Enhanced Breakdown Strength and Thermal Conductivity of BN/EP Nanocomposites with Bipolar Nanosecond Pulse DBD Plasma Modified BNNSs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
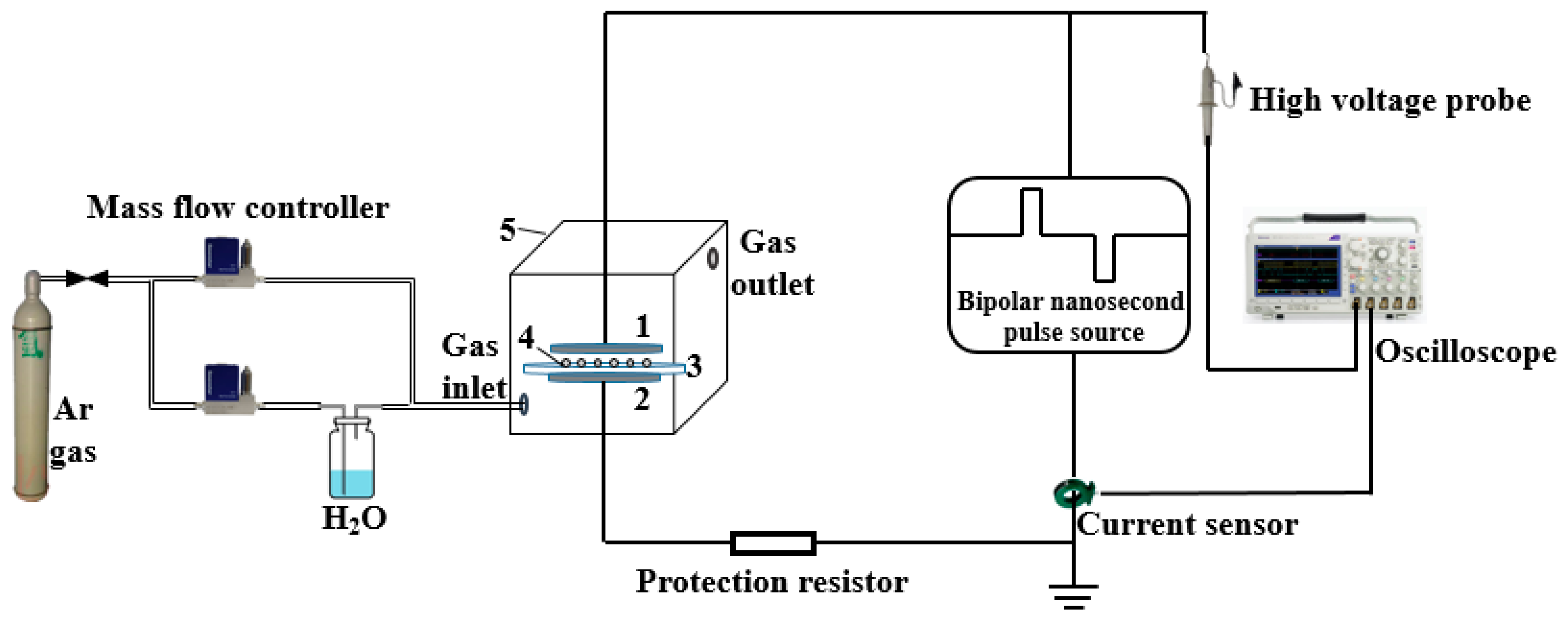
2.2. Plasma Modification of Epoxy Resin (EP) with BN Nanosheets (BNNSs)
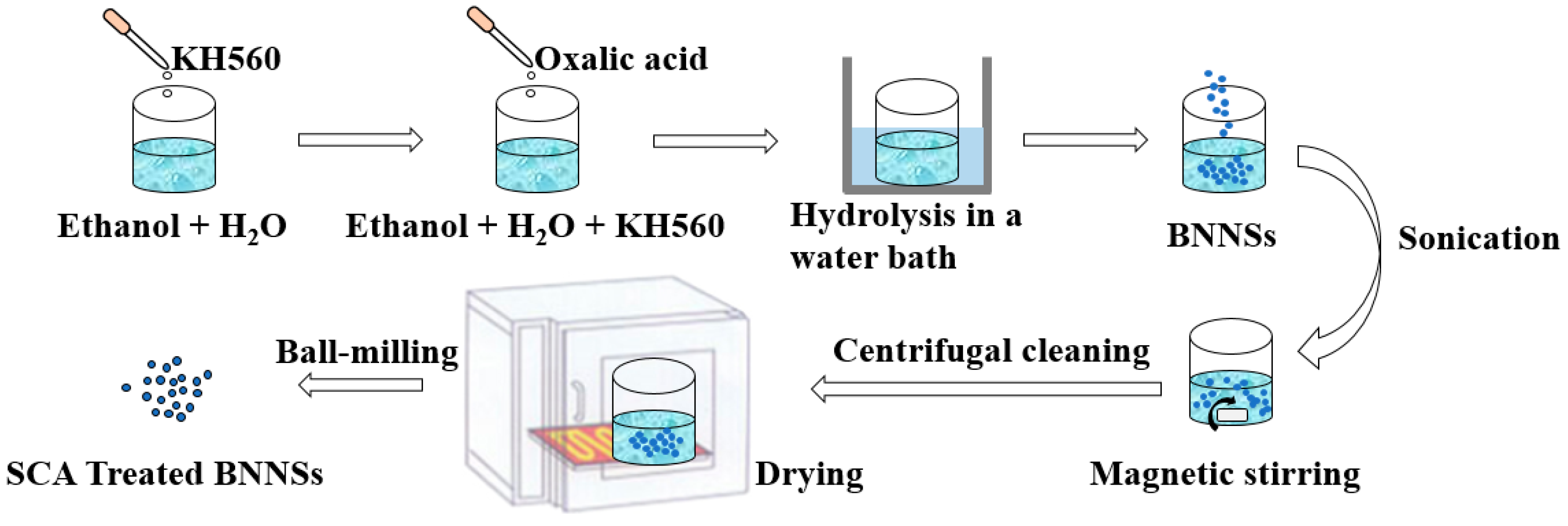
2.3. SCA Treated BNNSs
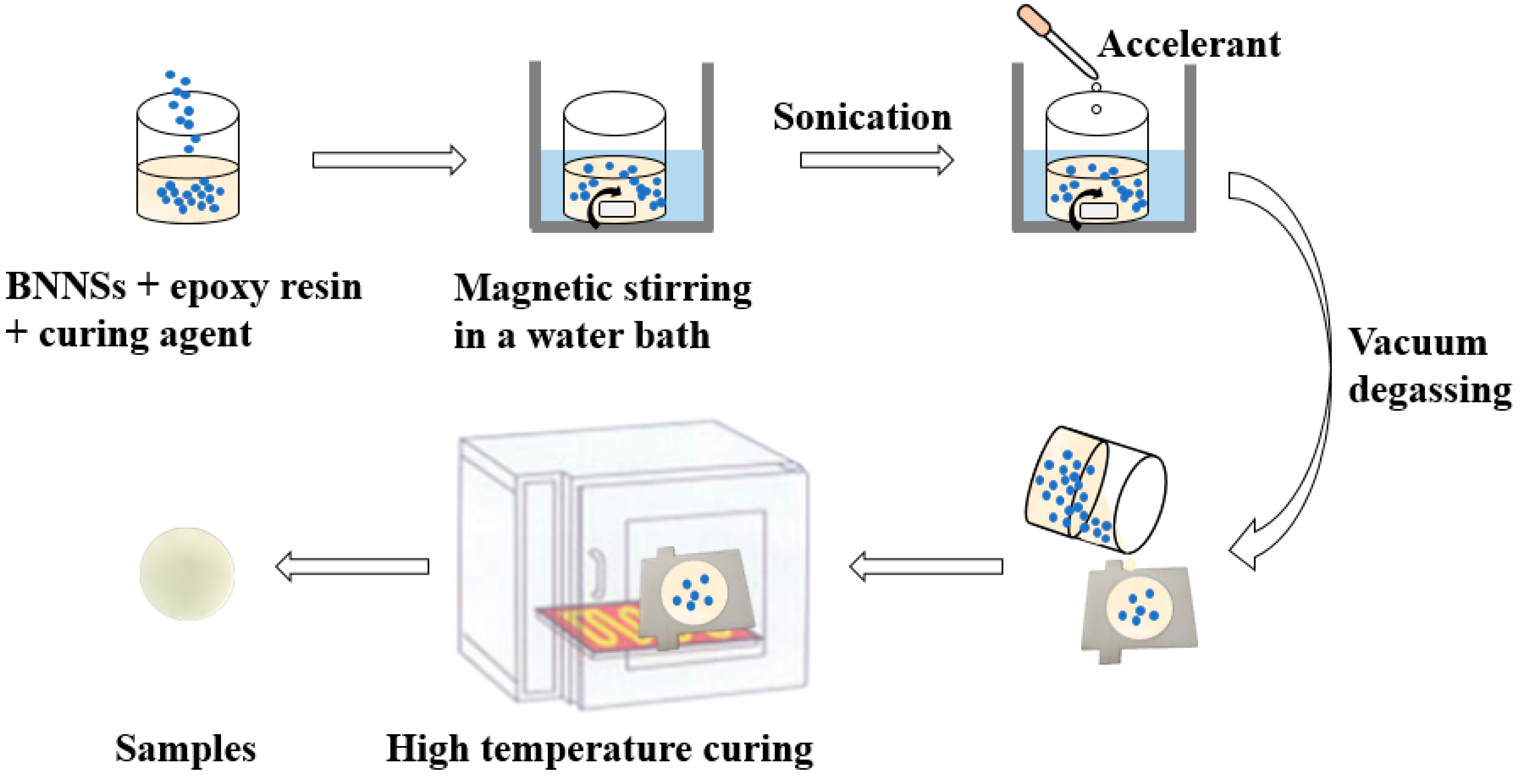
2.4. Preparation of the Epoxy Composites
2.5. Characterization
2.6. Statistical Analysis
3. Results and Discussion
3.1. X-Ray Photoelectron Spectroscopy (XPS)
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Thermogravimetric Analysis (TGA)
3.4. Breakdown Strength
3.5. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, X.Y.; Jiang, P.K.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Zhi, C.Y.; Bando, Y.; Terao, T.; Tang, C.C.; Kuwahara, H.; Golberg, D. Towards Thermoconductive, Electrically Insulating Polymeric Composites with Boron Nitride Nanotubes as Fillers. Adv. Funct. Mater. 2009, 19, 1857–1862. [Google Scholar] [CrossRef]
- Xu, Y.S.; Chung, D.D.L.; Mroz, C. Thermally conducting aluminum nitride polymer-matrix composites. Compos. Part A Appl. Sci. Manuf. 2001, 32, 1749–1757. [Google Scholar] [CrossRef]
- He, H.; Fu, R.; Han, Y.; Shen, Y.; Wang, D. High thermal conductive Si3N4 particle filled epoxy composites with a novel structure. J. Electron. Packag. 2007, 129, 469–472. [Google Scholar] [CrossRef]
