Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Filled with Carboplatin as a Novel Drug Nanocarrier against Murine Lung Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanovectors Construction
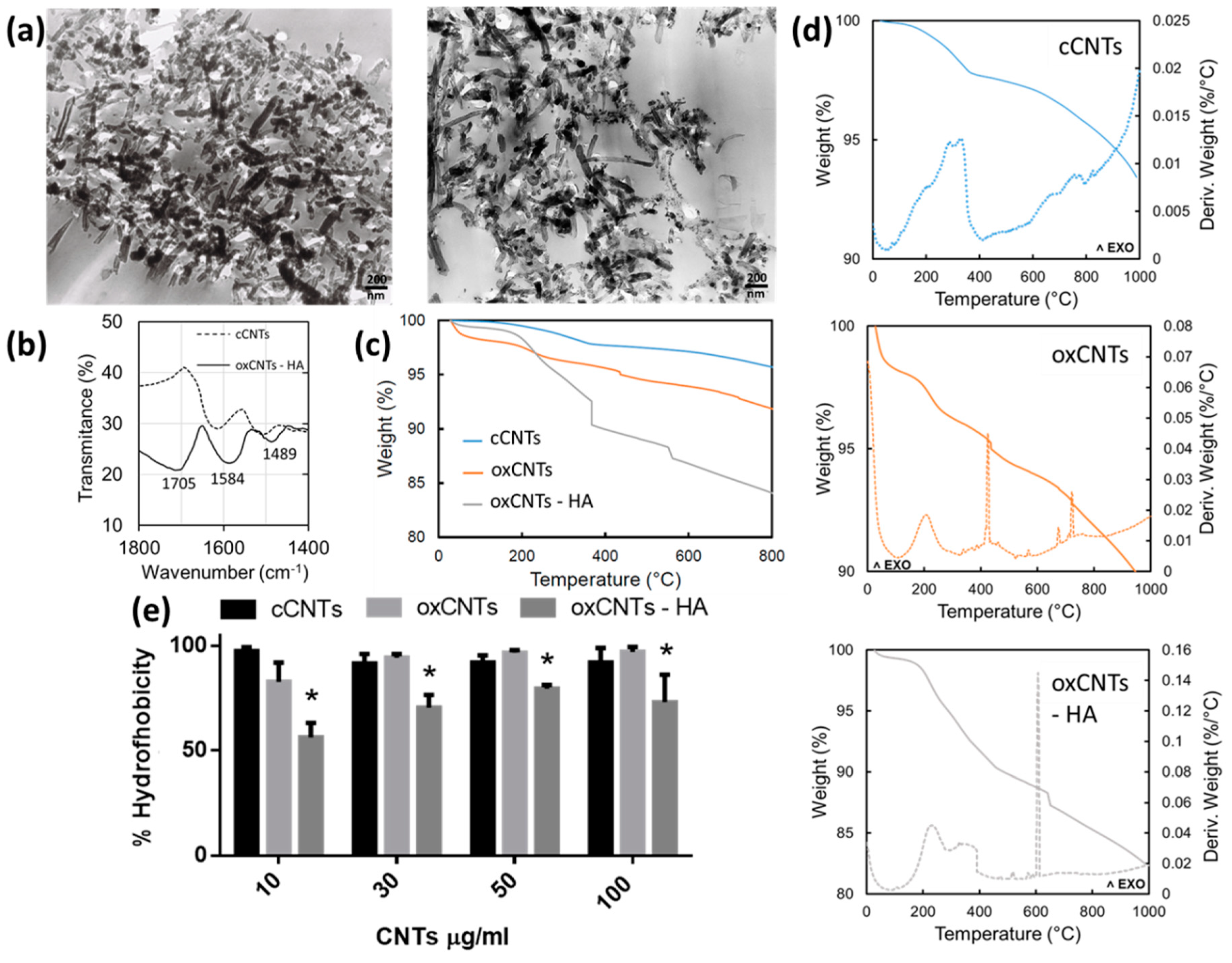
2.2. Characterization of the Constructed CNTs Using Transmission Electron Microscopy (TEM), Infrared Spectroscopy (FTIR), Thermogravimetric Analysis (TGA) and Hydrophobicity Index Test
2.3. Cell Culture
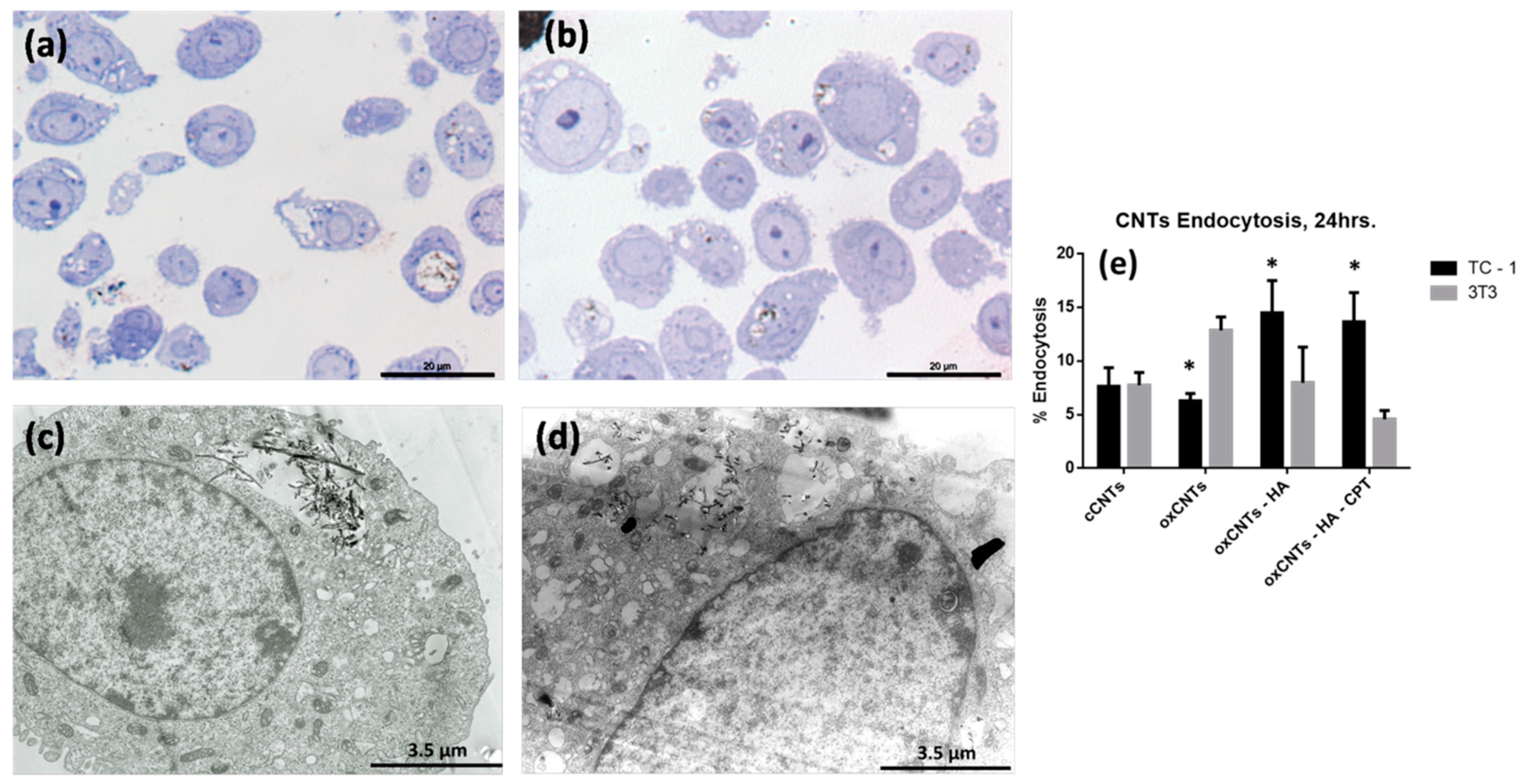
2.4. Evaluation of Cell Uptake of the Nanovectors in the Tumor and Non-Tumor Cell Lines by Light Microscopy and TEM
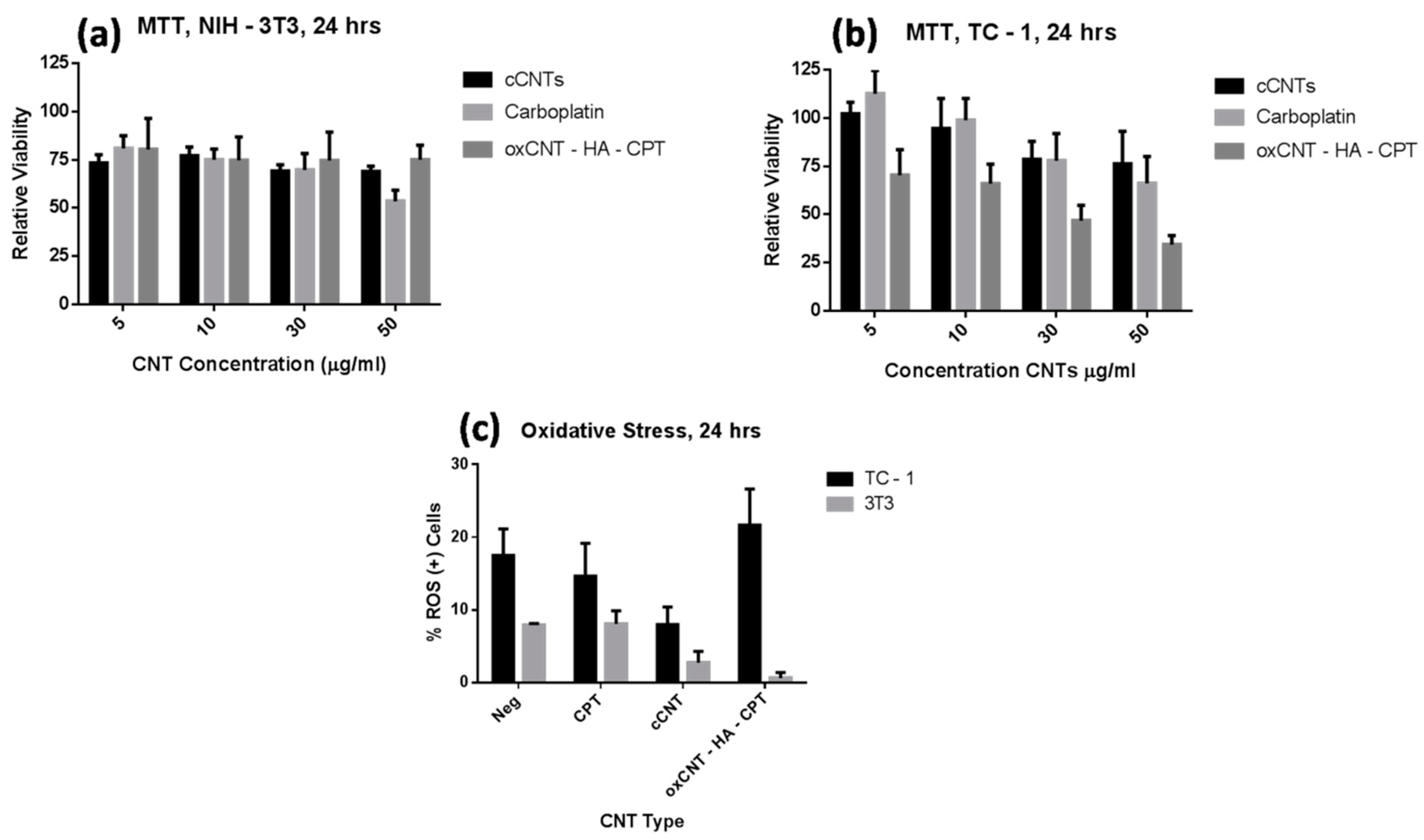
2.5. MTT Assay
2.6. Determination of ROS
2.7. Statistic Analysis
3. Results
3.1. Morphological and Chemical Characterization of Constructed Nanovectors
3.2. Analysis of Constructed Nanovectors Uptake by TC-1 and NIH/3T3 Cell Lines
3.3. Therapeutic Efficacy of Constructed Nanovectors in Tumor and Non-Tumor Cells
3.4. Oxidative Stress Induction by Constructed Nanovectors in TC-1 and NIH/3T3 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomedicine 2016, 12, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Sanginario, A.; Miccoli, B.; Demarchi, D. Carbon Nanotubes as an Effective Opportunity for Cancer Diagnosis and Treatment. Biosensors 2017, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Martincic, M.; Tobias, G. Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin. Drug Deliv. 2014, 12, 563–581. [Google Scholar] [CrossRef]
- Tsukahara, T.; Haniu, H. Cellular cytotoxic response induced by highly purified multi-wall carbon nanotube in human lung cells. Mol. Cell. Biochem. 2011, 352, 57–63. [Google Scholar] [CrossRef]
- Maruyama, K.; Haniu, H.; Saito, N.; Matsuda, Y.; Tsukahara, T.; Kobayashi, S.; Tanaka, M.; Aoki, K.; Takanashi, S.; Okamoto, M.; et al. Endocytosis of multiwalled carbon nanotubes in bronchial epithelial and mesothelial cells. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. The Role of Autophagy as a Mechanism of Toxicity Induced by Multi-Walled Carbon Nanotubes in Human Lung Cells. Int. J. Mol. Sci. 2015, 16, 40–48. [Google Scholar] [CrossRef]
