Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium Discs
2.2. Protein Adsorption
2.2.1. Surface Pre-Conditioning
2.2.2. Bradford Assay
2.3. Osteoblasts Adhesion Analysis
2.3.1. Cell Culture
2.3.2. Surface Conditioning
2.3.3. Adhesion Assay
2.3.4. Cell Morphology and Focal Adhesion Expression
2.3.5. Morphological Analysis of Osteoblast/Titanium Relationship
2.4. Statistical Analysis
3. Results
3.1. Serum Proteins Firmly Bind to Titanium Surfaces
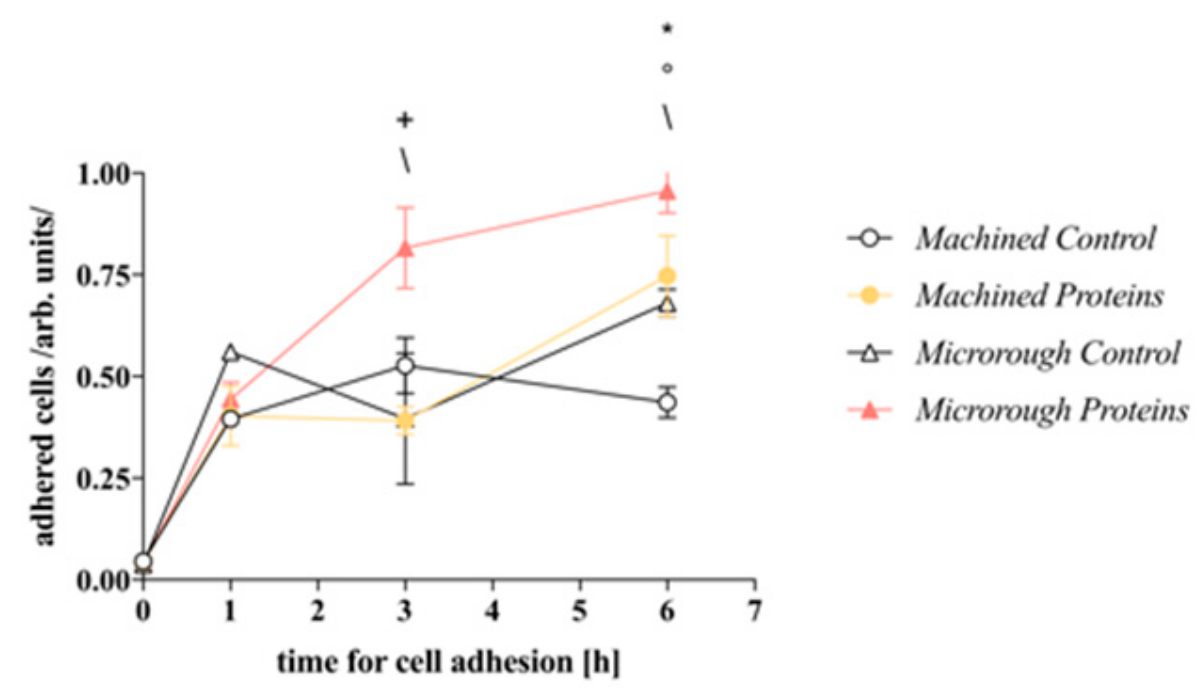
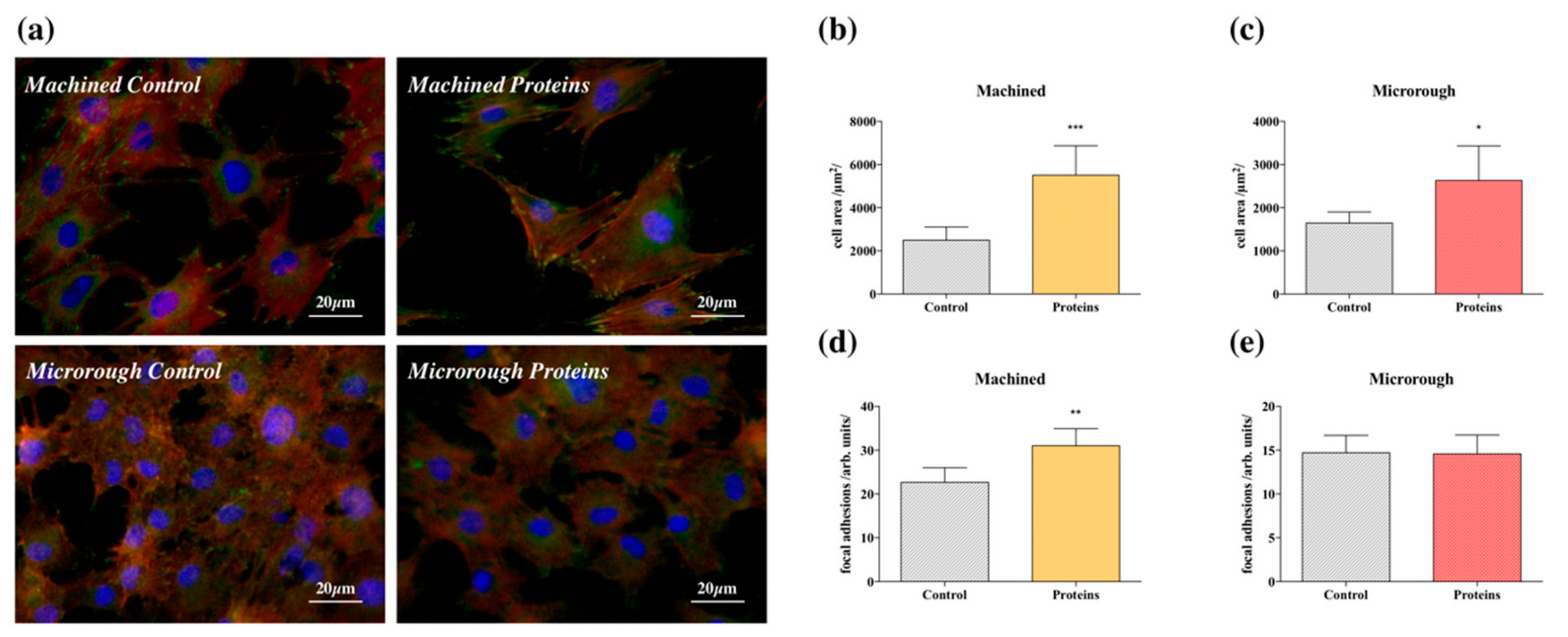
3.2. Serum Proteins at Titanium Interface Increase the Strength of Cell Adhesion and Focal Adhesions Expression
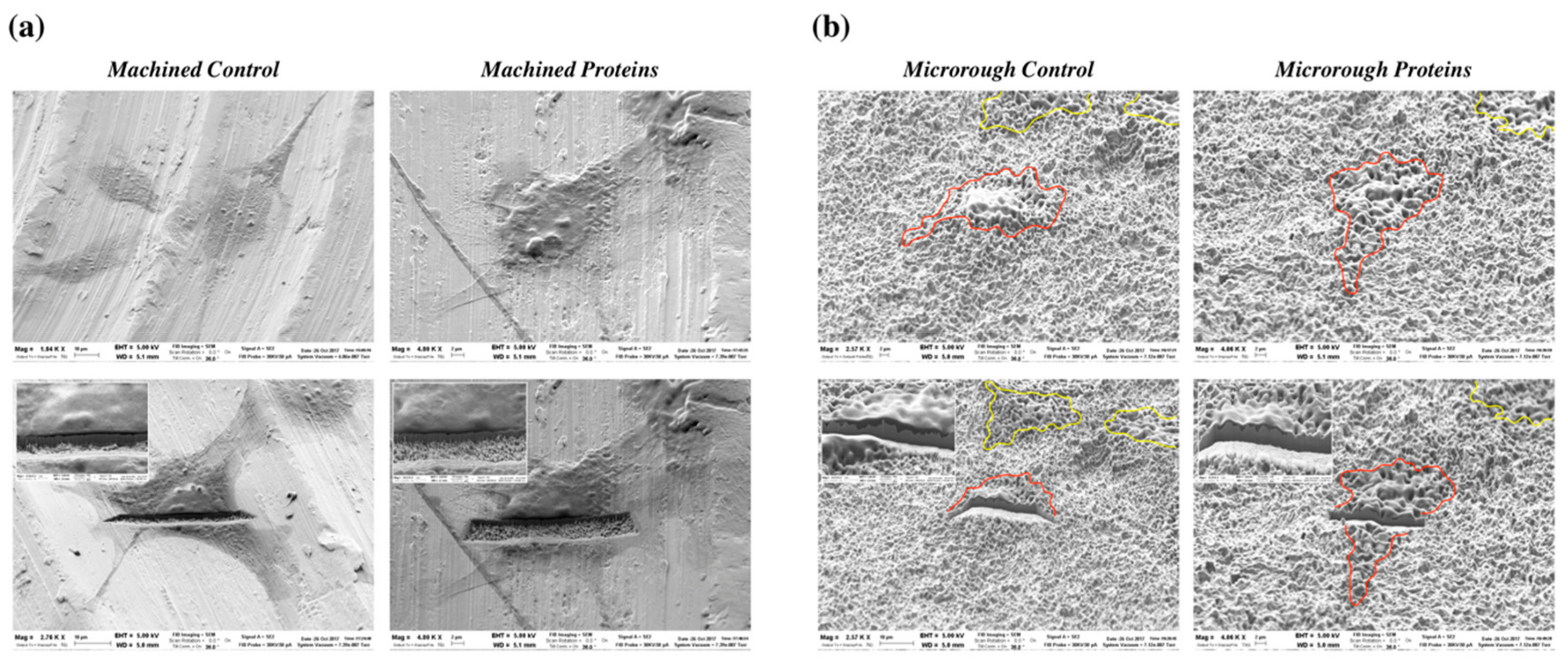
3.3. Serum Proteins at Titanium Interface Did Not Affect Cell Morphology at SEM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwartz, Z.; Boyan, B.D. Underlying mechanisms at the bone-biomaterial interface. J. Cell. Biochem. 1994, 56, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Karoussis, I.K.; Bragger, U.; Salvi, G.E.; Burgin, W.; Lang, N.P. Effect of implant design on survival and success rates of titanium oral implants: A 10-year prospective cohort study of the ITI® Dental Implant System. Clin. Oral Implant. Res. 2004, 15, 8–17. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in canine mandibles. Clin. Oral Implant. Res. 2008, 19, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [PubMed]