- Yu, J.H.; Huang, X.Y.; Wu, C.; Wu, X.F.; Wang, G.L.; Jiang, P.K. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Zhu, B.L.; Ma, J.; Wu, J.; Yung, K.C.; Xie, C.S. Study on the Properties of the Epoxy-Matrix Composites Filled with Thermally Conductive AlN and BN Ceramic Particles. J. Appl. Polym. Sci. 2010, 118, 2754–2764. [Google Scholar] [CrossRef]
- Chiang, T.H.; Hsieh, T.E. A study of encapsulation resin containing hexagonal boron nitride (hBN) as inorganic filler. J. Inorg. Organomet. Polym. Mater. 2006, 16, 175–183. [Google Scholar] [CrossRef]
- Marx, P.; Wanner, A.J.; Zhang, Z.C.; Jin, H.F.; Tsekmes, I.A.; Smit, J.J.; Kern, W.; Wiesbrock, F. Effect of Interfacial Polarization and Water Absorption on the Dielectric Properties of Epoxy-Nanocomposites. Polymers 2017, 9, 195. [Google Scholar] [CrossRef]
- Wang, Z.B.; Iizuka, T.; Kozako, M.; Ohki, Y.; Tanaka, T. Development of Epoxy/BN Composites with High Thermal Conductivity and Sufficient Dielectric Breakdown Strength Part I—Sample Preparations and Thermal Conductivity. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1963–1972. [Google Scholar] [CrossRef]
- Liu, D.; Hoang, A.T.; Pourrahimi, A.M.; Pallon, L.K.H.; Nilsson, F.; Gubanski, S.M.; Olsson, T.; Hedenqvist, M.S.; Gedde, U.W. Influence of Nanoparticle Surface Coating on Electrical Conductivity of LDPE/Al2O3 Nanocomposites for HVDC Cable Insulations. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1396–1404. [Google Scholar] [CrossRef]