- Goornavar, V.; Biradar, S.; Ezeagwu, C.; Ezeagwu, D.; Hall, J.C.; Ramesh, G.T. Toxicity of Raw and Purified Single-Walled Carbon Nanotubes in Rat ’ s Lung Epithelial and Cervical Cancer Cells. J. Nanosci. Nanotechology 2015, 15, 2105–2114. [Google Scholar] [CrossRef]
- Muzi, L.; Ménard-Moyon, C.; Russier, J.; Li, J.; Chin, C.F.; Ang, W.H.; Pastorin, G.; Risuleo, G.; Bianco, A. Diameter-dependent release of a cisplatin pro-drug from small and large functionalized carbon nanotubes. Nanoscale 2015, 7, 5383–5394. [Google Scholar] [CrossRef]
- Imran, M.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar]
- Hussain, S.; Ji, Z.; Taylor, A.J.; Degraff, L.M.; George, M.; Tucker, C.J.; Chang, C.H.; Li, R.; Bonner, J.C.; Garantziotis, S. Multiwalled Carbon Nanotube Functionalization with High Molecular Weight Hyaluronan Significantly Reduces Pulmonary Injury. ACS Nano 2016, 10, 7675–7688. [Google Scholar] [CrossRef] [PubMed]
- Bhirde, A.A.; Sousa, A.A.; Patel, V.; Azari, A.A.; Gutkind, J.S.; Leapman, R.D.; Rusling, J.F. Imaging the distribution of individual platinum-based anticancer drug molecules attached to single-wall carbon nanotubes. Nanomedicine 2009, 4, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Shrivastava, C.; Jain, A.K. Hyaluronic acid conjugated multi-walled carbon nanotubes for colon cancer targeting. Int. J. Biol. Macromol. 2019, 123, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Advances in use of functionalized carbon nanotubes for drug design and discovery. Expert Opin. Drug Discov. 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meliţă, E.D.; Purcel, G.; Grumezescu, A.M. Carbon nanotubes for cancer therapy and neurodegenerative diseases. Rom J Morphol Embryol 2015, 56, 349–356. [Google Scholar]
- Yoong, S.L.; Wong, B.S.; Zhou, Q.L.; Chin, C.F.; Li, J.; Venkatesan, T.; Ho, H.K.; Yu, V.; Ang, W.H.; Pastorin, G. Enhanced cytotoxicity to cancer cells by mitochondria-targeting MWCNTs containing platinum(IV) prodrug of cisplatin. Biomaterials 2014, 35, 748–759. [Google Scholar] [CrossRef]