- Keselowsky, B.G.; Collard, D.M.; Garcia, A.J. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. Part A 2003, 66, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Mortisen, D.J.; Henry, S.M.; Anseth, K.S. Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration. J. Biomed. Mater. Res. 2001, 57, 217–223. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.H.; McFarland, C.D.; Jenkins, M.L.; Rezania, A.; Steele, J.G.; Healy, K.E. The role of vitronectin in the attachment and spatial distribution of bone-derived cells on materials with patterned surface chemistry. J. Biomed. Mater. Res. 1997, 37, 81–93. [Google Scholar] [CrossRef]
- Lagonegro, P.; Trevisi, G.; Nasi, L.; Parisi, L.; Manfredi, E.; Lumetti, S.; Rossi, F.; Macaluso, G.M.; Salviati, G.; Galli, C. Osteoblasts preferentially adhere to peaks on micro-structured titanium. Dent. Mater. J. 2018, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Flasker, A.; Lokar, M.; Mrak-Poljsak, K.; Mazare, A.N.; Artenjak, A.; Cucnik, S.; Kralj, S.; Velikonja, A.; Schmuki, P.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar] [CrossRef]
- Zaqout, M.S.K.; Sumizawa, T.; Igisu, H.; Higashi, T.; Myojo, T. Binding of human serum proteins to titanium dioxide particles in vitro. J. Occup. Health 2011, 53, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bloom, L.; Ingham, K.C.; Hynes, R.O. Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13. Mol. Biol. Cell 1999, 10, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- McFarland, C.D.; Thomas, C.H.; DeFilippis, C.; Steele, J.G.; Healy, K.E. Protein adsorption and cell attachment to patterned surfaces. J. Biomed. Mater. Res. 2000, 49, 200–210. [Google Scholar] [CrossRef]
