Fluoride-Ion Batteries: On the Electrochemical Stability of Nanocrystalline La0.9Ba0.1F2.9 against Metal Electrodes
Abstract
:1. Introduction
2. Materials and Methods
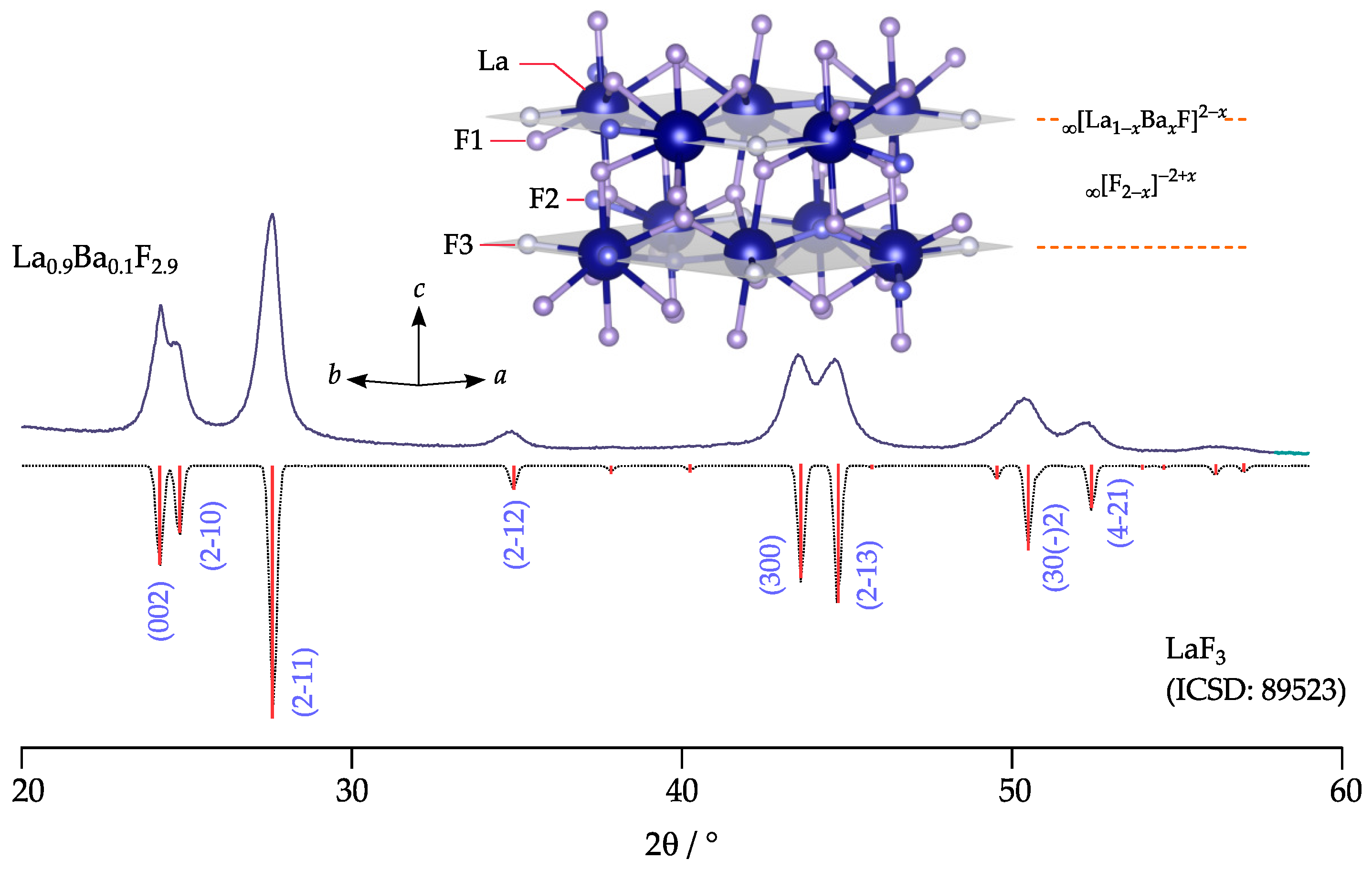
2.1. Mechanochemical Synthesis
2.2. X-Ray Powder Diffraction
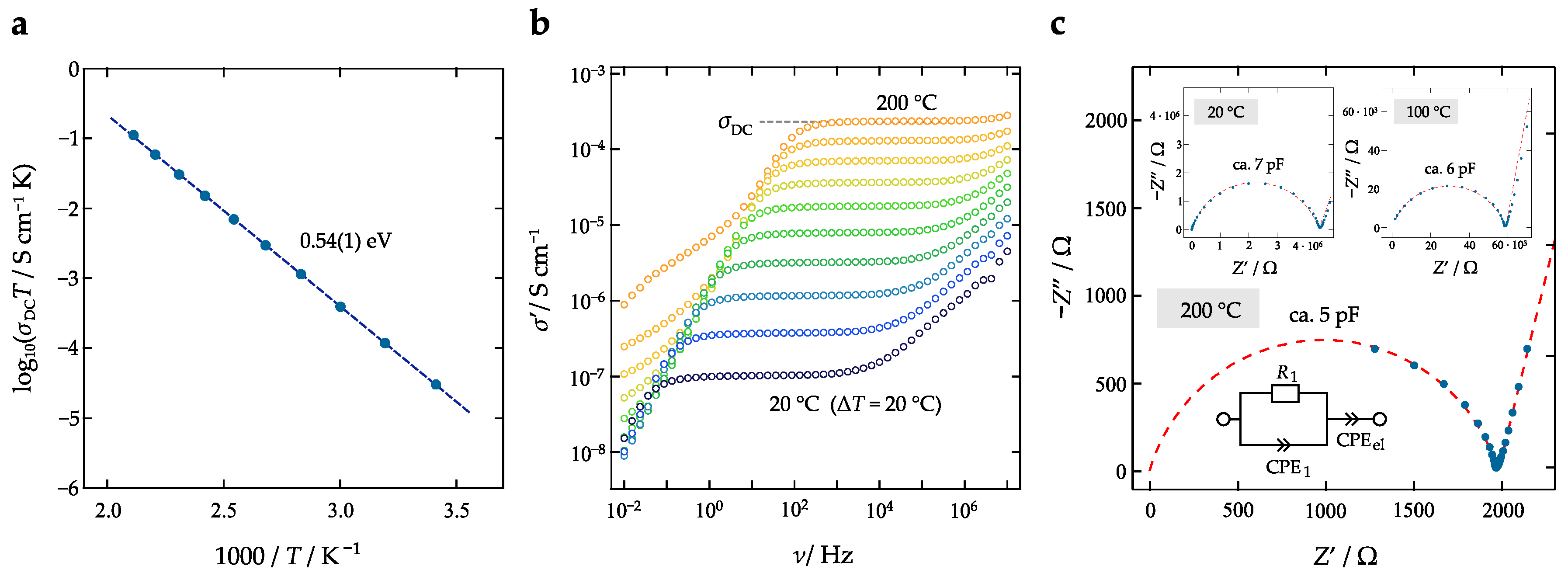
2.3. Broadband Impedance Spectroscopy
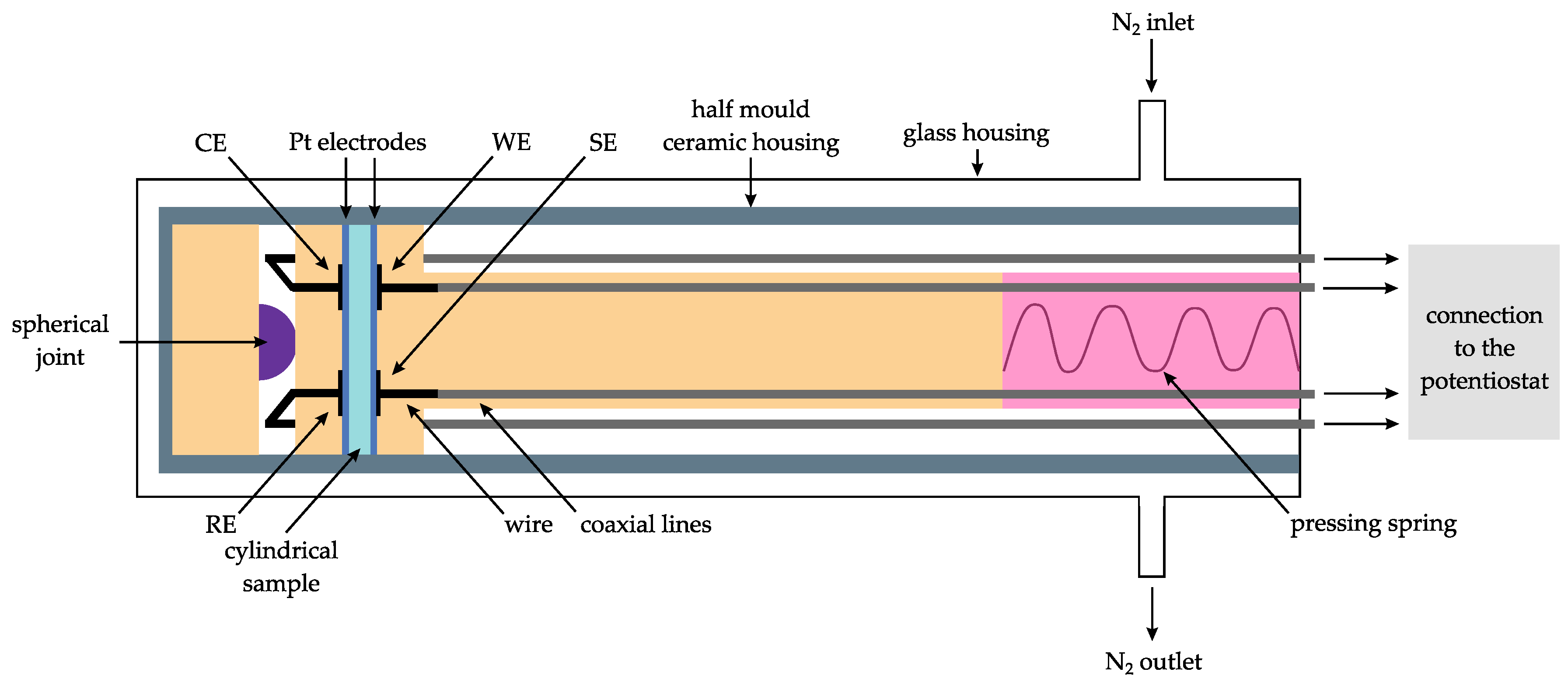
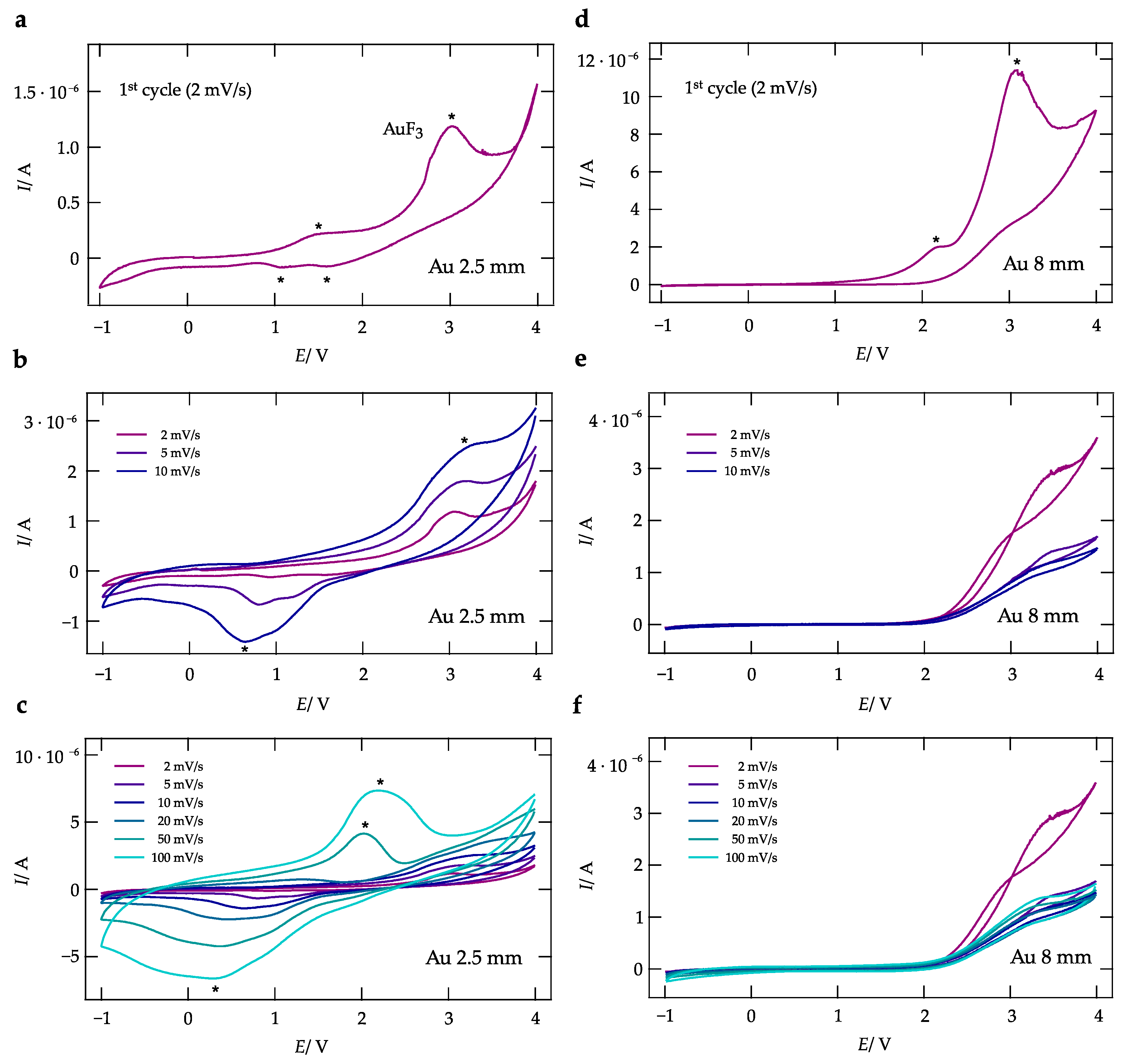
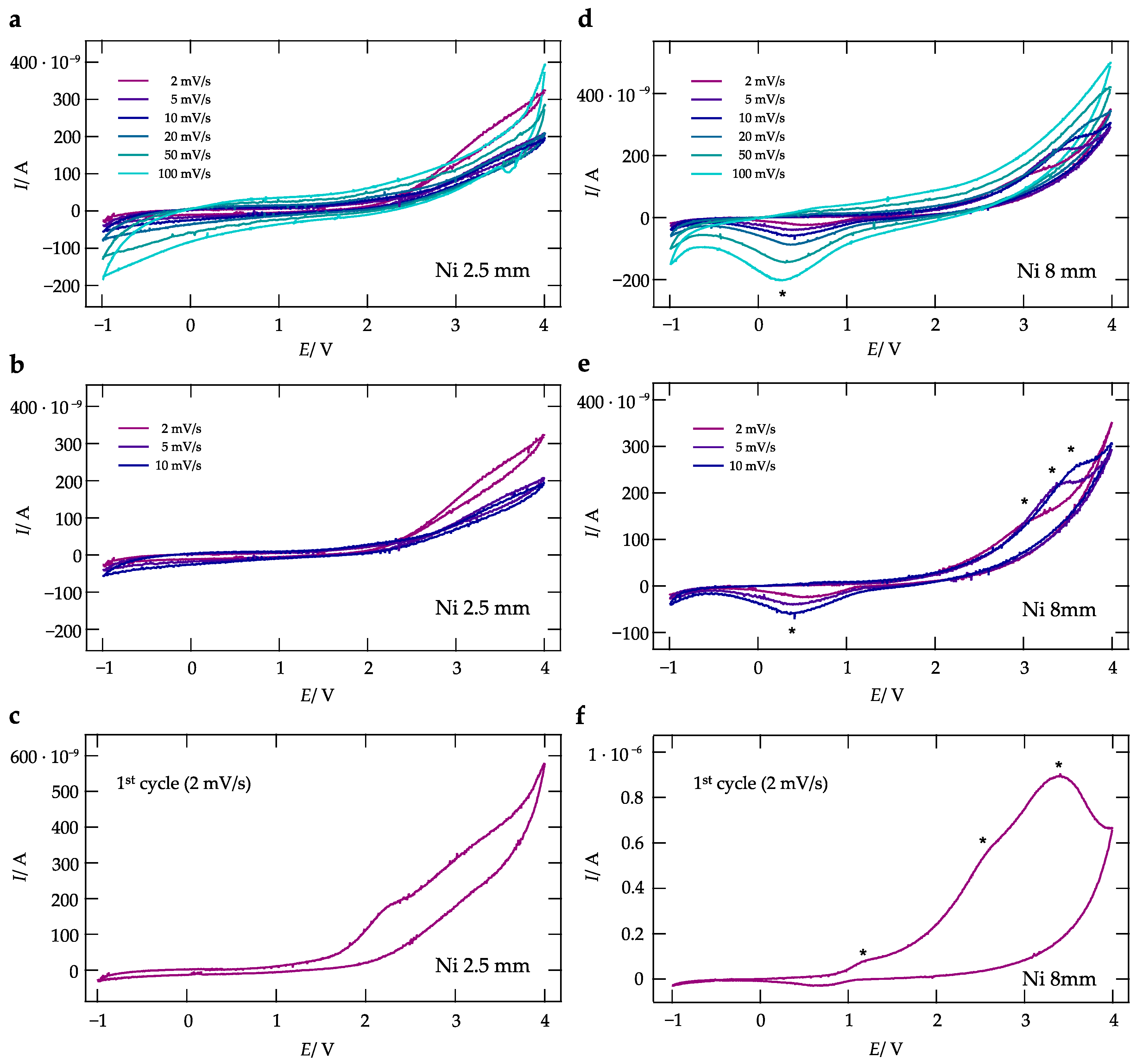
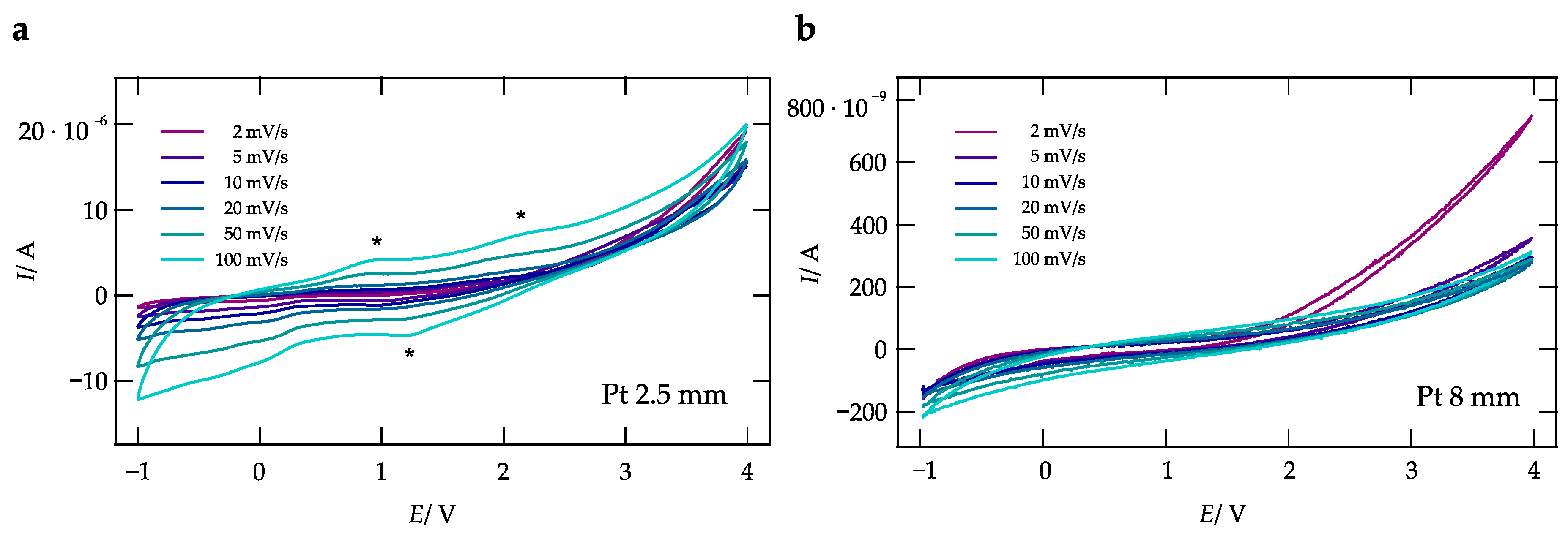
2.4. Cyclic Voltammetry
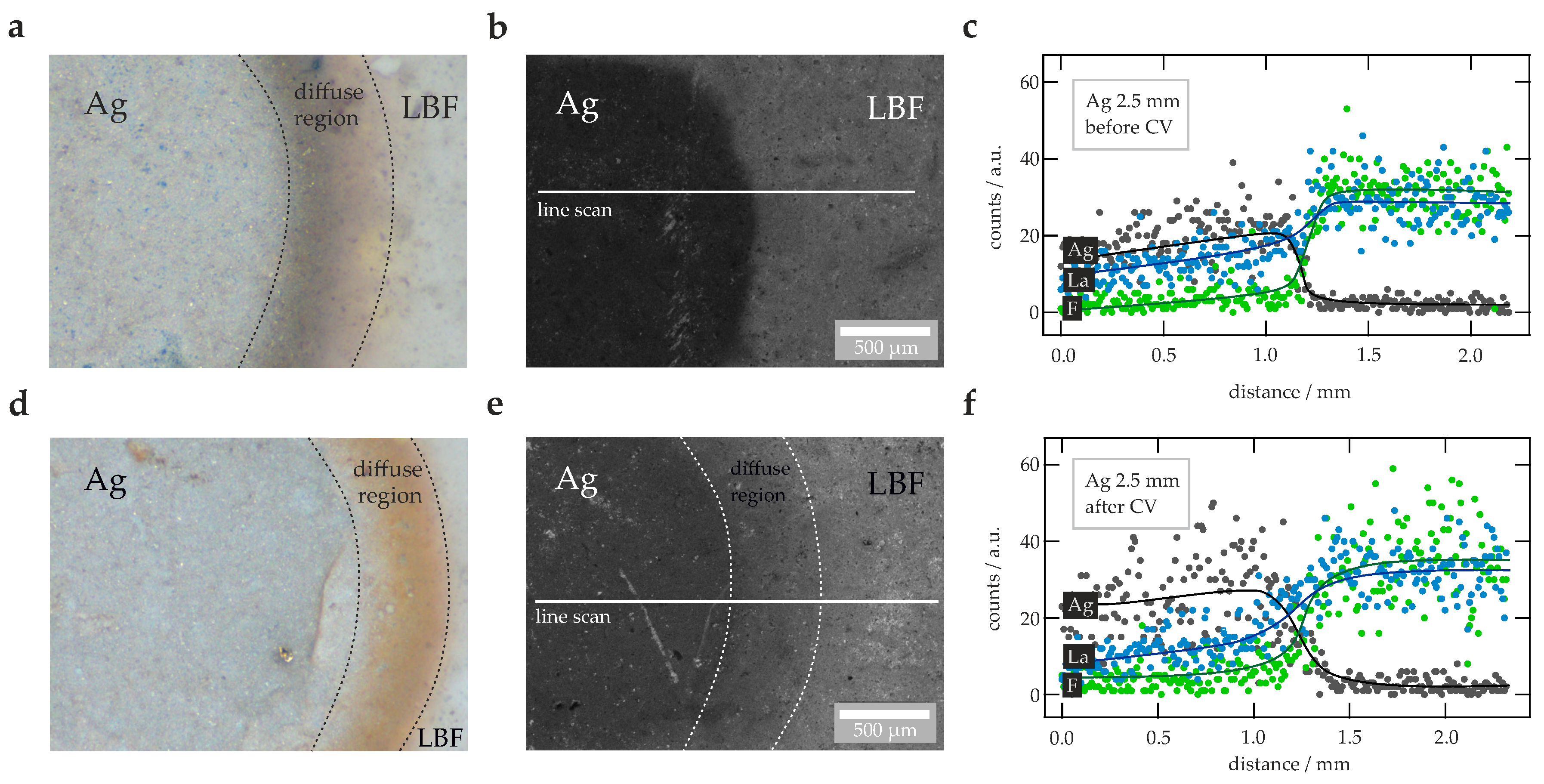
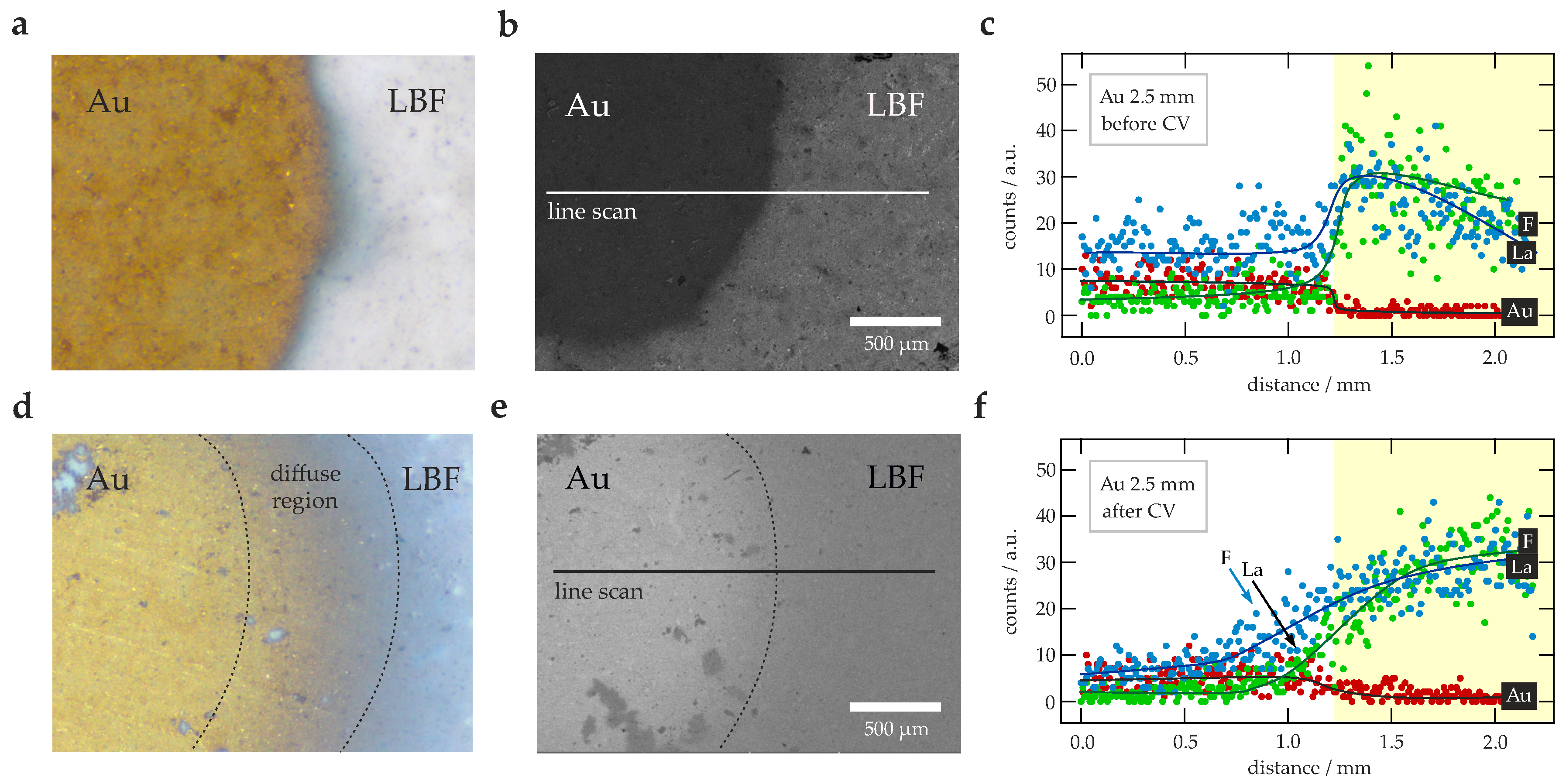
2.5. Scanning Electron Microscopy
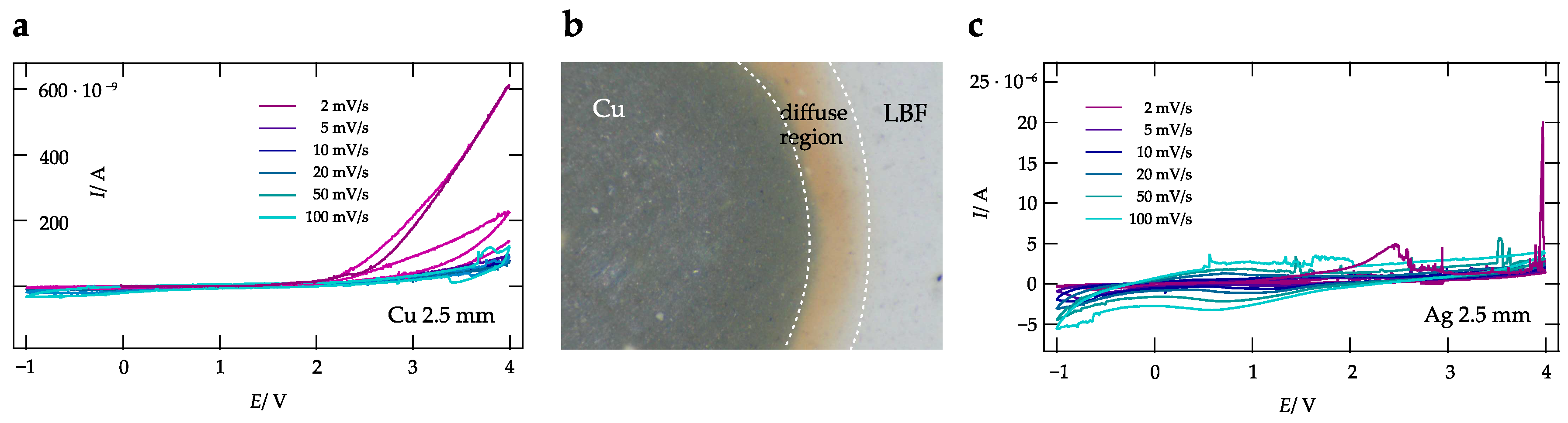
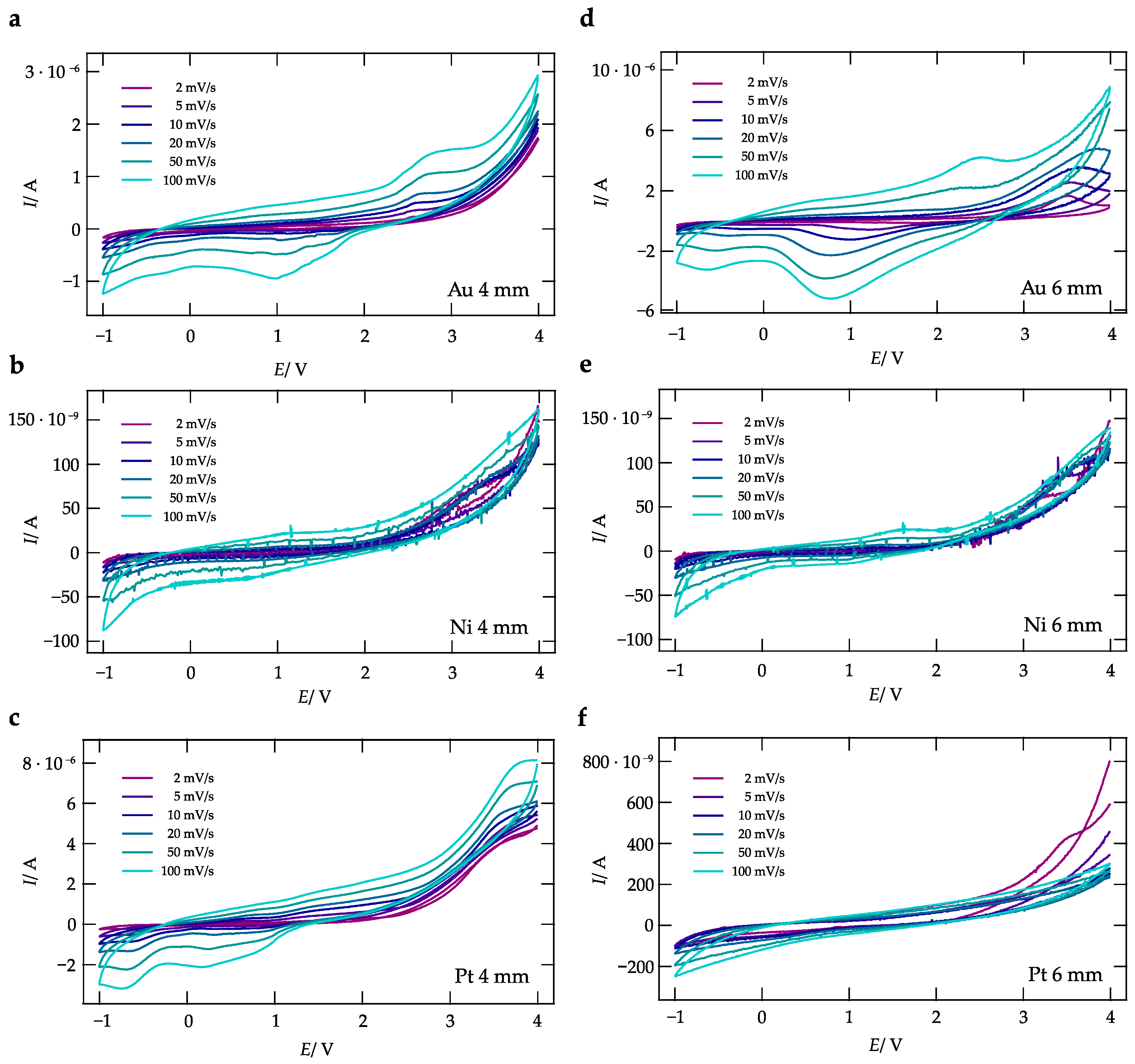
3. Results and Discussion
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| FIB | Fluorine Ion Battery |
| CV | Cyclic Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| SEM | Scanning Electron Microscopy |
| CE | Counter Electrode |
| WE | Working Electrode |
| EDX | Energy-Dispersive X-ray Spectroscopy |
| SE | Secondary Electrons |
| BSE | Backscattered Electrons |
Appendix A
References
