Elongated Flexuous Plant Virus-Derived Nanoparticles Functionalized for Autoantibody Detection
Abstract
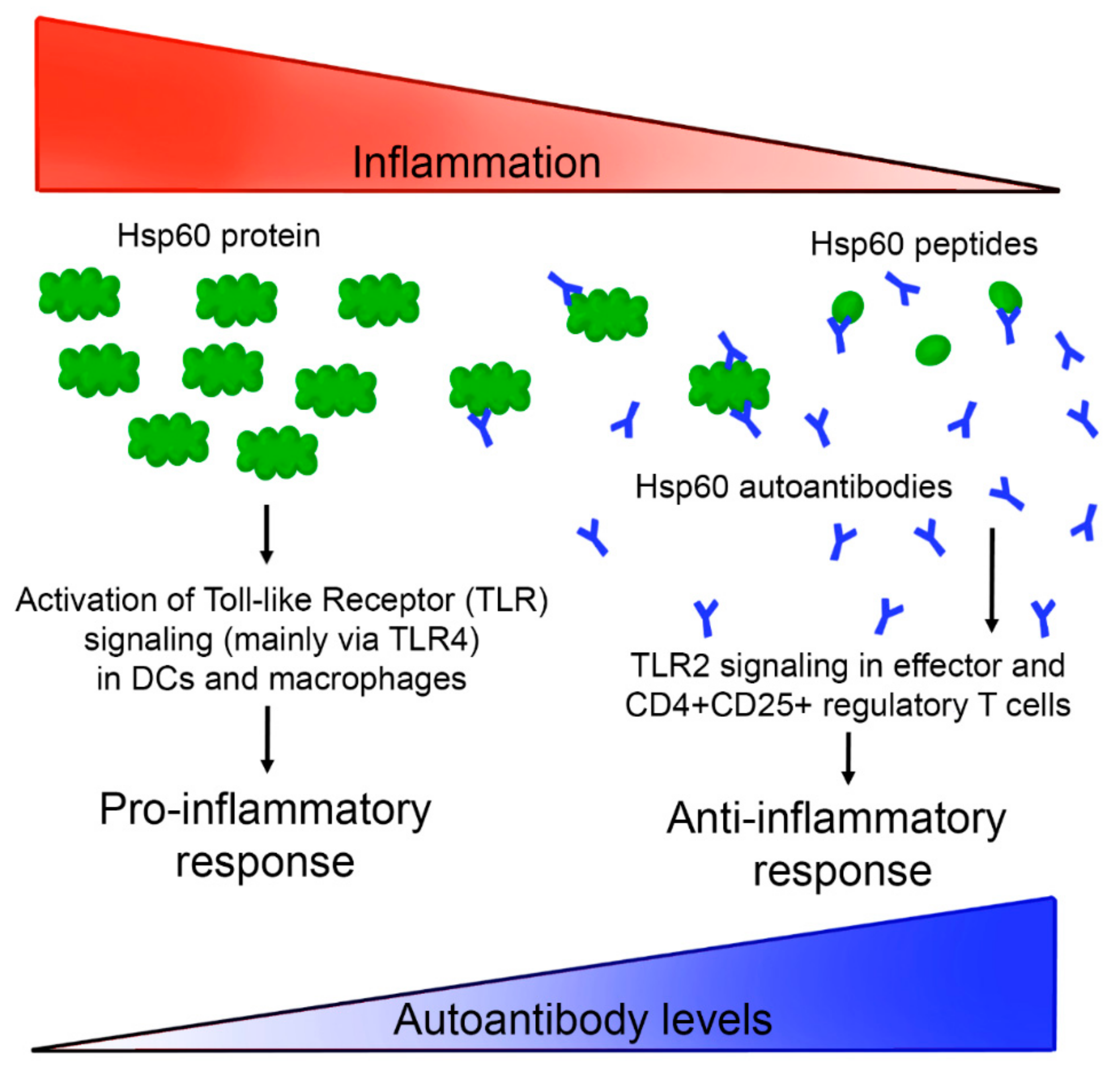
1. Introduction
2. Materials and Methods
2.1. Construction of CP and Hsp60-CP Expression Plasmids
2.2. Production and Purification of VLPs
2.3. VLP Characterization
2.4. IBD Murine Model
2.5. Immunoassays
3. Results
3.1. Production and Characterization of Hsp60-VLPs
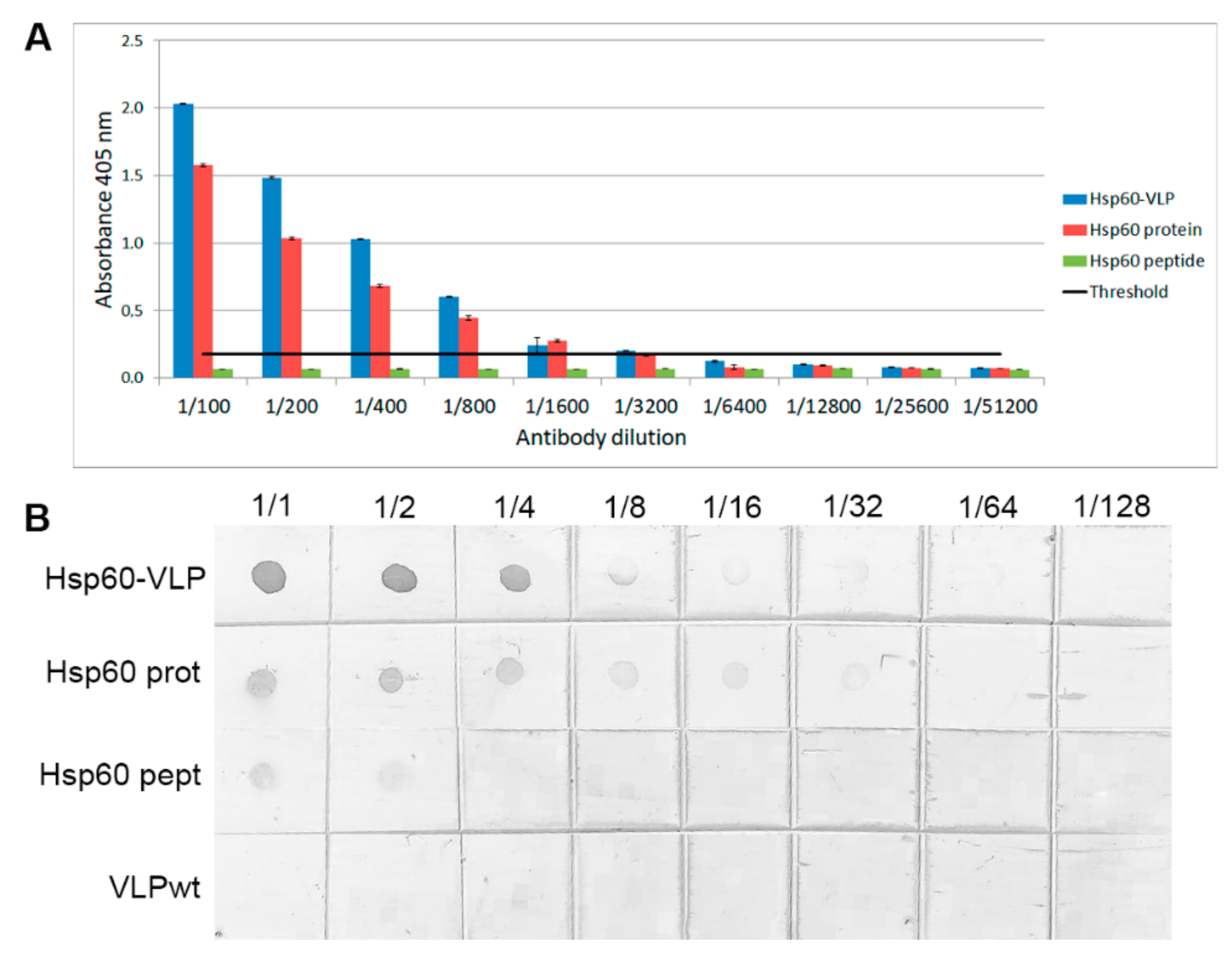
3.2. Increased Sensitivity of Hsp60 Peptide Detection by Specific Hsp60 Antibodies
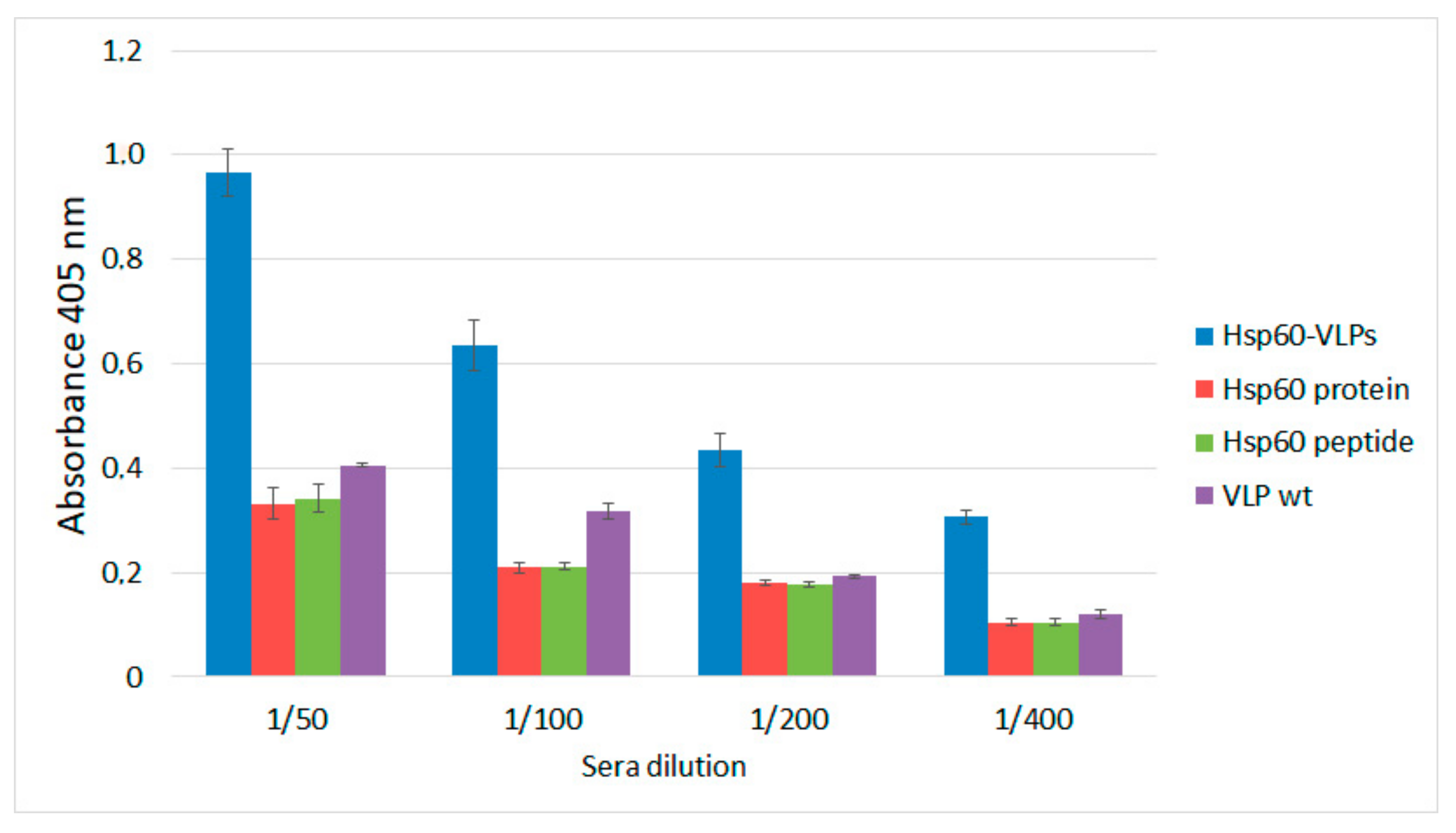
3.3. Hsp60-VLPs Autoantibody Detection
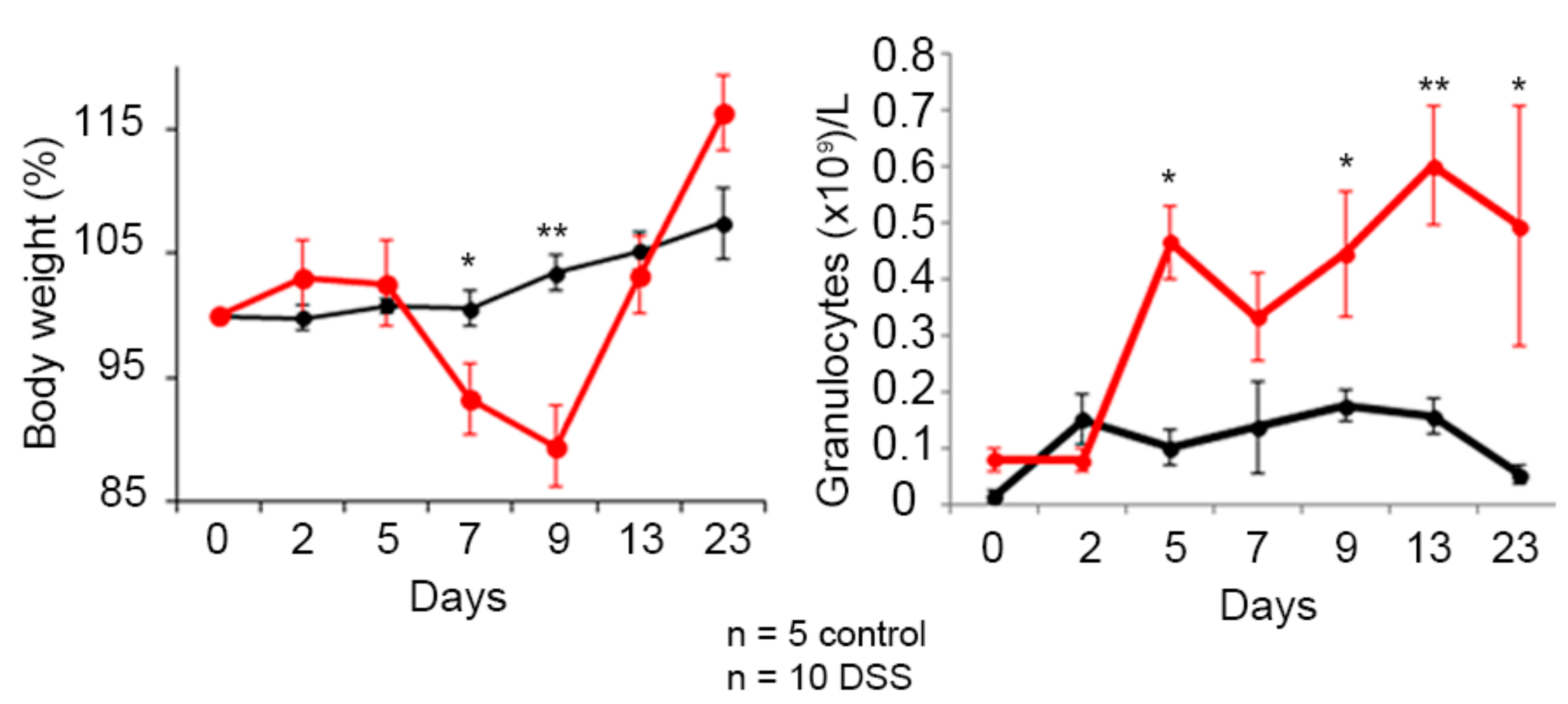
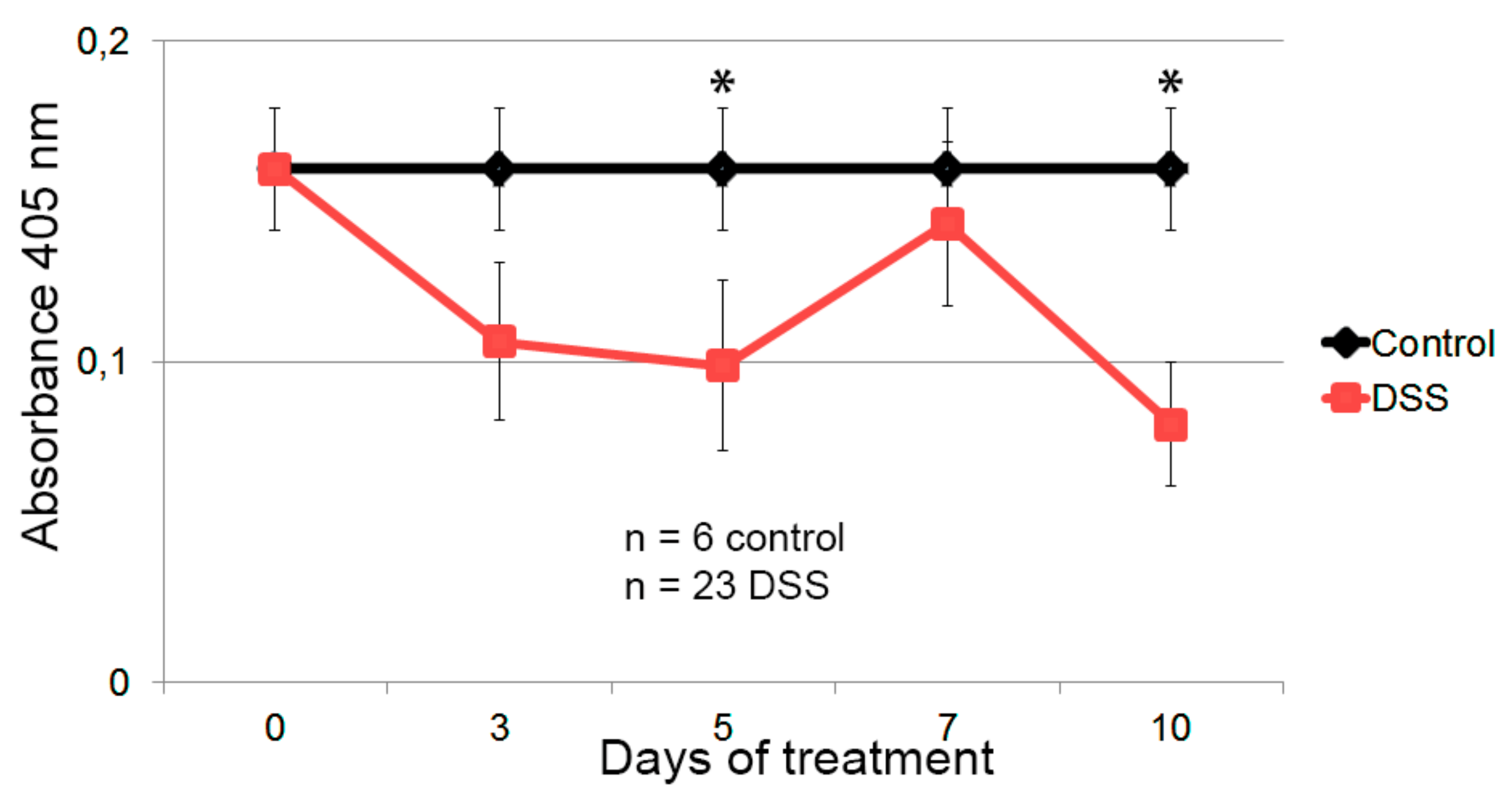
3.4. Hsp60-VLPs for Autoantibody Detection in DSS-Induced Colitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steele, J.F.C.; Peyret, H.; Saunders, K.; Castells-Graells, R.; Marsian, J.; Meshcheriakova, Y.; Lomonossoff, G.P. Synthetic plant virology for nanobiotechnology and nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1447. [Google Scholar] [CrossRef] [PubMed]
- Rohovie, M.J.; Nagasawa, M.; Swartz, J.R. Virus-like particles: Next-generation nanoparticles for targeted therapeutic delivery. Bioeng. Transl. Med. 2017, 2, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Twyman, R.M.; Fiering, S.; Steinmetz, N.F. Virus-based nanoparticles as platform technologies for modern vaccines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 554–578. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.; Douglas, T. Development of virus-like particles for diagnostic and prophylactic biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Lomonossoff, G.P.; Evans, D.J. Applications of plant viruses in bionanotechnology. Curr. Top. Microbiol. Immunol. 