- Singer, I.I. Association of fibronectin and vinculin with focal contacts and stress fibers in stationary hamster fibroblasts. J. Cell Biol. 1982, 92, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, A.; Parisi, L.; Tatti, R.; Lorenzi, A.; Verucchi, R.; Lumetti, S.; Manfredi, E.; Macaluso, G.M. Thermal-induced hydrophilicity enhancement of titanium dental implant surfaces. J. Oral Sci. 2019, in press. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, L.; Toffoli, A.; Cutrera, M.; Bianchi, M.G.; Lumetti, S.; Bussolati, O.; Macaluso, G.M. Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization. Nanomaterials 2019, 9, 1407. https://doi.org/10.3390/nano9101407
Parisi L, Toffoli A, Cutrera M, Bianchi MG, Lumetti S, Bussolati O, Macaluso GM. Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization. Nanomaterials. 2019; 9(10):1407. https://doi.org/10.3390/nano9101407
Chicago/Turabian StyleParisi, Ludovica, Andrea Toffoli, Miriam Cutrera, Massimiliano G. Bianchi, Simone Lumetti, Ovidio Bussolati, and Guido M. Macaluso. 2019. "Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization" Nanomaterials 9, no. 10: 1407. https://doi.org/10.3390/nano9101407
APA StyleParisi, L., Toffoli, A., Cutrera, M., Bianchi, M. G., Lumetti, S., Bussolati, O., & Macaluso, G. M. (2019). Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization. Nanomaterials, 9(10), 1407. https://doi.org/10.3390/nano9101407