- Turcheniuk, K.; Bondarev, D.; Singhal, V.; Yushin, G. Ten Years Left to Redesign Lithium-Ion Batteries. Nature 2018, 559, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A Cost and Resource Analysis of Sodium-Ion Batteries. Nat. Rev. Chem. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Faraday, M. Experimental Researches in Electricity, Art. 1339; Taylor and Francis: London, UK, 1893. [Google Scholar]
- O’Keeffe, M. Phase Transitions and Translational Freedom in Solid Electrolytes. In Superionic Conductors; Springer: Boston, MA, USA, 1976; pp. 101–114. [Google Scholar]
- Funke, K. Solid State Ionics: From Michael Faraday to green energy—The European dimension. Sci. Technol. Adv. Mater. 2013, 14, 043502. [Google Scholar] [CrossRef]
- Takahashi, T. High Conductivity Solid Ionic Conductors: The Past and the Present. In High Conductivity Solid Ionic Conductors; World Scientific: Singapore, 1989; pp. 1–16. [Google Scholar]
- Tubandt, C. Über Elektrizitätsleitung in festen kristallisierten Verbindungen. Zweite Mitteilung. Überführung und Wanderung der Ionen in einheitlichen festen Elektrolyten. Z. Anorg. Allg. Chem. 1921, 115, 105–126. [Google Scholar] [CrossRef]