2014, 375, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, J.; Tullman-Ercek, D. Production and applications of engineered viral capsids. Appl. Microbiol. Biotechnol. 2014, 98, 5847–5858. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lai, H. Plant-derived virus-like particles as vaccines. Hum. Vaccines Immunother. 2013, 9, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Gorzny, M.L.; Bittner, A.M. The physics of tobacco mosaic virus and virus-based devices in biotechnology. Trends Biotechnol. 2013, 31, 530–538. [Google Scholar] [CrossRef]
- Yildiz, I.; Shukla, S.; Steinmetz, N.F. Applications of viral nanoparticles in medicine. Curr. Opin. Biotechnol. 2011, 22, 901–908. [Google Scholar] [CrossRef]
- Pokorski, J.K.; Steinmetz, N.F. The art of engineering viral nanoparticles. Mol. Pharm. 2011, 8, 29–43. [Google Scholar] [CrossRef]
- Plummer, E.M.; Manchester, M. Viral nanoparticles and virus-like particles: Platforms for contemporary vaccine design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 174–196. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Wagner, R. Virus-like particles-universal molecular toolboxes. Curr. Opin. Biotechnol. 2007, 18, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, S.; Mansilla, C.; Aguado, M.; Yuste-Calvo, C.; Sánchez, F.; Sánchez-Montero, J.M.; Ponz, F. Nanonets derived from Turnip mosaic virus as scaffolds for increased enzymatic activity of immobilized Candida antarctica lipase B. Front. Plant Sci. 2016, 7, 464. [Google Scholar] [CrossRef] [PubMed]
- Yuste-Calvo, C.; González-Gamboa, I.; Pacios, L.F.; Sánchez, F.; Ponz, F. Structure-based multifunctionalization of flexuous elongated viral nanoparticles. ACS Omega 2019, 4, 5019–5028. [Google Scholar] [CrossRef]