- Gao, M.Z.; Zhang, P.H. Relationship between dielectric properties and nanoparticle dispersion of nano-SiO2/epoxy composite. Acta Phys. Sin. 2016, 65, 192–199. [Google Scholar] [CrossRef]
- Yang, G.Q.; Cui, J.; Ohki, Y.; Wang, D.Y.; Li, Y.; Tao, K. Dielectric and relaxation properties of composites of epoxy resin and hyperbranched-polyester-treated nanosilica. RSC Adv. 2018, 8, 30669–30677. [Google Scholar] [CrossRef]
- Jesionowski, T.; Krysztafkiewicz, A. Influence of silane coupling agents on surface properties of precipitated silicas. Appl. Surf. Sci. 2001, 172, 18–32. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; So, J.I.; Kim, C.L.; Kim, M.; Sang, E.S. Treatment of Atmospheric-Pressure Radio Frequency Plasma on Boron Nitride for Improving Thermal Conductivity of Polydimethylsiloxane Composites. Macromol. Res. 2018, 26, 864–867. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Xin, W.; Lo, S.; Yuan, H. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol–gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Hwang, Y.; Kim, J. Chemically modified boron nitride-epoxy terminated dimethylsiloxane composite for improving the thermal conductivity. Ceram. Int. 2014, 40, 2047–2056. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhi, C.Y.; Jiang, P.K.