [CrossRef]
- Vollmer, F.; Yang, L. Review label-free detection with high-Q microcavities: A review of biosensing mechanisms for integrated devices. Nanophotonics 2012, 1, 267–291. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Fazio, B.; D’Andrea, C.; Bonaccorso, F.; Irrera, A.; Calogero, G.; Vasi, C.; Gucciardi, P.G.; Allegrini, M.; Toma, A.; Chiappe, D.; et al. Re-radiation enhancement in polarized surface-enhanced resonant Raman scattering of randomly oriented molecules on self-organized gold nanowires. ACS Nano 2011, 5, 5945–5956. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Petralia, S.; Fazio, B.; Musumeci, P.; Conoci, S.; Irrera, A.; Priolo, F. Ultrasensitive label- and PCR-free genome detection based on cooperative hybridization of silicon nanowires optical biosensors. ACS Sens. 2018, 3, 1690–1697. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzò, G.; Musumeci, P.; Fazio, B.; et al. New generation of ultrasensitive label-free optical si nanowire-based biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface enhanced Raman scattering. ACS Nano 2019. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy and Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780444527790. [Google Scholar]
- Le Ru, E.C.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface enhanced Raman scattering enhancement factors: A comprehensive study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- D’Andrea, C.; Bochterle, J.; Toma, A.; Huck, C.; Neubrech, F.; Messina, E.; Fazio, B.; Maragò, O.M.; Di Fabrizio, E.; Lamy de La Chapelle, M.; et al. Optical nanoantennas for multiband surface-enhanced infrared and Raman spectroscopy. ACS Nano 2013, 7, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Cottat, M.; D’Andrea, C.; Yasukuni, R.; Malashikhina, N.; Grinyte, R.; Lidgi-Guigui, N.; Fazio, B.; Sutton, A.; Oudar, O.; Charnaux, N.; et al. High sensitivity, high selectivity SERS detection of MnSOD using optical nanoantennas functionalized with aptamers. J. Phys. Chem. C 2015, 119, 15532–15540. [Google Scholar] [CrossRef]
- D’Andrea, C.; Fazio, B.; Gucciardi, P.G.; Giordano, M.C.; Martella, C.; Chiappe, D.; Toma, A.; Buatier de Mongeot, F.; Tantussi, F.; Vasanthakumar, P.; et al. SERS enhancement and field confinement in nanosensors based on self-organized gold nanowires produced by Ion-Beam sputtering. J. Phys. Chem. C 2014, 118, 8571–8580. [Google Scholar] [CrossRef]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtuluş, Ö.; Lee, S.H.; Lindquist, N.C.; Oh, S.-H.; Haynes, C.L. Recent progress in SERS biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551. [Google Scholar] [CrossRef]
- Xie, W.; Schlücker, S. Medical applications of surface-enhanced Raman scattering. Phys. Chem. Chem. Phys. 2013, 15, 5329. [Google Scholar] [CrossRef]
- Kneipp, K.; Haka, A.S.; Kneipp, H.; Badizadegan, K.; Yoshizawa, N.; Boone, C.; Shafer-Peltier, K.E.; Motz, J.T.; Dasari, R.R.; Feld, M.S. Surface-enhanced Raman spectroscopy in single living cells using gold nanoparticles. Appl. Spectrosc. 2002, 56, 150–154. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- D’Andrea, C.; Faro, M.J.L.; Bertino, G.; Ossi, P.M.; Neri, F.; Trusso, S.; Musumeci, P.; Galli, M.; Cioffi, N.; Irrera, A.; et al. Decoration of silicon nanowires with silver nanoparticles for ultrasensitive surface enhanced Raman scattering. Nanotechnology 2016, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mulazimoglu, E.; Nogay, G.; Turan, R.; Emrah Unalan, H. Enhanced localized surface plasmon resonance obtained in two step etched silicon nanowires decorated with silver nanoparticles. Appl. Phys. Lett. 2013, 103, 143124. [Google Scholar] [CrossRef]