- González-Gamboa, I.; Manrique, P.; Sánchez, F.; Ponz, F. Plant-made potyvirus-like particles used for log-increasing antibody sensing capacity. J. Biotechnol. 2017, 254, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Sáez, M.; Lunello, P.; Ponz, F. Plant viral elongated nanoparticles modified for log-increases of foreign peptide immunogenicity and specific antibody detection. J. Biotechnol. 2013, 168, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, B.; De Hertogh, G.; Sagaert, X. Inflammatory Bowel Diseases. In Pathobiology of Human Disease; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: San Diego, CA, USA, 2014; Volume 19, pp. 1297–1304. [Google Scholar]
- Denmark, V.K.; Mayer, L. Chapter 60—Inflammatory Bowel Diseases. In The Autoimmune Diseases, 5th ed.; Rose, N.R., Mackay, I.R., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 873–888. [Google Scholar]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Van Eden, W.; Jansen, M.A.A.; Ludwig, I.S.; Leufkens, P.; van der Goes, M.C.; van Laar, J.M.; Broere, F. Heat shock proteins can be surrogate autoantigens for induction of antigen specific therapeutic tolerance in rheumatoid arthritis. Front. Immunol. 2019, 10, 279. [Google Scholar] [CrossRef]
- Mantej, J.; Polasik, K.; Piotrowska, E.; Tukaj, S. Autoantibodies to heat shock proteins 60, 70, and 90 in patients with rheumatoid arthritis. Cell Stress Chaperones 2019, 24, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Gonciarz, W.; Matusiak, A.; Rudnicka, K.; Rechcinski, T.; Chalubinski, M.; Czkwianianc, E.; Broncel, M.; Gajewski, A.; Chmiela, M. Autoantibodies to a specific peptide epitope of human Hsp60 (ATVLA) with homology to Helicobacter pylori HspB in H. pylori-infected patients. APMIS 2019, 127, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Ulmansky, R.; Naparstek, Y. Protective antibodies against HSP60 for autoimmune inflammatory diseases. Clin. Immunol. 