- Baukal, W. Ger. Offen. Galvanische Zelle mit dotiertem Calciumfluorid als Festelektrolyt. Germany Patent GWXXBX DE 2017128, 7 April 1977. [Google Scholar]
- Kennedy, J.H.; Hunter, J.C. Thin-Film Galvanic Cell Pb/PbF2/PbF2,CuF2/Cu. J. Electrochem. Soc. 1976, 123, 10–14. [Google Scholar] [CrossRef]
- Schoonman, J. A Solid-State Galvanic Cell with Fluoride-Conducting Electrolytes. J. Electrochem. Soc. 1976, 123, 1772–1775. [Google Scholar] [CrossRef]
- Schoonman, J.; Wapenaar, K.; Overluizen, G.; Dirksen, G. Fluoride-Conducting Solid Electrolytes in Galvanic Cells. J. Electrochem. Soc. 1979, 126, 709–713. [Google Scholar] [CrossRef]
- Schoonman, J.; Wolfert, A. Solid-State Galvanic Cells with Fast Fluoride Conducting Electrolytes. Solid State Ion. 1981, 3–4, 373–379. [Google Scholar] [CrossRef]
- Anji Reddy, M.; Fichtner, M. Batteries based on Fluoride Shuttle. J. Mater. Chem. 2011, 21, 17059–17062. [Google Scholar] [CrossRef]
- Reddy, M.A.; Fichtner, M. Fluoride Cathodes for Secondary Batteries. In Advanced Fluoride-Based Materials for Energy Conversion; Nakajima, T., Groult, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 51–76. [Google Scholar]