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral Oligosilsesquioxane-Modified Boron Nitride Nanotube Based Epoxy Nanocomposites: An Ideal Dielectric Material with High Thermal Conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.H.; Yang, N.; Qin, L.L.; Grami, M.E.; Zhang, Q.X.; Wang, N.Y.; Qu, X.W. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Wu, X.H.; Wu, G.N.; Yang, Y.; Zhang, X.T.; Lei, Y.X.; Zhong, X.; Zhu, J. Influence of Nanoparticle Plasma Modification on Trap Properties of Polyimide Composite Films. Proc. CSEE 2018, 38, 3410–3418. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, C.; Long, K.H.; Zhang, D.D.; Wang, J.; Yan, P.; Zhou, Y.X. Surface modification of polyimide films using unipolar nanosecond-pulse DBD in atmospheric air. Appl. Surf. Sci. 2010, 256, 3888–3894. [Google Scholar] [CrossRef]
- Shao, T.; Yu, Y.; Zhang, C.; Zhang, D.D.; Niu, Z.; Wang, J.; Yan, P.; Zhou, Y.X. Excitation of Atmospheric Pressure Uniform Dielectric Barrier Discharge Using Repetitive Unipolar Nanosecond-pulse Generator. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1830–1837. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, W.C.; Yang, D.Z.; Zhao, Z.L.; Zhang, L.; Wang, S. Atmospheric air dielectric barrier discharge excited by nanosecond pulse and AC used for improving the hydrophilicity of aramid fibers. Plasma Sci. Technol. 2017, 19, 125401. [Google Scholar] [CrossRef]