- Casiello, M.; Picca, R.R.A.; Fusco, C.; D’Accolti, L.; Leonardi, A.A.A.; Lo Faro, M.J.M.; Irrera, A.; Trusso, S.; Cotugno, P.; Sportelli, M.C.M.; et al. Catalytic activity of silicon nanowires decorated with gold and copper nanoparticles deposited by pulsed laser ablation. Nanomaterials 2018, 8, 78. [Google Scholar] [CrossRef]
- Wallace, G.Q.; Lagugné-Labarthet, F. Advancements in fractal plasmonics: Structures, optical properties, and applications. Analyst 2019, 144, 13–30. [Google Scholar] [CrossRef]
- Lafuente, M.; Berenschot, E.; Tiggelaar, R.; Mallada, R.; Tas, N.; Pina, M. 3D Fractals as SERS active platforms: Preparation and evaluation for gas phase detection of G-nerve agents. Micromachines 2018, 9, 60. [Google Scholar] [CrossRef]
- Butenko, A.V.; Shalaev, V.M.; Stockman, M.I. Fractals: Giant impurity nonlinearities in optics of fractal clusters. Z. Phys. D Atoms Mol. Clust. 1988, 10, 81–92. [Google Scholar] [CrossRef]
- Podolskiy, V.A.; Shalaev, V.M. Giant optical responses in microcavity-fractal composites | Request PDF. Laser Phys. 2001, 11, 26–30. [Google Scholar]
- Neubrech, F.; Huck, C.; Weber, K.; Pucci, A.; Giessen, H. Surface-enhanced infrared spectroscopy using resonant nanoantennas. Chem. Rev. 2017, 117, 5110–5145. [Google Scholar] [CrossRef]
- Aslan, E.; Aslan, E.; Wang, R.; Hong, M.K.; Erramilli, S.; Turkmen, M.; Saracoglu, O.G.; Dal Negro, L. Multispectral cesaro-type fractal plasmonic nanoantennas. ACS Photonics 2016, 3, 2102–2111. [Google Scholar] [CrossRef]
- Dies, H.; Raveendran, J.; Escobedo, C.; Docoslis, A. In situ assembly of active surface-enhanced Raman scattering substrates via electric field-guided growth of dendritic nanoparticle structures. Nanoscale 2017, 9, 7847–7857. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Qiu, Y.; Li, Z.; Yang, D.; Ding, S.; Cheng, G.; Hao, Z.; Wang, Q. Fabrication of silver dendrite fractal structures for enhanced second harmonic generation and surface-enhanced Raman scattering. Opt. Mater. Express 2019, 9, 860. [Google Scholar] [CrossRef]
- Fu, L.; Tamanna, T.; Hu, W.J.; Yu, A. Chemical preparation and applications of silver dendrites. Chem. Pap. 2014, 68, 1283–1297. [Google Scholar] [CrossRef]
- Kaniyankandy, S.; Nuwad, J.; Thinaharan, C.; Dey, G.K.; Pillai, C.G.S. Electrodeposition of silver nanodendrites. Nanotechnology 2007, 18, 1–6. [Google Scholar] [CrossRef]
- Tang, S.; Meng, X.; Lu, H.; Zhu, S. PVP-assisted sonoelectrochemical growth of silver nanostructures with various shapes. Mater. Chem. Phys. 2009, 116, 464–468. [Google Scholar] [CrossRef]
- Fang, J.; Hahn, H.; Krupke, R.; Schramm, F.; Scherer, T.; Ding, B.; Song, X. Silver nanowires growth via branch fragmentation of electrochemically grown silver dendrites. Chem. Commun. 2009, 1130–1132. [Google Scholar] [CrossRef]
- Xiao, J.P.; Xie, Y.; Tang, R.; Chen, M.; Tian, X.B. Novel ultrasonically assisted templated synthesis of palladium and silver dendritic nanostructures. Adv. Mater. 2001, 13, 1887–1891. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Speth, T.F.; Varma, R.S. Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 2011, 44, 469–478. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X. Self-assembled synthesis of Ag nanodendrites and their applications to SERS. J. Mol. Struct. 2011, 997, 64–69. [Google Scholar] [CrossRef]