- de Sousa, G.F.; Wlodarczyk, S.R.; Monteiro, G. Carboplatin: Molecular mechanisms of action associated with chemoresistance. Brazilian J. Pharm. Sci. 2014, 50, 693–702. [Google Scholar] [CrossRef]
- Arlt, M.; Haase, D.; Hampel, S.; Oswald, S.; Bachmatiuk, A.; Klingeler, R.; Schulze, R.; Ritschel, M.; Leonhardt, A.; Fuessel, S.; et al. Delivery of carboplatin by carbon-based nanocontainers mediates increased cancer cell death. Nanotechnology 2010, 21, 335101. [Google Scholar] [CrossRef]
- Balas, M.; Constanda, S.; Duma-Voiculet, A.; Prodana, M.; Hermenean, A.; Pop, S.; Demetrescu, I.; Dinischiotu, A. Fabrication and toxicity characterization of a hybrid material based on oxidized and aminated MWCNT loaded with carboplatin. Toxicol. Vitr. 2016, 37, 189–200. [Google Scholar] [CrossRef]
- Cao, X.; Tao, L.; Wen, S.; Hou, W.; Shi, X. Hyaluronic acid-modified multiwalled carbon nanotubes for targeted delivery of doxorubicin into cancer cells. Carbohydr. Res. 2015, 405, 70–77. [Google Scholar] [CrossRef]
- Wu, L.; Man, C.; Wang, H.; Lu, X.; Ma, Q.; Cai, Y.; Ma, W. PEGylated multi-walled carbon nanotubes for encapsulation and sustained release of oxaliplatin. Pharm. Res. 2013, 30, 412–423. [Google Scholar] [CrossRef]
- Wang, X.; Xia, T.; Ntim, S.A.; Ji, Z.; George, S.; Meng, H.; Zhang, H.; Castranova, V.; Mitra, S.; Nel, A.E. Quantitative techniques for assessing and controlling the dispersion and biological effects of multiwalled carbon nanotubes in mammalian tissue culture cells. ACS Nano 2010, 4, 7241–7252. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Moreno, M.J.; Duarte-Jurado, A.P.; Gopar-Cuevas, Y.; Gonzalez-Alcocer, A.; Loera-Arias, M.J.; Saucedo-Cardenas, O.; Montes de Oca-Luna, R.; Rodriguez-Rocha, H.; Garcia-Garcia, A. Autophagy Stimulation Decreases Dopaminergic Neuronal Death Mediated by Oxidative Stress. Mol. Neurobiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Matsuda, Y.; Usui, Y.; Haniu, H. Highly purified, multi-wall carbon nanotubes induce light-chain 3B expression in human lung cells. Biochem. Biophys. Res. Commun. 