- Wu, S.; Xu, H.; Lu, X.; Pan, Y. Effect of Pulse Rising Time of Pulse dc Voltage on Atmospheric Pressure Non-Equilibrium Plasma. Plasma Process Polym. 2013, 10, 136–140. [Google Scholar] [CrossRef]
- Liu, Y.; Chunqiang, S.U.; Xiang, R.; Fan, C.; Zhou, W.; Feng, W.; Ding, W. Experimental study on surface modification of PET films under bipolar nanosecond-pulse dielectric barrier discharge in atmospheric air. Appl. Surf. Sci. 2014, 313, 53–59. [Google Scholar] [CrossRef]
- Yang, D.Z.; Yang, Y.; Li, S.Z.; Nie, D.X.; Zhang, S.; Wang, W.C. A homogeneous dielectric barrier discharge plasma excited by a bipolar nanosecond pulse in nitrogen and air. Plasma Sources Sci. Technol. 2012, 21, 035004. [Google Scholar] [CrossRef]
- Mi, Y.; Wan, H.; Bian, C.H.; Peng, W.C.; Gui, L. An MMC-based Modular Unipolar/Bipolar High-voltage Nanosecond Pulse Generator with Adjustable Rise/Fall Time. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 515–522. [Google Scholar] [CrossRef]
- Ma, X.K.; Lee, N.H.; Oh, H.J.; Jung, S.C.; Lee, W.J.; Kim, S.J. Morphology control of hexagonal boron nitride by a silane coupling agent. J. Cryst. Growth 2011, 316, 185–190. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Plasma-Assisted Interface Engineering of Boron Nitride Nanostructure Films. ACS Nano 2014, 8, 10631–10639. [Google Scholar] [CrossRef]
- Park, G.; Lee, H.; Kim, G.; Lee, J.K. Global Model of He/O2 and Ar/O2 Atmospheric Pressure Glow Discharges. Plasma Process. Polym. 2010, 5, 569–576. [Google Scholar] [CrossRef]