2018, 186, 63. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, T.; Venkatachala, S.K.; Ramanjappa, M.; Kakkar, V.V.; Mundkur, L.A. Inverse association of ApoB and HSP60 antibodies with coronary artery disease in Indian population. Heart Asia 2018, 10, e011018. [Google Scholar] [CrossRef] [PubMed]
- Selli, M.E.; Wick, G.; Wraith, D.C.; Newby, A.C. Autoimmunity to HSP60 during diet induced obesity in mice. Int. J. Obes. (Lond.) 2017, 41, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Jung, K.Y.; Hwang, E.; Jang, Y.J. Characterization of human anti-heat shock protein 60 monoclonal autoantibody Fab fragments in atherosclerosis: Genetic and functional analysis. Mol. Immunol. 2013, 54, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Sakurai, T.; Yamada, M.; Koumura, A.; Hayashi, Y.; Tanaka, Y.; Hozumi, I.; Takemura, M.; Seishima, M.; Inuzuka, T. Elevated anti-heat shock protein 60 antibody titer is related to white matter hyperintensities. J. Stroke Cerebrovasc. Dis. 2012, 21, 305–309. [Google Scholar] [CrossRef]
- Quintana, F.J.; Cohen, I.R. The HSP60 immune system network. Trends Immunol. 2011, 32, 89–95. [Google Scholar] [CrossRef]
- Khalil, A.A.; Kabapy, N.F.; Deraz, S.F.; Smith, C. Heat shock proteins in oncology: Diagnostic biomarkers or therapeutic targets? Biochim. Biophys. Acta 2011, 1816, 89–104. [Google Scholar] [CrossRef]
- Cappello, F.; Mazzola, M.; Jurjus, A.; Zeenny, M.N.; Jurjus, R.; Carini, F.; Leone, A.; Bonaventura, G.; Tomasello, G.; Bucchieri, F.; et al. Hsp60 as a novel target in IBD management: A prospect. Front. Pharmacol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Tomasello, G.; Sciume, C.; Rappa, F.; Rodolico, V.; Zerilli, M.; Martorana, A.; Cicero, G.; De Luca, R.; Damiani, P.; Accardo, F.M.; et al. Hsp10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur. J. Histochem. 