- Taleb, A.; Mangeney, C.; Ivanova, V. Electrochemical synthesis using a self-assembled Au nanoparticle template of dendritic films with unusual wetting properties. Nanotechnology 2011, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Mandal, T.K. Synthesis and catalytic application of nanostructured silver dendrites. J. Phys. Chem. C 2007, 111, 16750–16760. [Google Scholar] [CrossRef]
- Lu, L.; Kobayashi, A.; Kikkawa, Y.; Tawa, K.; Ozaki, Y. Oriented attachment-based assembly of dendritic silver nanostructures at room temperature. J. Phys. Chem. B 2006, 110, 23234–23241. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xie, Y.-T.; Mak, W.C.; Cheung, K.Y.; Li, X.-Y.; Renneberg, R.; Yang, S. Dendritic nanostructures of silver: Facile synthesis, structural characterizations, and sensing applications. Langmuir 2006, 22, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Guo, S.; Dong, S.; Wang, E. A simple route for the synthesis of morphology-controlled and SERS-active Ag dendrites with near-infrared absorption. J. Phys. Chem. C 2011, 115, 10315–10320. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; de Boor, J.; Gösele, U. Metal-assisted chemical etching of silicon: A review. Adv. Mater. 2011, 23, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-L.; Peng, K.-Q.; Fan, X.; Jie, J.-S.; Zhang, R.-Q.; Lee, S.-T.; Wong, N.-B. Preparation of large-area uniform silicon nanowires arrays through metal-assisted chemical etching. J. Phys. Chem. C 2008, 112, 4444–4450. [Google Scholar] [CrossRef]
- Irrera, A.; Lo Faro, M.J.; D’Andrea, C.; Alessio Leonardi, A.; Artoni, P.; Fazio, B.; Anna Picca, R.; Cioffi, N.; Trusso, S.; Franzò, G.; et al. Light-emitting silicon nanowires obtained by metal-assisted chemical etching. Semicond. Sci. Technol. 2017, 32, 043004. [Google Scholar] [CrossRef]
- Fazio, B.; D’Andrea, C.; Foti, A.; Messina, E.; Irrera, A.; Donato, M.G.; Villari, V.; Micali, N.; Maragò, O.M.; Gucciardi, P.G. SERS detection of biomolecules at physiological pH via aggregation of gold nanorods mediated by optical forces and plasmonic heating. Sci. Rep. 2016, 6, 26952. [Google Scholar] [CrossRef] [Green Version]
- Foti, A.; D’Andrea, C.; Villari, V.; Micali, N.; Donato, M.; Fazio, B.; Maragò, O.; Gillibert, R.; Lamy de la Chapelle, M.; Gucciardi, P. Optical aggregation of gold nanoparticles for SERS detection of proteins and toxins in liquid environment: Towards ultrasensitive and selective detection. Materials 2018, 11, 440. [Google Scholar] [CrossRef] [Green Version]
- Donato, M.G.; Brzobohatý, O.; Simpson, S.H.; Irrera, A.; Leonardi, A.A.; Lo Faro, M.J.; Svak, V.; Maragò, O.M.; Zemánek, P. Optical trapping, optical binding, and rotational dynamics of silicon nanowires in counter-propagating beams. Nano Lett. 2019, 19, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Pecora, E.F.; Lawrence, N.; Gregg, P.; Trevino, J.; Artoni, P.; Irrera, A.; Priolo, F.; Negro, L.D. Nanopatterning of silicon nanowires for enhancing visible photoluminescence. Nanoscale 2012, 4, 2863. [Google Scholar] [CrossRef] [PubMed]
- Lo Faro, M.J.; Leonardi, A.A.; D’Andrea, C.; Morganti, D.; Musumeci, P.; Vasi, C.; Priolo, F.; Fazio, B.; Irrera, A. Low cost synthesis of silicon nanowires for photonic applications. J. Mater. Sci. Mater. Electron. 2019, 1–7. [Google Scholar] [CrossRef]
- McPeak, K.M.; Jayanti, S.V.; Kress, S.J.P.; Meyer, S.; Iotti, S.; Rossinelli, A.; Norris, D.J. Plasmonic films can easily be better: Rules and recipes. ACS Photonics 2015, 2, 326–333. [Google Scholar] [CrossRef] [PubMed]
- M.N. Polyanskiy Citing RefractiveIndex. INFO. Available online: https://refractiveindex.info/cite.php (accessed on 23 September 2019).