- Liu, D.X.; Sun, B.W.; Iza, F.; Xu, D.H.; Wang, X.H.; Rong, M.Z.; Kong, M.G. Main species and chemical pathways in cold atmospheric-pressure Ar+ H2O plasmas. Plasma Sources Sci. Technol. 2017, 26, 045009. [Google Scholar] [CrossRef]
- Joni, I.M.; Balgis, R.; Ogi, T.; Iwaki, T.; Okuyama, K. Surface functionalization for dispersing and stabilizing hexagonal boron nitride nanoparticle by bead milling. Colloids Surf. A Physicochem. Eng. Asp. 2011, 388, 49–58. [Google Scholar] [CrossRef]
- Seyhan, A.T.; Goncu, Y.; Durukan, O.; Akay, A.; Ay, N. Silanization of boron nitride nanosheets (BNNSs) through microfluidization and their use for producing thermally conductive and electrically insulating polymer nanocomposites. J. Solid State Chem. 2017, 249, 98–107. [Google Scholar] [CrossRef]
- Tuncer, E.; James, D.R.; Sauers, I.; Ellis, A.R.; Pace, M.O. On dielectric breakdown statistics. J. Phys. D Appl. Phys. 2006, 39, 4257–4268. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric nanocomposites with insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Wang, X.Y.; Andritsch, T.; Chen, G.; Virtanen, S. The Role of the Filler Surface Chemistry on the Dielectric and Thermal Properties of Polypropylene Aluminium Nitride Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1009–1017. [Google Scholar] [CrossRef]