- Thieu, D.T.; Fawey, M.H.; Bhatia, H.; Diemant, T.; Chakravadhanula, V.S.K.; Behm, R.J.; Kübel, C.; Fichtner, M. CuF2 as Reversible Cathode for Fluoride Ion Batteries. Adv. Fuct. Mater. 2017, 27, 1701051. [Google Scholar] [CrossRef]
- Bhatia, H.; Thieu, D.T.; Pohl, A.H.; Chakravadhanula, V.S.K.; Fawey, M.H.; Kübel, C.; Fichtner, M. Conductivity Optimization of Tysonite-type La1-xBaxF3-x Solid Electrolytes for Advanced Fluoride Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 23707–23715. [Google Scholar] [CrossRef] [PubMed]
- Wapenaar, K.; Koesveld, J.V.; Schoonman, J. Conductivity Enhancement in Fluorite-Structured Ba1-xLaxF2+x Solid Solutions. Solid State Ion. 1981, 2, 145–154. [Google Scholar] [CrossRef]
- Gschwind, F.; Zao-Karger, Z.; Fichtner, M. A Fluoride-Doped PEG Matrix as an Electrolyte for Anion Transportation in a Room-Temperature Fluoride Ion Battery. J. Mater. Chem. A 2014, 2, 1214–1218. [Google Scholar] [CrossRef]