2011, 55, e38. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Rodolico, V.; Zerilli, M.; Martorana, A.; Bucchieri, F.; Pitruzzella, A.; Marino Gammazza, A.; David, S.; Rappa, F.; Zummo, G.; et al. Changes in immunohistochemical levels and subcellular localization after therapy and correlation and colocalization with CD68 suggest a pathogenetic role of Hsp60 in ulcerative colitis. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Rodolico, V.; Tomasello, G.; Zerilli, M.; Martorana, A.; Pitruzzella, A.; Gammazza, A.M.; David, S.; Zummo, G.; Damiani, P.; Accomando, S.; et al. Hsp60 and Hsp10 increase in colon mucosa of Crohn’s disease and ulcerative colitis. Cell Stress Chaperones 2010, 15, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Kolinski, T.; Marek-Trzonkowska, N.; Trzonkowski, P.; Siebert, J. Heat shock proteins (HSPs) in the homeostasis of regulatory T cells (Tregs). Cent. Eur. J. Immunol. 2016, 41, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.E.; Pers, J.O.; Youinou, P.; Jamin, C. Chapter 41—Heat shock protein autoantibodies. In Autoantibodies, 3rd ed.; Shoenfeld, Y., Meroni, P.L., Gershwin, M.E., Eds.; Elsevier: San Diego, CA, USA, 2014; pp. 343–348. [Google Scholar]
- Grundtman, C.; Kreutmayer, S.B.; Almanzar, G.; Wick, M.C.; Wick, G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Habich, C.; Burkart, V. Heat shock protein 60: Regulatory role on innate immune cells. Cell. Mol. Life Sci. 2007, 64, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Pinar, O.; Ozden, Y.A.; Omur, E.; Muhtesem, G. Heat shock proteins in multiple sclerosis. In Multiple Sclerosis: Bench to Bedside: Global Perspectives on a Silent Killer; Asea, A.A.A., Geraci, F., Kaur, P., Eds.; Springer International Publishing Ag: Champaign, IL, USA, 2017; Volume 958, pp. 29–42. [Google Scholar]