2013, 440, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Hampel, S.; Kunze, D.; Haase, D.; Krämer, K.; Rauschenbach, M.; Ritschel, M.; Leonhardt, A.; Thomas, J.; Oswald, S.; Hoffmann, V.; et al. Carbon nanotubes filled with a chemotherapeutic agent: a nanocarrier mediates inhibition of tumor cell growth. Nanomedicine (Lond) 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroǧlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: biomedical applications. Int. J. Nanomedicine 2012, 7, 5361–5374. [Google Scholar]
- Datir, S.R.; Das, M.; Singh, R.P.; Jain, S. Hyaluronate tethered, “smart” multiwalled carbon nanotubes for tumor-targeted delivery of doxorubicin. Bioconjug. Chem. 2012, 23, 2201–2213. [Google Scholar] [CrossRef]
- Li, R.; Wu, R.; Zhao, L.; Hu, Z.; Guo, S.; Pan, X.; Zou, H. Folate and iron difunctionalized multiwall carbon nanotubes as dual-targeted drug nanocarrier to cancer cells. Carbon N. Y. 2011, 49, 1797–1805. [Google Scholar] [CrossRef]
- Dhar, S.; Liu, Z.; Thomale, J.; Dai, H.; Lippard, S.J. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 2008, 130, 11467–11476. [Google Scholar] [CrossRef]
- Ji, Z.; Lin, G.; Lu, Q.; Meng, L.; Shen, X.; Dong, L.; Fu, C.; Zhang, X. Targeted therapy of SMMC-7721 liver cancer in vitro and in vivo with carbon nanotubes based drug delivery system. J. Colloid Interface Sci. 2012, 365, 143–149. [Google Scholar] [CrossRef]
- Saucedo-Cárdenas, O.; García-Garza, R.; Ramírez-Durón, R.; Muñoz-Maldonado, G.E.; Villanueva-Olivo, A.; Montes-de-Oca-Luna, R.; Soto-Domínguez, A. Invasion of TC-1 cells to skeletal muscle fibers protect them from peroxisomicine A1 (T-514) treatment in a murine model of cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 8062–8071. [Google Scholar]