- Davis, V.K.; Bates, C.M.; Omichi, K.; Savoie, B.M.; Momčilović, N.; Xu, Q.; Wolf, W.J.; Webb, M.A.; Billings, K.J.; Chou, N.H.; et al. Room-Temperature Cycling of Metal Fluoride Electrodes: Liquid Electrolytes for High-Energy Fluoride Ion Cells. Science 2018, 362, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Rongeat, C.; Anji Reddy, M.; Diemant, T.; Behm, R.J.; Fichtner, M. Development of New Anode Composite Materials for Fluoride Ion Batteries. J. Mater. Chem. A 2014, 2, 20861–20872. [Google Scholar] [CrossRef]
- Zhang, L.; Reddy, M.A.; Fichtner, M. Electrochemical Performance of All-Solid-State Fluoride-Ion Batteries based on Thin-Film Electrolyte using Alternative Conductive Additives and Anodes. J. Solid State Electrochem. 2018, 22, 997–1006. [Google Scholar] [CrossRef]
- Nowroozi, M.A.; Ivlev, S.; Rohrer, J.; Clemens, O. La2CoO4: A New Intercalation based Cathode Material for Fluoride Ion Batteries with Improved Cycling Stability. J. Mater. Chem. A 2018, 6, 4658–4669. [Google Scholar] [CrossRef]
- Mohammad, I.; Chable, J.; Witter, R.; Fichtner, M.; Reddy, M.A. Synthesis of Fast Fluoride-Ion-Conductive Fluorite-Type Ba1-xSbxF2+x (0.1 <x< 0.4): A Potential Solid Electrolyte for Fluoride-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 17249–17256. [Google Scholar]