- Ulmansky, R.; Landstein, D.; Moallem, E.; Loeb, V.; Levin, A.; Meyuhas, R.; Katzavian, G.; Yair, S.; Naparstek, Y. A humanized monoclonal antibody against Heat Shock Protein 60 suppresses murine arthritis and colitis and skews the cytokine balance toward an anti-inflammatory response. J. Immunol. 2015, 194, 5103–5109. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Ou, L.S.; Yeh, K.W.; Lee, W.I.; Huang, J.L. Serum heat shock protein 60 can predict remission of flare-up in juvenile idiopathic arthritis. Clin. Rheumatol. 2011, 30, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Touriño, A.; Sánchez, F.; Fereres, A.; Ponz, F. High expression of foreign proteins from a biosafe viral vector derived from Turnip mosaic virus. Span. J. Agric. Res. 2008, 6, 48–58. [Google Scholar] [CrossRef]
- D’Aoust, M.A.; Lavoie, P.O.; Couture, M.M.; Trepanier, S.; Guay, J.M.; Dargis, M.; Mongrand, S.; Landry, N.; Ward, B.J.; Vezina, L.P. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 2008, 6, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Liu, L.; Lomonossoff, G.P. Cowpea mosaic virus-based systems for the expression of antigens and antibodies in plants. Methods Mol. Biol. 2009, 483, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Duval, F.; Cruz-Vega, D.E.; González-Gamboa, I.; González-Garza, M.T.; Ponz, F.; Sánchez, F.; Alarcon-Galvan, G.; Moreno-Cuevas, J.E. Detection of autoantibodies to vascular endothelial growth factor receptor-3 in bile duct ligated rats and correlations with a