- Li, S.T.; Min, D.M.; Wang, W.W.; Chen, G. Linking Traps to Dielectric Breakdown through Charge Dynamics for Polymer Nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2777–2785. [Google Scholar] [CrossRef]
- Gu, J.W.; Zhang, Q.Y.; Dang, J.; Xie, C. Thermal conductivity epoxy resin composites filled with boron nitride. Polym. Adv. Technol. 2012, 23, 1025–1028. [Google Scholar] [CrossRef]
- Gu, J.W.; Liang, C.B.; Dang, J.; Dong, W.C.; Zhang, Q.Y. Ideal dielectric thermally conductive bismaleimide nanocomposites filled with polyhedral oligomeric silsesquioxane functionalized nanosized boron nitride. RSC Adv. 2016, 6, 35809–35814. [Google Scholar] [CrossRef]
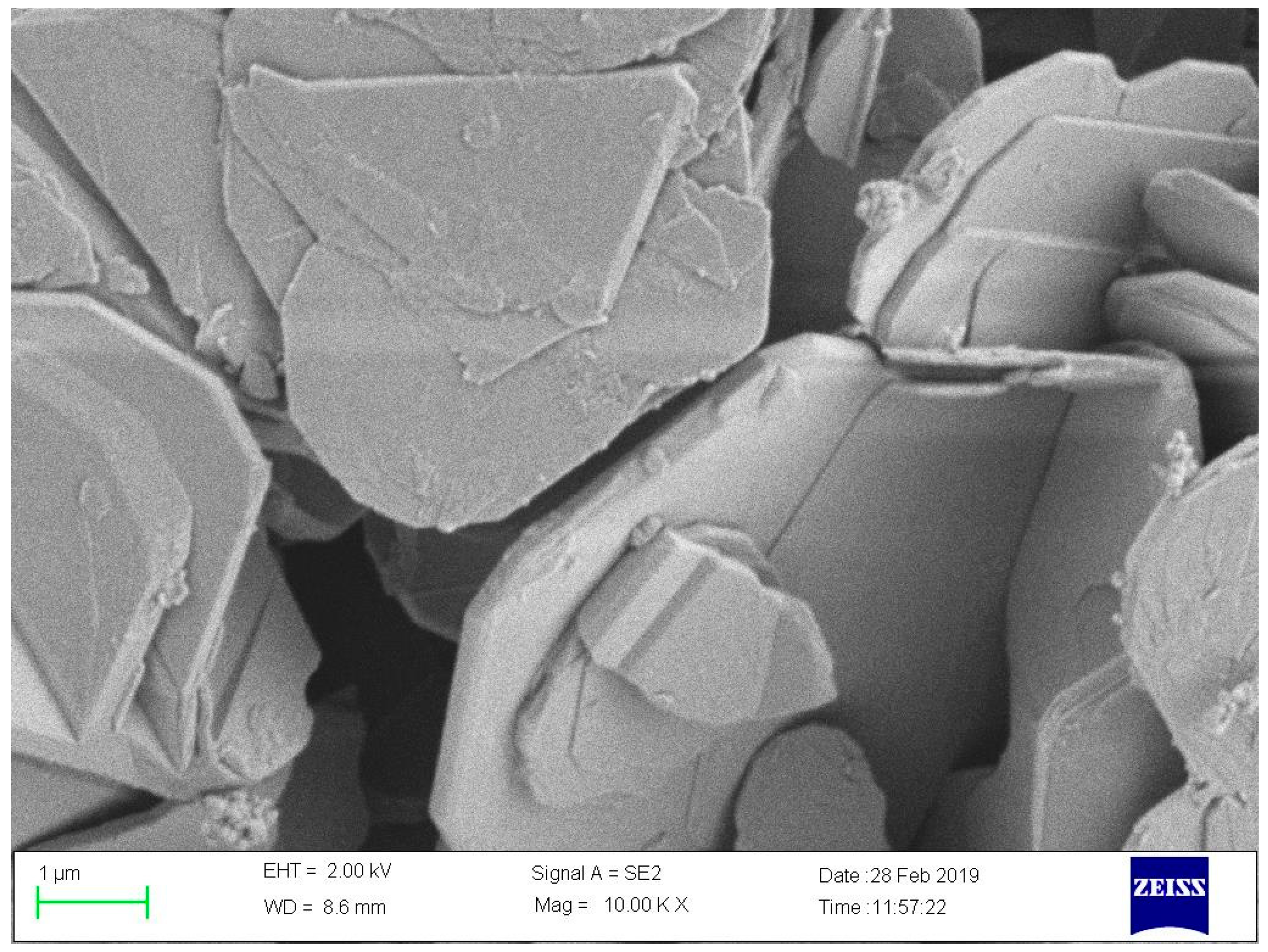

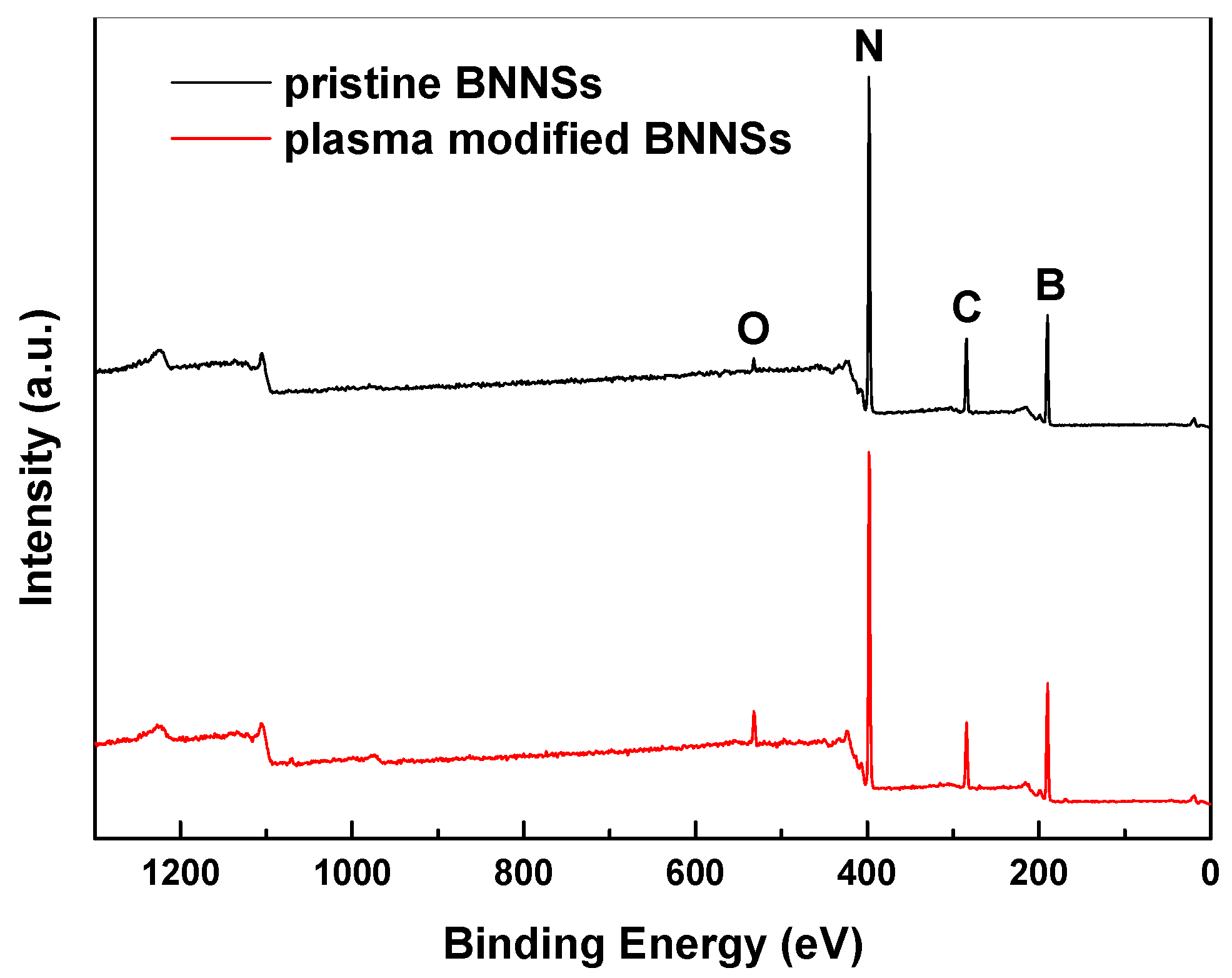
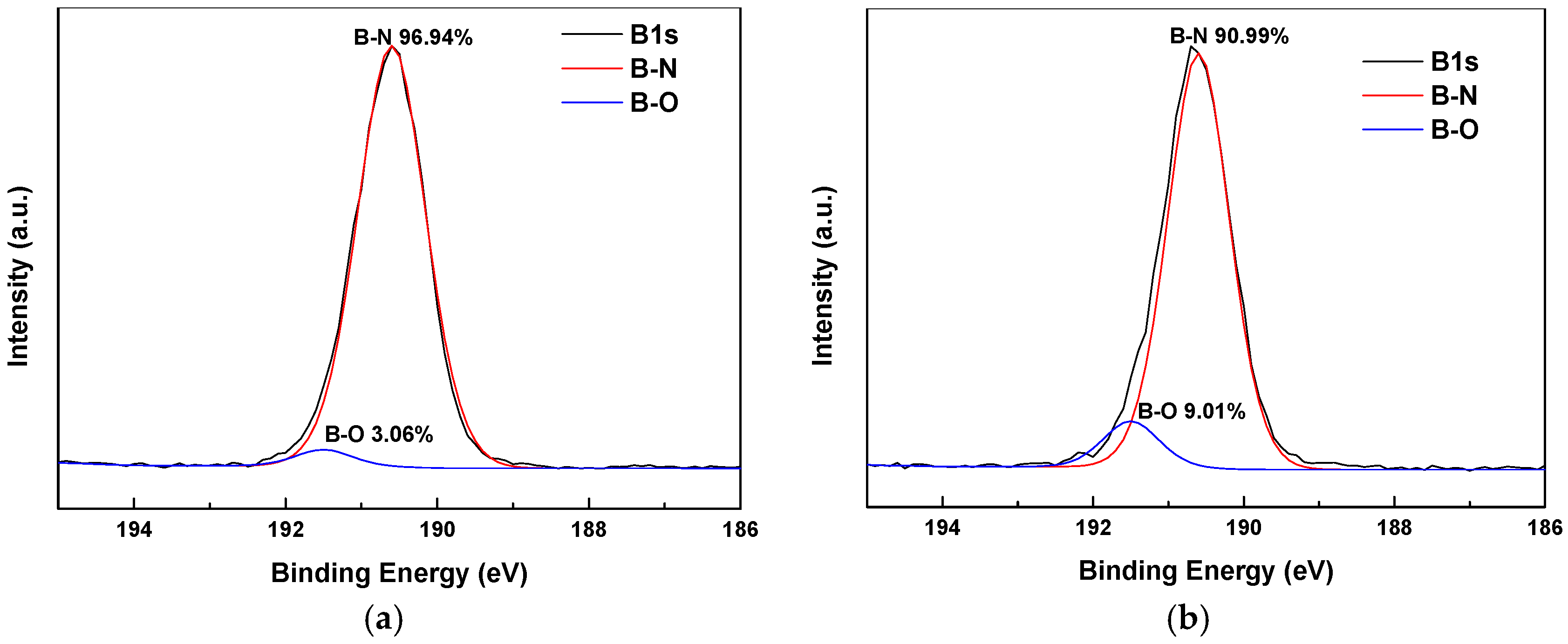

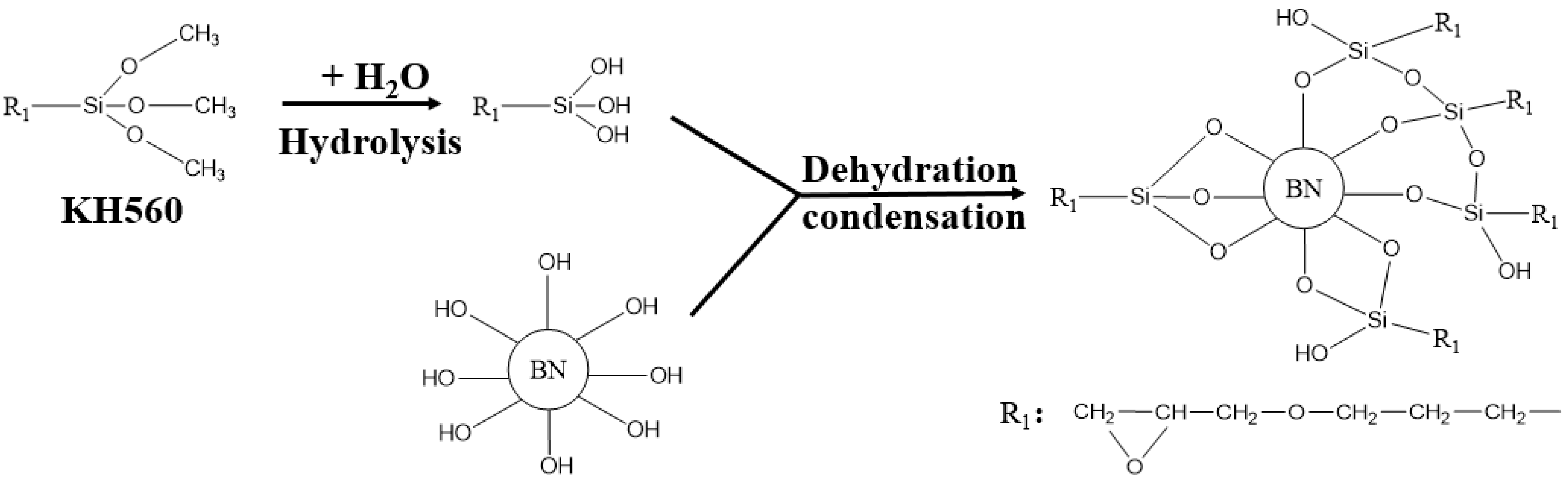
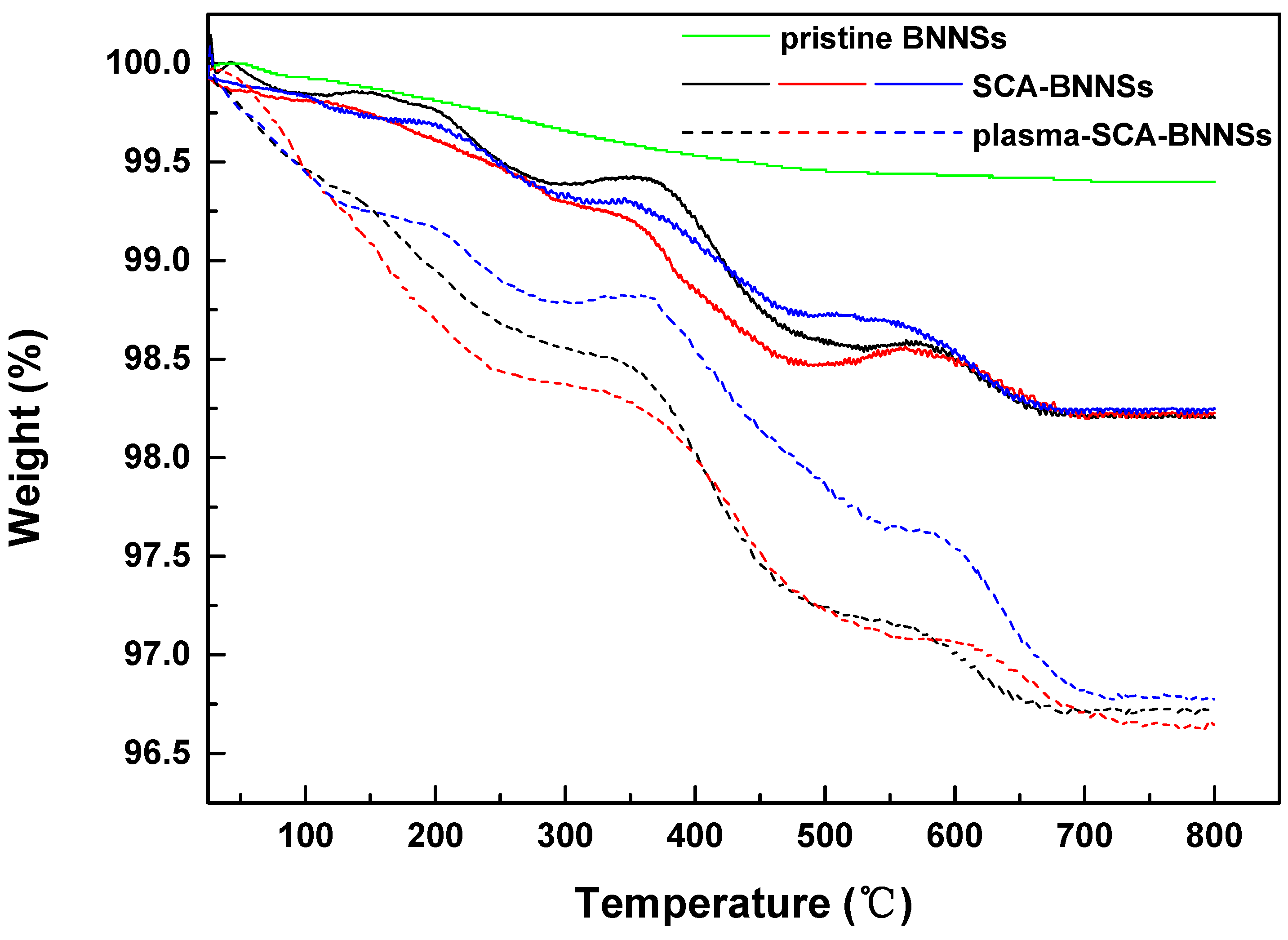
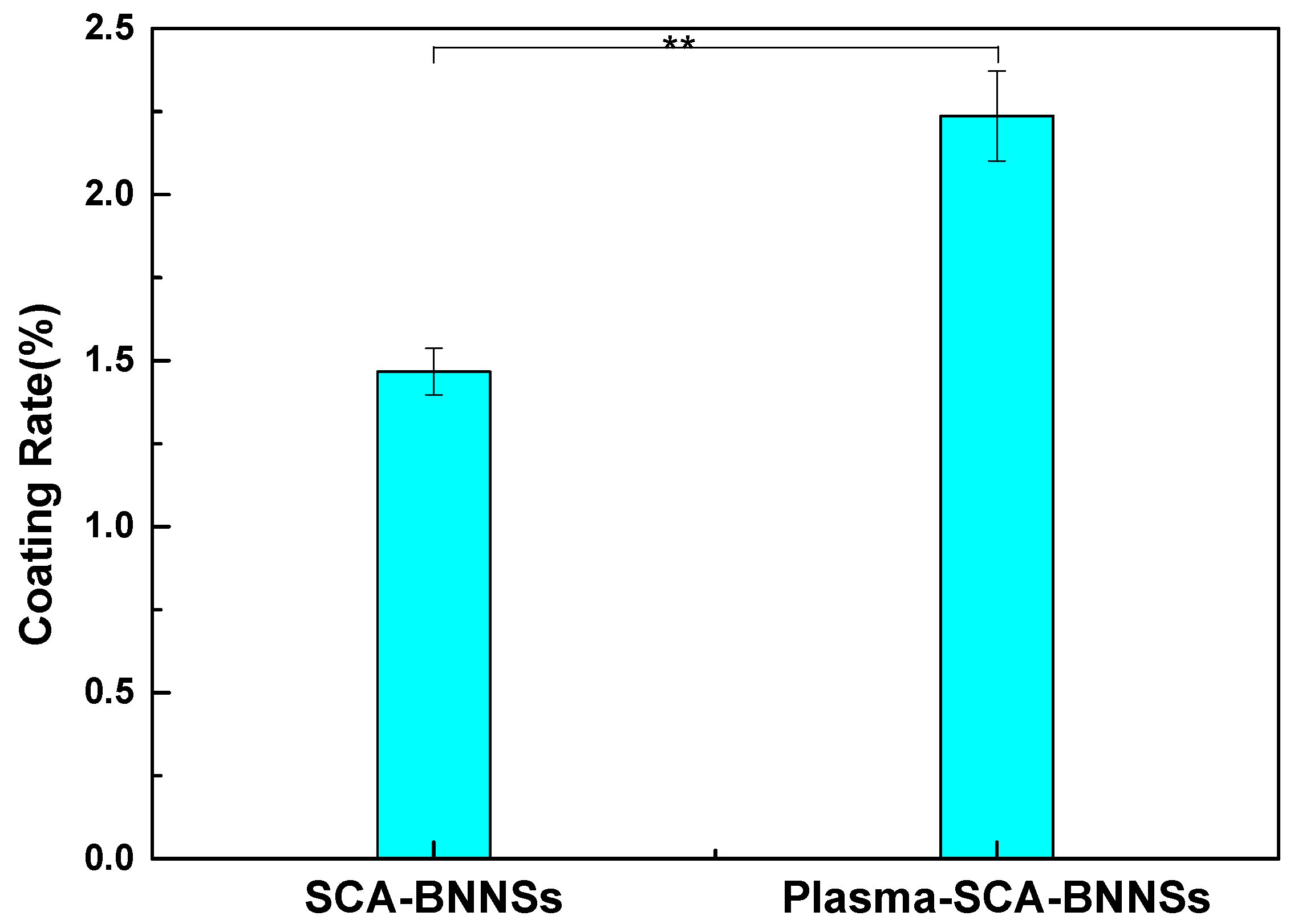
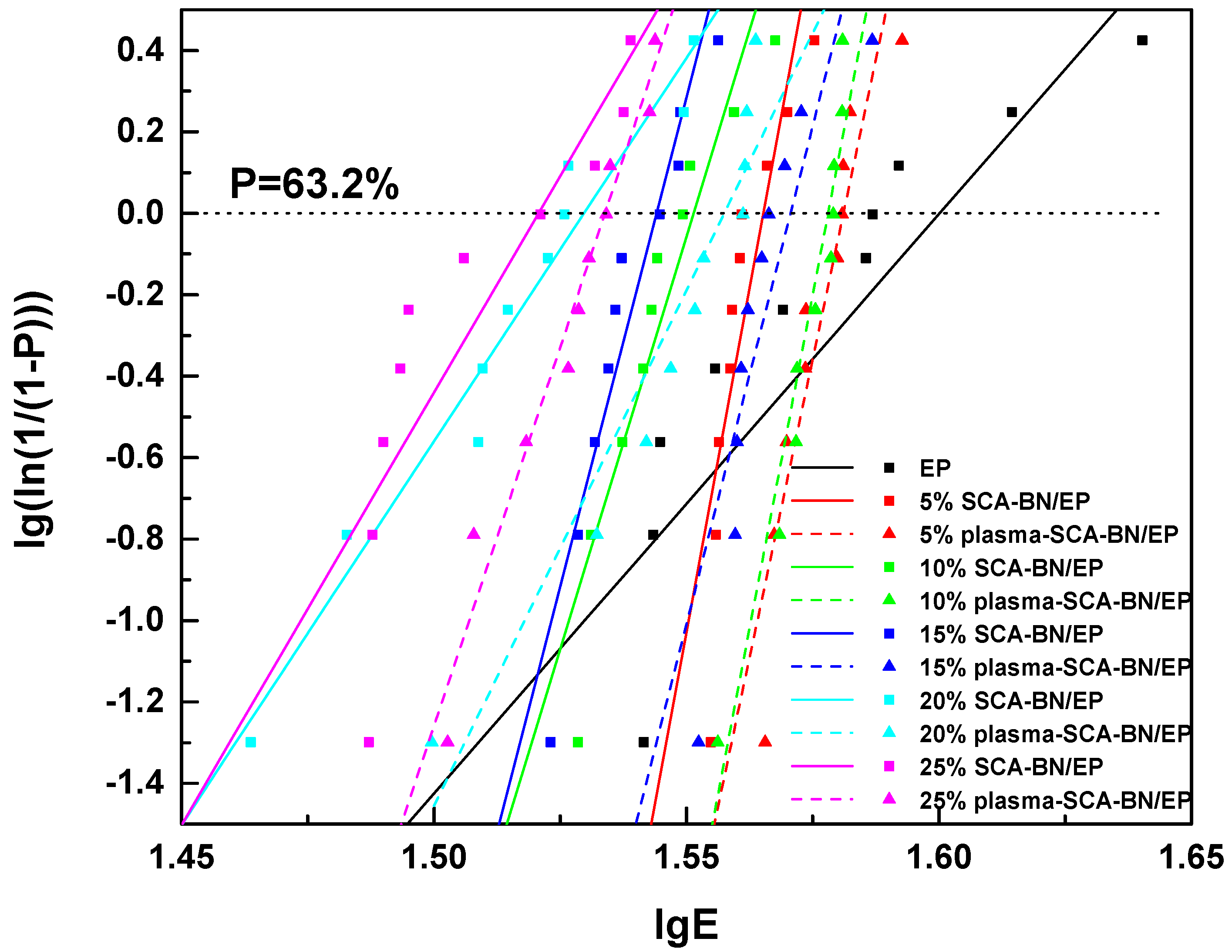
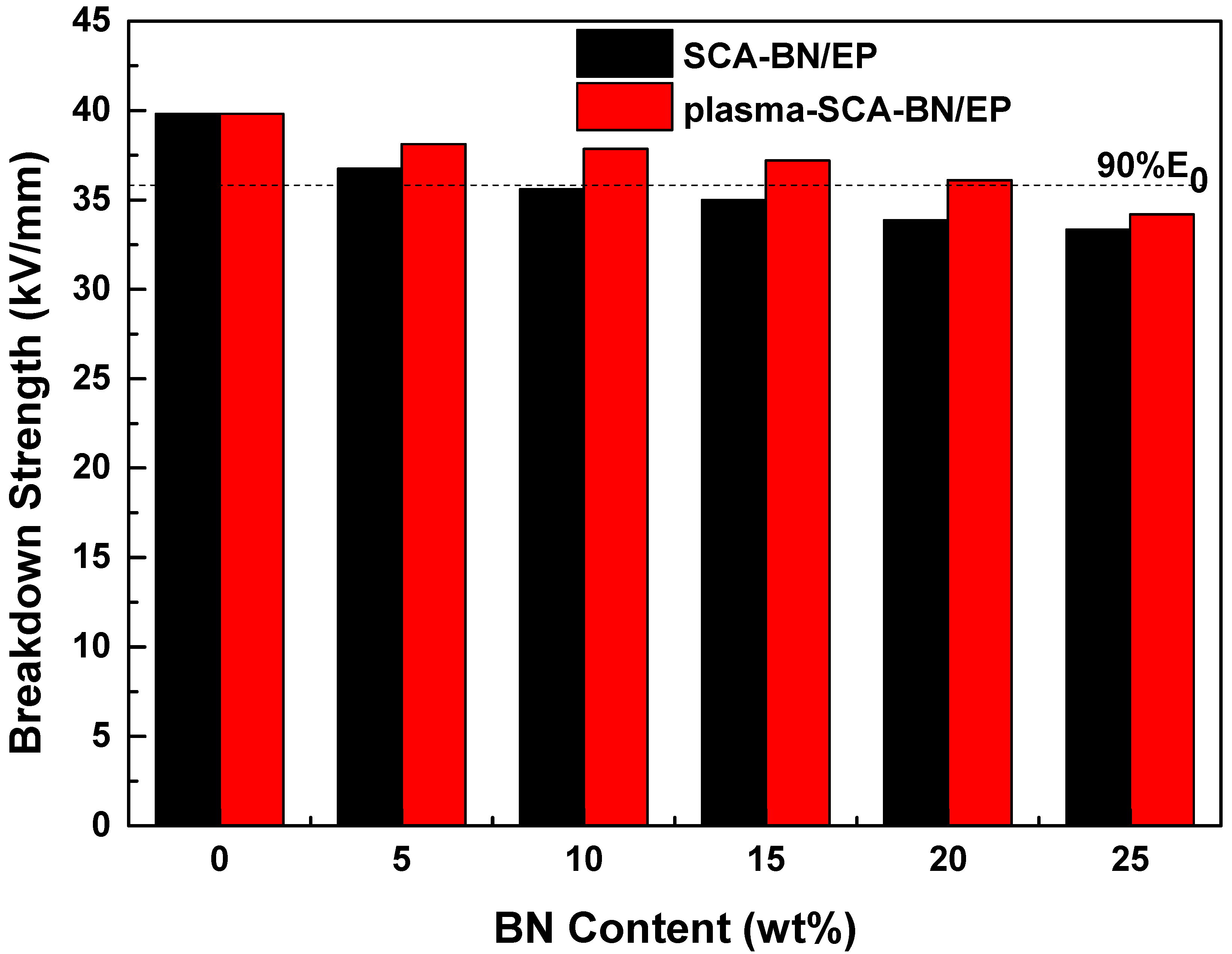
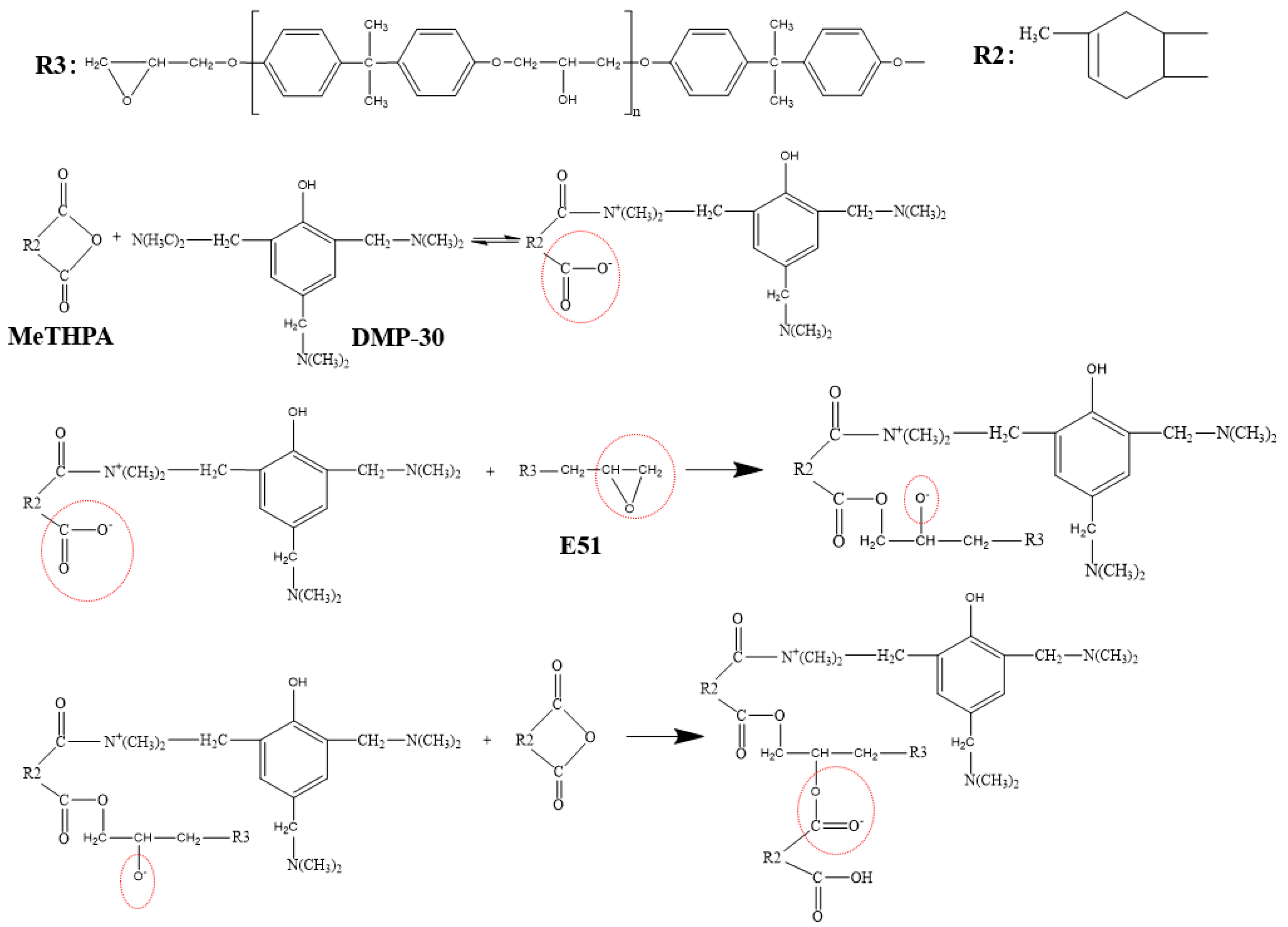

| Sample Number | SCA-BNNSs | Plasma-SCA-BNNSs |
|---|---|---|
| 1 | 1.45% | 2.39% |
| 2 | 1.56% | 2.26% |
| 3 | 1.39% | 2.06% |
| Average | 1.47% | 2.24% |
| Samples | α (kV/mm) | β | R2 |
|---|---|---|---|
| EP | 39.81 | 14.23 | 0.8134 |
| 5% SCA-BN/EP | 36.75 | 67.53 | 0.8493 |
| 5% plasma-SCA-BN/EP | 38.11 | 58.70 | 0.8690 |
| 10% SCA-BN/EP | 35.60 | 40.55 | 0.8885 |