- Dieudonne, B.; Chable, J.; Body, M.; Legein, C.; Durand, E.; Mauvy, F.; Fourcade, S.; Leblanc, M.; Maisonneuve, V.; Demourgues, A. The Key Role of the Composition and Structural Features in Fluoride Ion Conductivity in Tysonite Ce1-xSrxF3-x Solid Solutions. Dalton Trans. 2017, 46, 3761–3769. [Google Scholar] [CrossRef]
- Dieudonne, B.; Chable, J.; Mauvy, F.; Fourcade, S.; Durand, E.; Lebraud, E.; Leblanc, M.; Legein, C.; Body, M.; Maisonneuve, V.; et al. Exploring the Sm1-xCaxF3-x Tysonite Solid Solution as a Solid-State Electrolyte: Relationships between Structural Features and Fast Ionic Conductivity. J. Phys. Chem. C 2015, 119, 25170–25179. [Google Scholar] [CrossRef]
- Raccichini, R.; Amores, M.; Hinds, G. Critical Review of the Use of Reference Electrodes in Li-Ion Batteries: A Diagnostic Perspective. Batteries 2019, 5, 12. [Google Scholar] [CrossRef]
- Breuer, S.; Gombotz, M.; Pregartner, V.; Hanzu, I.; Wilkening, M. Heterogeneous F Anion Transport, Local Dynamics and Electrochemical Stability of Nanocrystalline La1-xBaxF3-x. Energy Storage Mater. 2019, 16, 481–490. [Google Scholar] [CrossRef]