panel of traditional markers of liver diseases. Dis. Markers 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Van Eden, W.; van Herwijnen, M.; Wagenaar, J.; van Kooten, P.; Broere, F.; van der Zee, R. Stress proteins are used by the immune system for cognate interactions with anti-inflammatory regulatory T cells. FEBS Lett. 2013, 587, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.B.; Vermeire, S. Differential diagnosis of inflammatory bowel disease: Imitations and complications. Lancet Gastroenterol. Hepatol. 2018, 3, 644–653. [Google Scholar] [CrossRef]
- Quintana, F.J.; Farez, M.F.; Viglietta, V.; Iglesias, A.H.; Merbl, Y.; Izquierdo, G.; Lucas, M.; Basso, A.S.; Khoury, S.J.; Lucchinetti, C.F.; et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2008, 105, 18889–18894. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, G.S. Protein/peptide drug delivery: Advanced drug delivery systems (DDSs). Biotech Int. 2010, 22, 10–13. [Google Scholar]
- Lico, C.; Benvenuto, E.; Baschieri, S. The two-faced Potato virus X: From plant pathogen to smart nanoparticle. Front. Plant Sci. 2015, 6, 1009. [Google Scholar] [CrossRef]
- Higa, A.M.; Mambrini, G.P.; Ierich, J.C.M.; Garcia, P.S.; Scramin, J.A.; Peroni, L.A.; Okuda-Shinagawa, N.M.; Machini, M.T.; Trivinho-Strixino, F.; Leite, F.L. Peptide-conjugated silver nanoparticle for autoantibody recognition. J. Nanosci. Nanotechnol. 2019, 19, 7564–7573. [Google Scholar] [CrossRef]
- Poletaev, A.; Osipenko, L. General network of natural autoantibodies as immunological homunculus (Immunculus). Autoimmun. Rev. 2003, 2, 264–271. [Google Scholar] [CrossRef]
- Cohen, I.R. Autoantibody repertoires, natural biomarkers, and system controllers. Trends Immunol. 2013, 34, 620–625. [Google Scholar] [CrossRef]