- Li, J.; Pant, A.; Chin, C.F.; Ang, W.H.; Ménard-Moyon, C.; Nayak, T.R.; Gibson, D.; Ramaprabhu, S.; Panczyk, T.; Bianco, A.; et al. In vivo biodistribution of platinum-based drugs encapsulated into multi-walled carbon nanotubes. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Santana-Davila, R.; Szabo, A.; Arce-Lara, C.; Williams, C.D.; Kelley, M.J.; Whittle, J. Cisplatin versus carboplatin-based regimens for the treatment of patients with metastatic lung cancer. An analysis of Veterans Health Administration data. J. Thorac. Oncol. 2014, 9, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yap, S.Q.; Yoong, S.L.; Nayak, T.R.; Chandra, G.W.; Ang, W.H.; Panczyk, T.; Ramaprabhu, S.; Vashist, S.K.; Sheu, F.S.; et al. Carbon nanotube bottles for incorporation, release and enhanced cytotoxic effect of cisplatin. Carbon N. Y. 2012, 50, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Tan, L.; Zheng, R.; Tan, H.; Zheng, L. Targeted delivery and controlled release of Paclitaxel for the treatment of lung cancer using single-walled carbon nanotubes. Mater. Sci. Eng. C 2016, 68, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Sowerst, L.C.; Loeb, L.A. Reverse chemical mutagenesis: Identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA (oxygen free radicals/DNA damage/cardnogens/mutagns/human ummunodeficency virus reverse transcriptase). Proc. Natl. Acad. Sci. 1994, 91, 6609–6613. [Google Scholar] [CrossRef]
- Oberley, T.D. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997, 12, 525–535. [Google Scholar]
- Vales, G.; Rubio, L.; Marcos, R. Genotoxic and cell-transformation effects of multi-walled carbon nanotubes (MWCNT) following in vitro sub-chronic exposures. J. Hazard. Mater. 2016, 306, 193–202. [Google Scholar] [CrossRef]
- Cheng, C.F.; Juan, S.H.; Chen, J.J.; Chao, Y.C.; Chen, H.H.; Lian, W.S.; Lu, C.Y.; Chang, C.I.; Chiu, T.H.; Lin, H. Pravastatin attenuates carboplatin-induced cardiotoxicity via inhibition of oxidative stress associated apoptosis. Apoptosis 2008, 13, 883–894. [Google Scholar] [CrossRef]
- Galano, A. Carbon nanotubes: Promising agents against free radicals. Nanoscale 2010, 2, 373–380. [Google Scholar] [CrossRef]