- Setup of The Impedance Measuring Cell (Novocontrol). Available online: https://www.novocontrol.de/brochures/BDS1200.pdf (accessed on 21 October 2019).
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Gombotz, M.; Lunghammer, S.; Breuer, S.; Hanzu, I.; Preishuber-Pflügl, F.; Wilkening, H.M.R. Spatial Confinement—Rapid 2D F- Diffusion in Micro- and Nanocrystalline RbSn2F5. Phys. Chem. Chem. Phys. 2019, 21, 1872–1883. [Google Scholar] [CrossRef]
- Preishuber-Pflügl, F.; Bottke, P.; Pregartner, V.; Bitschnau, B.; Wilkening, M. Correlated Fluorine Diffusion and Ionic conduction in the Nanocrystalline F- Solid Electrolyte Ba0.6La0.4F2.4 – 19F T1(ρ) NMR Relaxation vs. Conductivity Measurements. Phys. Chem. Chem. Phys. 2014, 16, 9580–9590. [Google Scholar] [CrossRef]
- Narayanan, S.; Epp, V.; Wilkening, M.; Thangadurai, V. Macroscopic and Microscopic Li+ Transport Parameters in Cubic Garnet-Type “Li6.5La2.5Ba0.5ZrTaO12” as Probed by Impedance Spectroscopy and NMR. RSC Adv. 2012, 2, 2553–2561. [Google Scholar] [CrossRef]