- Papuc, E.; Krupski, W.; Kurys-Denis, E.; Rejdak, K. Antibodies against small heat-shock proteins in Alzheimer’s disease as a part of natural human immune repertoire or activation of humoral response? J. Neural Transm. (Vienna) 2016, 123, 455–461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, Y.; Tang, H.; Wang, X.; Zeng, Q.; Liu, Y.; Zhao, X.I.; Yu, K.; Shi, H.; Zhu, R.; Mao, X. Intranasal immunization with heat shock protein 60 induces CD4(+) CD25(+) GARP(+) and type 1 regulatory T cells and inhibits early atherosclerosis. Clin. Exp. Immunol. 2016, 183, 452–468. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuste-Calvo, C.; López-Santalla, M.; Zurita, L.; Cruz-Fernández, C.F.; Sánchez, F.; Garín, M.I.; Ponz, F. Elongated Flexuous Plant Virus-Derived Nanoparticles Functionalized for Autoantibody Detection. Nanomaterials 2019, 9, 1438. https://doi.org/10.3390/nano9101438
Yuste-Calvo C, López-Santalla M, Zurita L, Cruz-Fernández CF, Sánchez F, Garín MI, Ponz F. Elongated Flexuous Plant Virus-Derived Nanoparticles Functionalized for Autoantibody Detection. Nanomaterials. 2019; 9(10):1438. https://doi.org/10.3390/nano9101438
Chicago/Turabian StyleYuste-Calvo, Carmen, Mercedes López-Santalla, Lucía Zurita, César F. Cruz-Fernández, Flora Sánchez, Marina I. Garín, and Fernando Ponz. 2019. "Elongated Flexuous Plant Virus-Derived Nanoparticles Functionalized for Autoantibody Detection" Nanomaterials 9, no. 10: 1438. https://doi.org/10.3390/nano9101438
APA StyleYuste-Calvo, C., López-Santalla, M., Zurita, L., Cruz-Fernández, C. F., Sánchez, F., Garín, M. I., & Ponz, F. (2019). Elongated Flexuous Plant Virus-Derived Nanoparticles Functionalized for Autoantibody Detection. Nanomaterials, 9(10), 1438. https://doi.org/10.3390/nano9101438