| 10% plasma-SCA-BN/EP | 37.85 | 65.46 | 0.9345 |
| 15% SCA-BN/EP | 35.00 | 48.10 | 0.9172 |
| 15% plasma-SCA-BN/EP | 37.21 | 48.93 | 0.8715 |
| 20% SCA-BN/EP | 33.86 | 18.84 | 0.9612 |
| 20% plasma-SCA-BN/EP | 36.10 | 25.31 | 0.9174 |
| 25% SCA-BN/EP | 33.33 | 21.24 | 0.8079 |
| 25% plasma-SCA-BN/EP | 34.19 | 37.20 | 0.9622 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Gou, J.; Liu, L.; Ge, X.; Wan, H.; Liu, Q. Enhanced Breakdown Strength and Thermal Conductivity of BN/EP Nanocomposites with Bipolar Nanosecond Pulse DBD Plasma Modified BNNSs. Nanomaterials 2019, 9, 1396. https://doi.org/10.3390/nano9101396
Mi Y, Gou J, Liu L, Ge X, Wan H, Liu Q. Enhanced Breakdown Strength and Thermal Conductivity of BN/EP Nanocomposites with Bipolar Nanosecond Pulse DBD Plasma Modified BNNSs. Nanomaterials. 2019; 9(10):1396. https://doi.org/10.3390/nano9101396
Chicago/Turabian StyleMi, Yan, Jiaxi Gou, Lulu Liu, Xin Ge, Hui Wan, and Quan Liu. 2019. "Enhanced Breakdown Strength and Thermal Conductivity of BN/EP Nanocomposites with Bipolar Nanosecond Pulse DBD Plasma Modified BNNSs" Nanomaterials 9, no. 10: 1396. https://doi.org/10.3390/nano9101396
APA StyleMi, Y., Gou, J., Liu, L., Ge, X., Wan, H., & Liu, Q. (2019). Enhanced Breakdown Strength and Thermal Conductivity of BN/EP Nanocomposites with Bipolar Nanosecond Pulse DBD Plasma Modified BNNSs. Nanomaterials, 9(10), 1396. https://doi.org/10.3390/nano9101396