- Lunghammer, S.; Ma, Q.; Rettenwander, D.; Hanzu, I.; Tietz, F.; Wilkening, H. Bulk and Grain-Boundary Ionic Conductivity in Sodium Zirconophosphosilicate Na3Zr2(SiO4)2PO4 (NASICON). Chem. Phys. Lett. 2018, 701, 147–150. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gombotz, M.; Pregartner, V.; Hanzu, I.; Wilkening, H.M.R. Fluoride-Ion Batteries: On the Electrochemical Stability of Nanocrystalline La0.9Ba0.1F2.9 against Metal Electrodes. Nanomaterials 2019, 9, 1517. https://doi.org/10.3390/nano9111517
Gombotz M, Pregartner V, Hanzu I, Wilkening HMR. Fluoride-Ion Batteries: On the Electrochemical Stability of Nanocrystalline La0.9Ba0.1F2.9 against Metal Electrodes. Nanomaterials. 2019; 9(11):1517. https://doi.org/10.3390/nano9111517
Chicago/Turabian StyleGombotz, Maria, Veronika Pregartner, Ilie Hanzu, and H. Martin R. Wilkening. 2019. "Fluoride-Ion Batteries: On the Electrochemical Stability of Nanocrystalline La0.9Ba0.1F2.9 against Metal Electrodes" Nanomaterials 9, no. 11: 1517. https://doi.org/10.3390/nano9111517
APA StyleGombotz, M., Pregartner, V., Hanzu, I., & Wilkening, H. M. R. (2019). Fluoride-Ion Batteries: On the Electrochemical Stability of Nanocrystalline La0.9Ba0.1F2.9 against Metal Electrodes. Nanomaterials, 9(11), 1517. https://doi.org/10.3390/nano9111517