- D’Andrea, C.; Foti, A.; Cottat, M.; Banchelli, M.; Capitini, C.; Barreca, F.; Canale, C.; de Angelis, M.; Relini, A.; Maragò, O.M.; et al. Nanoscale discrimination between toxic and nontoxic protein misfolded oligomers with tip-enhanced Raman spectroscopy. Small 2018, 14, 1800890. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Kneipp, J. SERS probing of proteins in gold nanoparticle agglomerates. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Madzharova, F.; Heiner, Z.; Kneipp, J. Surface enhanced hyper-Raman scattering of the amino acids tryptophan, Histidine, Phenylalanine, and Tyrosine. J. Phys. Chem. C 2017, 121, 1235–1242. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, D.; Halas, N.J.; Hartgerink, J.D. Aromatic amino acids providing characteristic motifs in the Raman and SERS spectroscopy of peptides. J. Phys. Chem. B 2008, 112, 9158–9164. [Google Scholar] [CrossRef]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Part I: Surface-enhanced Raman spectroscopy investigation of amino acids and their homodipeptides adsorbed on colloidal silver. Appl. Spectrosc. 2004, 58, 570–580. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Etchegoin, P.G. Rigorous justification of the |E|4 enhancement factor in surface enhanced Raman spectroscopy. Chem. Phys. Lett. 2006, 423, 63–66. [Google Scholar] [CrossRef]