- Fenoglio, I.; Tomatis, M.; Lison, D.; Muller, J.; Fonseca, A.; Nagy, J.B.; Fubini, B. Reactivity of carbon nanotubes: Free radical generation or scavenging activity? Free Radic. Biol. Med. 2006, 40, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Sun, L.; Qiao, D.; Wang, D.; Zeng, X. Antioxidant acitivity of low molecular weight hyaluronic acid. Food Chem. Toxicol. 2011, 49, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Treviño, D.; Saucedo-Cárdenas, O.; Loera-Arias, M.d.J.; Rodríguez-Rocha, H.; García-García, A.; Montes-de-Oca-Luna, R.; Piña-Mendoza, E.I.; Contreras-Torres, F.F.; García-Rivas, G.; Soto-Domínguez, A. Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Filled with Carboplatin as a Novel Drug Nanocarrier against Murine Lung Cancer Cells. Nanomaterials 2019, 9, 1572. https://doi.org/10.3390/nano9111572
Salas-Treviño D, Saucedo-Cárdenas O, Loera-Arias MdJ, Rodríguez-Rocha H, García-García A, Montes-de-Oca-Luna R, Piña-Mendoza EI, Contreras-Torres FF, García-Rivas G, Soto-Domínguez A. Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Filled with Carboplatin as a Novel Drug Nanocarrier against Murine Lung Cancer Cells. Nanomaterials. 2019; 9(11):1572. https://doi.org/10.3390/nano9111572
Chicago/Turabian StyleSalas-Treviño, Daniel, Odila Saucedo-Cárdenas, María de Jesús Loera-Arias, Humberto Rodríguez-Rocha, Aracely García-García, Roberto Montes-de-Oca-Luna, Edgar I. Piña-Mendoza, Flavio F. Contreras-Torres, Gerardo García-Rivas, and Adolfo Soto-Domínguez. 2019. "Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Filled with Carboplatin as a Novel Drug Nanocarrier against Murine Lung Cancer Cells" Nanomaterials 9, no. 11: 1572. https://doi.org/10.3390/nano9111572
APA StyleSalas-Treviño, D., Saucedo-Cárdenas, O., Loera-Arias, M. d. J., Rodríguez-Rocha, H., García-García, A., Montes-de-Oca-Luna, R., Piña-Mendoza, E. I., Contreras-Torres, F. F., García-Rivas, G., & Soto-Domínguez, A. (2019). Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Filled with Carboplatin as a Novel Drug Nanocarrier against Murine Lung Cancer Cells. Nanomaterials, 9(11), 1572. https://doi.org/10.3390/nano9111572