- Le Ru, E.C.; Meyer, M.; Blackie, E.; Etchegoin, P.G. Advanced aspects of electromagnetic SERS enhancement factors at a hot spot. J. Raman Spectrosc. 2008, 39, 1127–1134. [Google Scholar] [CrossRef]
- Moskovits, M. Surface selection rules. J. Chem. Phys. 1982, 77, 4408–4416. [Google Scholar] [CrossRef]
- Deckert-Gaudig, T.; Rauls, E.; Deckert, V. Aromatic amino acid monolayers sandwiched between gold and silver: A combined tip-enhanced Raman and theoretical approach. J. Phys. Chem. C 2010, 114, 7412–7420. [Google Scholar] [CrossRef]
- Kocherbitov, V.; Latynis, J.; Misiunas, A.; Barauskas, J.; Niaura, G. Hydration of lysozyme studied by Raman spectroscopy. J. Phys. Chem. B 2013, 117, 4981–4992. [Google Scholar] [CrossRef] [PubMed]
- Frontzek, A.V.; Paccou, L.; Guinet, Y.; Hédoux, A. Study of the phase transition in lysozyme crystals by Raman spectroscopy. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urabe, H.; Sugawara, Y.; Ataka, M.; Rupprecht, A. Low-frequency raman spectra of lysozyme crystals and oriented DNA films: Dynamics of crystal water. Biophys. J. 1998, 74, 1533–1540. [Google Scholar] [CrossRef] [Green Version]
- Brubach, J.B.; Mermet, A.; Filabozzi, A.; Gerschel, A.; Lairez, D.; Krafft, M.P.; Roy, P. Dependence of water dynamics upon confinement size. J. Phys. Chem. B 2001, 105, 430–435. [Google Scholar] [CrossRef]
- Brubach, J.B.; Mermet, A.; Filabozzi, A.; Gerschel, A.; Roy, P. Signatures of the hydrogen bonding in the infrared bands of water. J. Chem. Phys. 2005, 122, 1–7. [Google Scholar] [CrossRef]
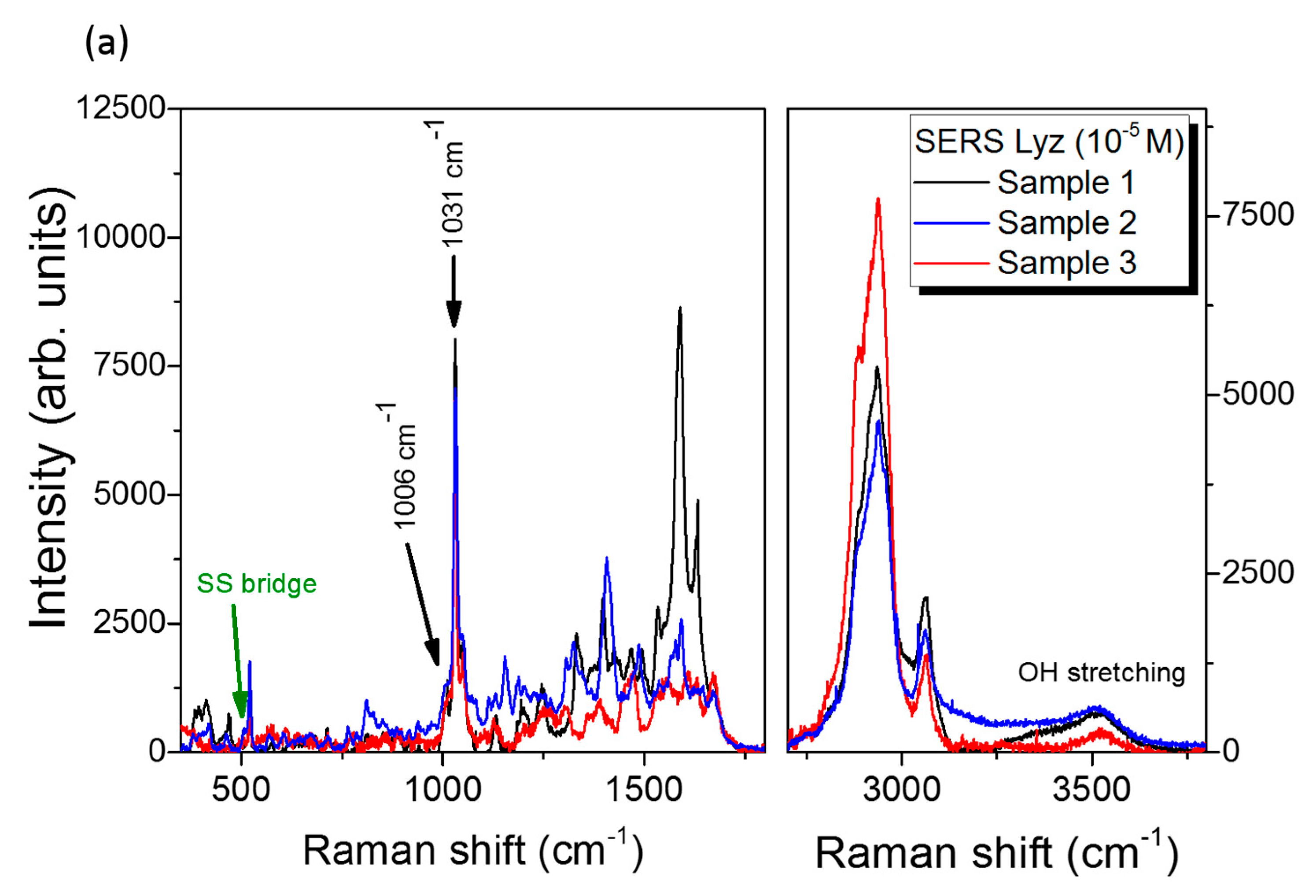
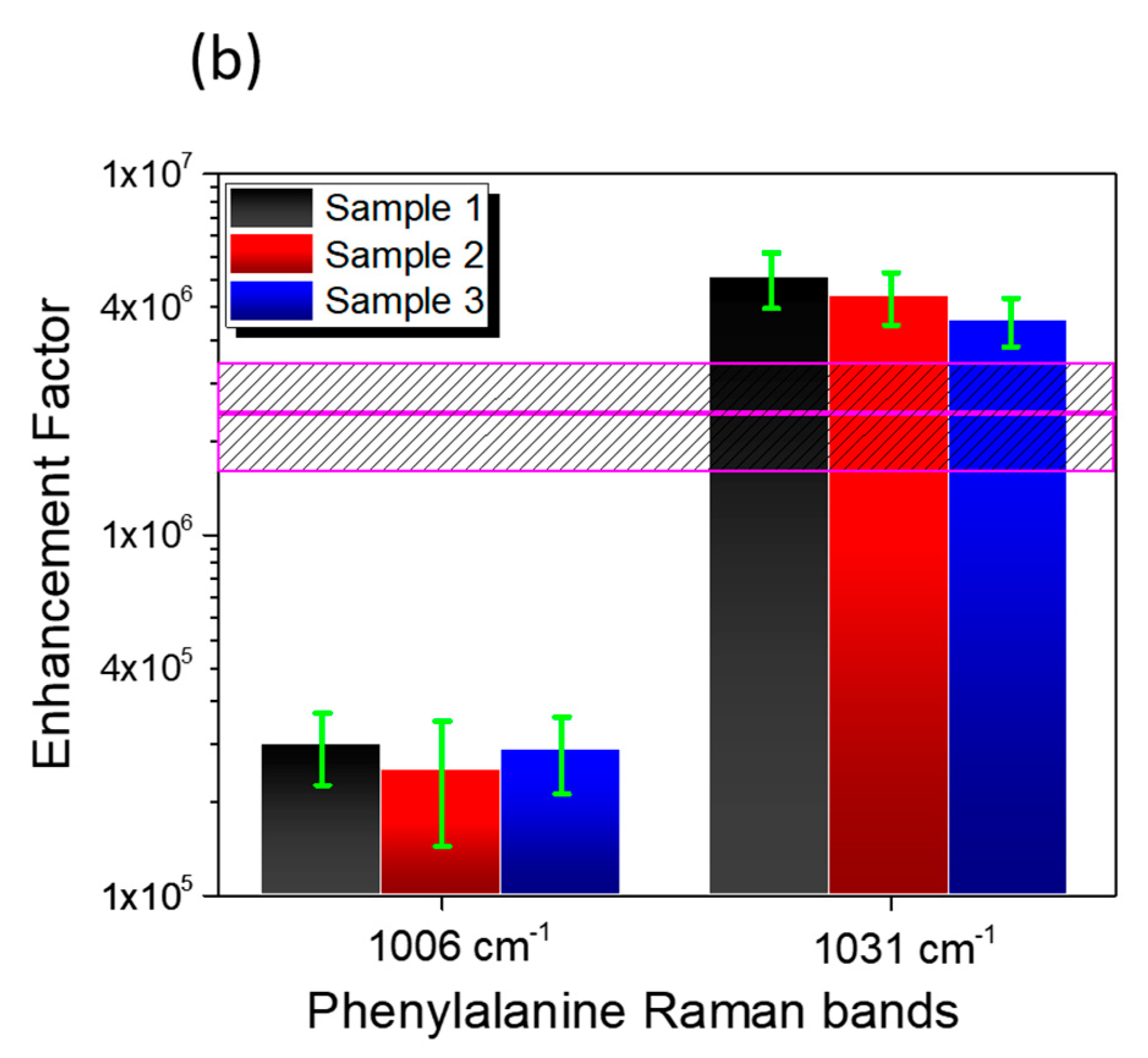
| Enhancement Factor | Peak 1006 cm−1 | Peak 1031 cm−1 |
|---|---|---|
| Sample 1 | 2.6 ± 0.6 × 105 | 5.1 ± 0.9 × 106 |
| Sample 2 | 2.2 ± 0.8 × 105 | 4.6 ± 0.8 × 106 |
| Sample 3 | 2.5 ± 0.6 × 105 | 3.9 ± 0.6 × 106 |
| Average | 2.4 ± 0.8 × 106 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Faro, M.J.; D’Andrea, C.; Leonardi, A.A.; Morganti, D.; Irrera, A.; Fazio, B. Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions. Nanomaterials 2019, 9, 1630. https://doi.org/10.3390/nano9111630
Lo Faro MJ, D’Andrea C, Leonardi AA, Morganti D, Irrera A, Fazio B. Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions. Nanomaterials. 2019; 9(11):1630. https://doi.org/10.3390/nano9111630
Chicago/Turabian StyleLo Faro, Maria José, Cristiano D’Andrea, Antonio Alessio Leonardi, Dario Morganti, Alessia Irrera, and Barbara Fazio. 2019. "Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions" Nanomaterials 9, no. 11: 1630. https://doi.org/10.3390/nano9111630
APA StyleLo Faro, M. J., D’Andrea, C., Leonardi, A. A., Morganti, D., Irrera, A., & Fazio, B. (2019). Fractal Silver Dendrites as 3D SERS Platform for Highly Sensitive Detection of Biomolecules in Hydration Conditions. Nanomaterials, 9(11), 1630. https://doi.org/10.3390/nano9111630







