The Synthesis of NiCo2O4–MnO2 Core–Shell Nanowires by Electrodeposition and Its Supercapacitive Properties
Abstract
1. Introduction
2. Experimental Section
2.1. The Synthesis of NiCo2O4–MnO2 Core–Shell Nanowires
2.2. Material Characterization
2.3. Hybrid Capacitor
3. Results and Discussion
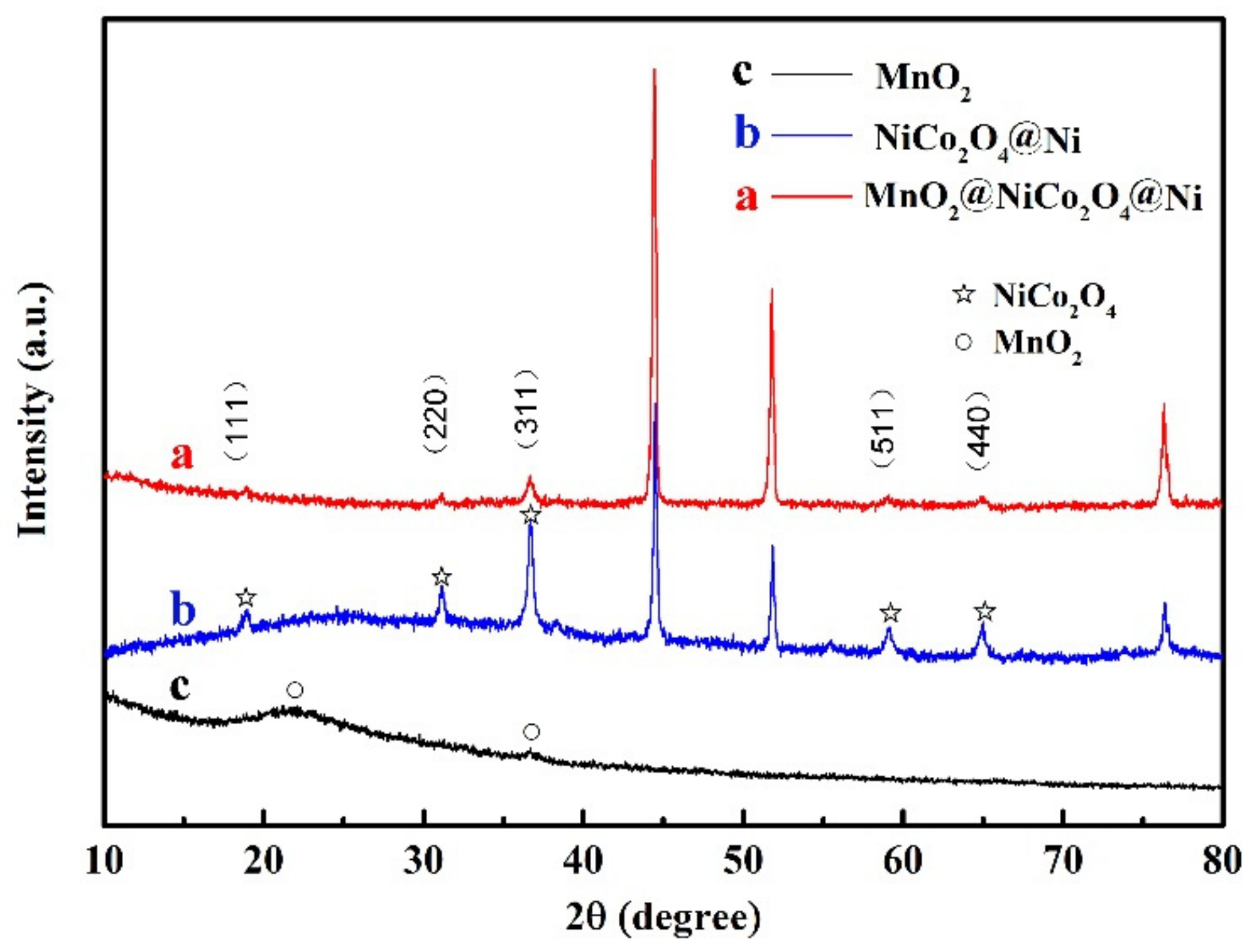
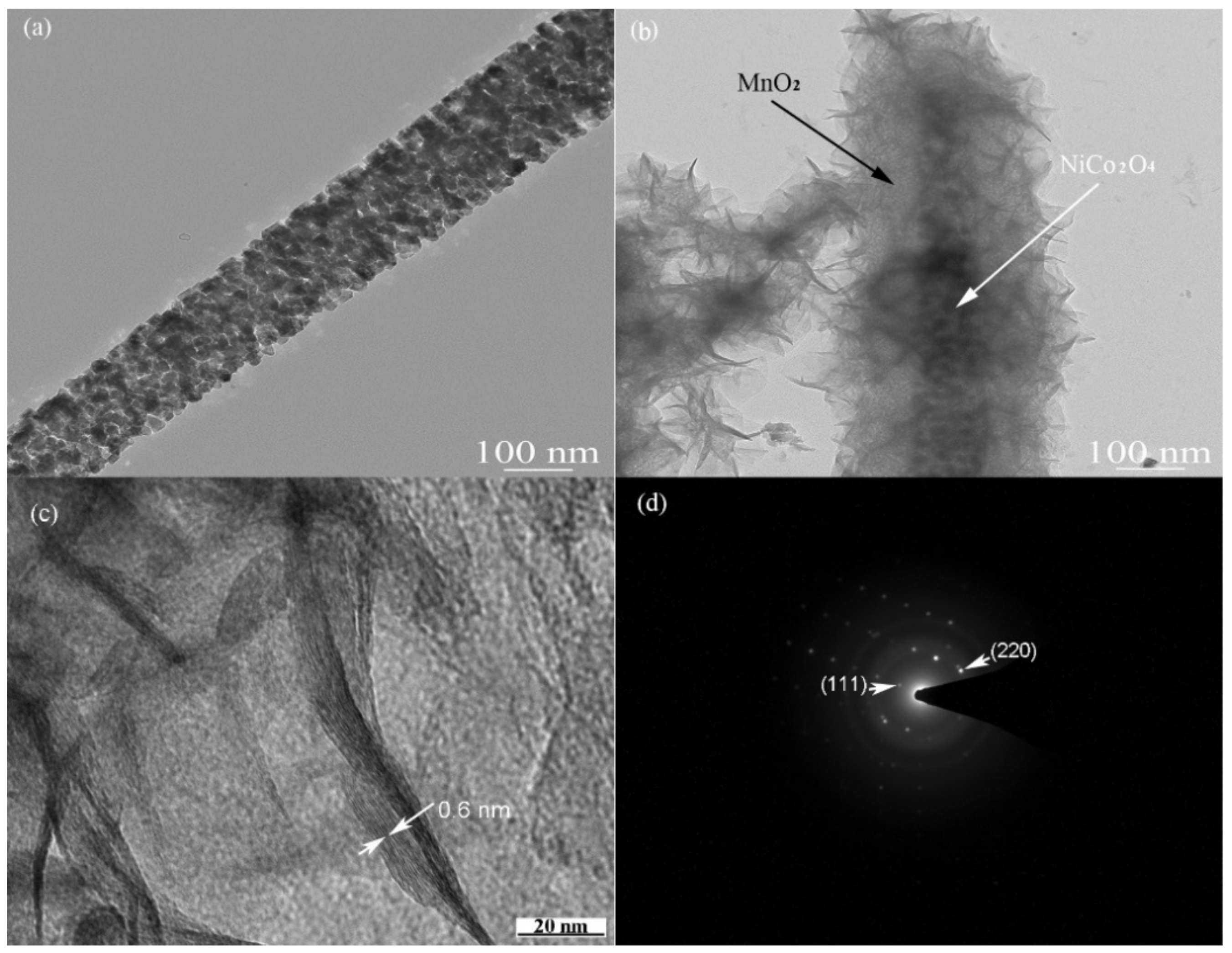
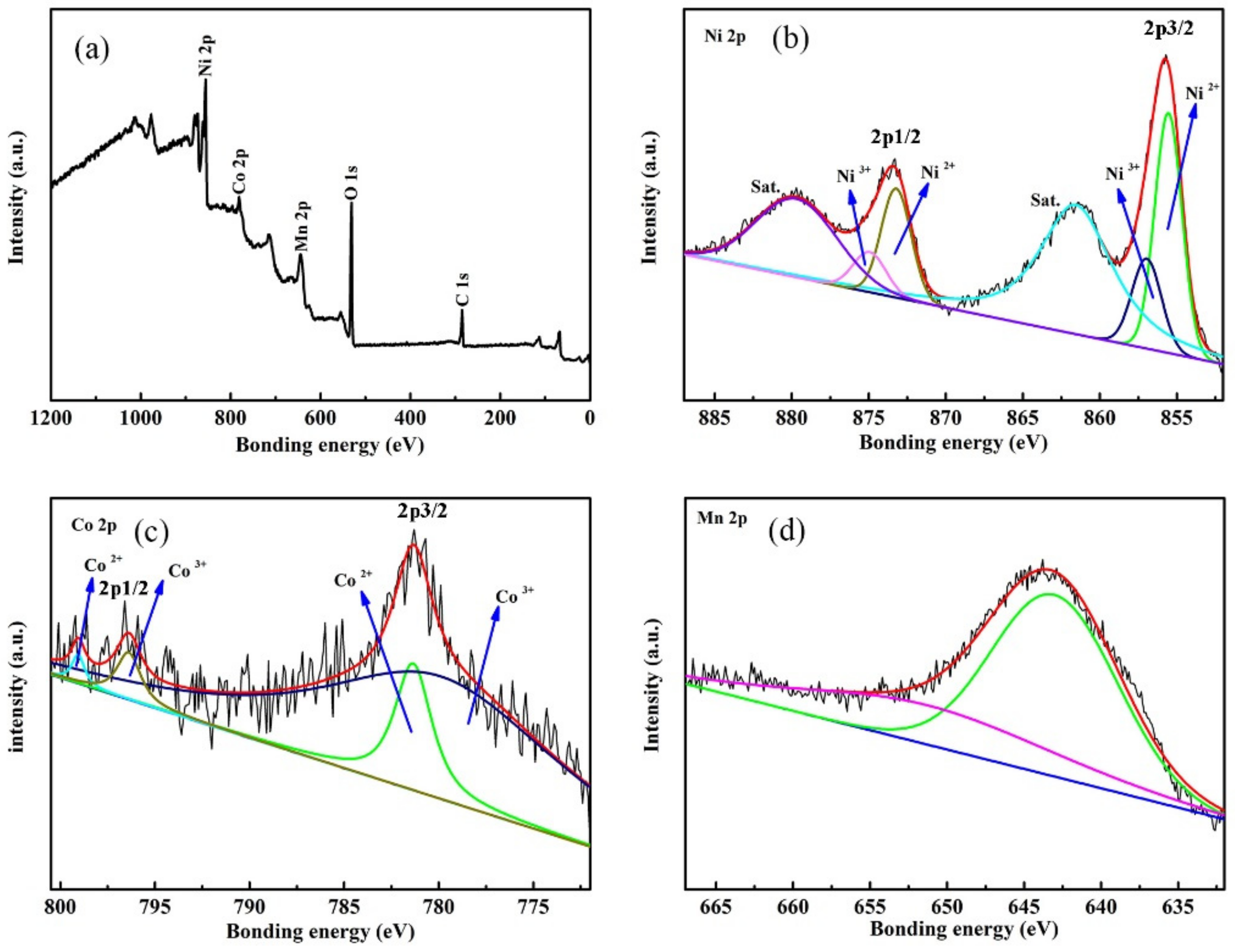
3.1. Structure and Chemical Analysis
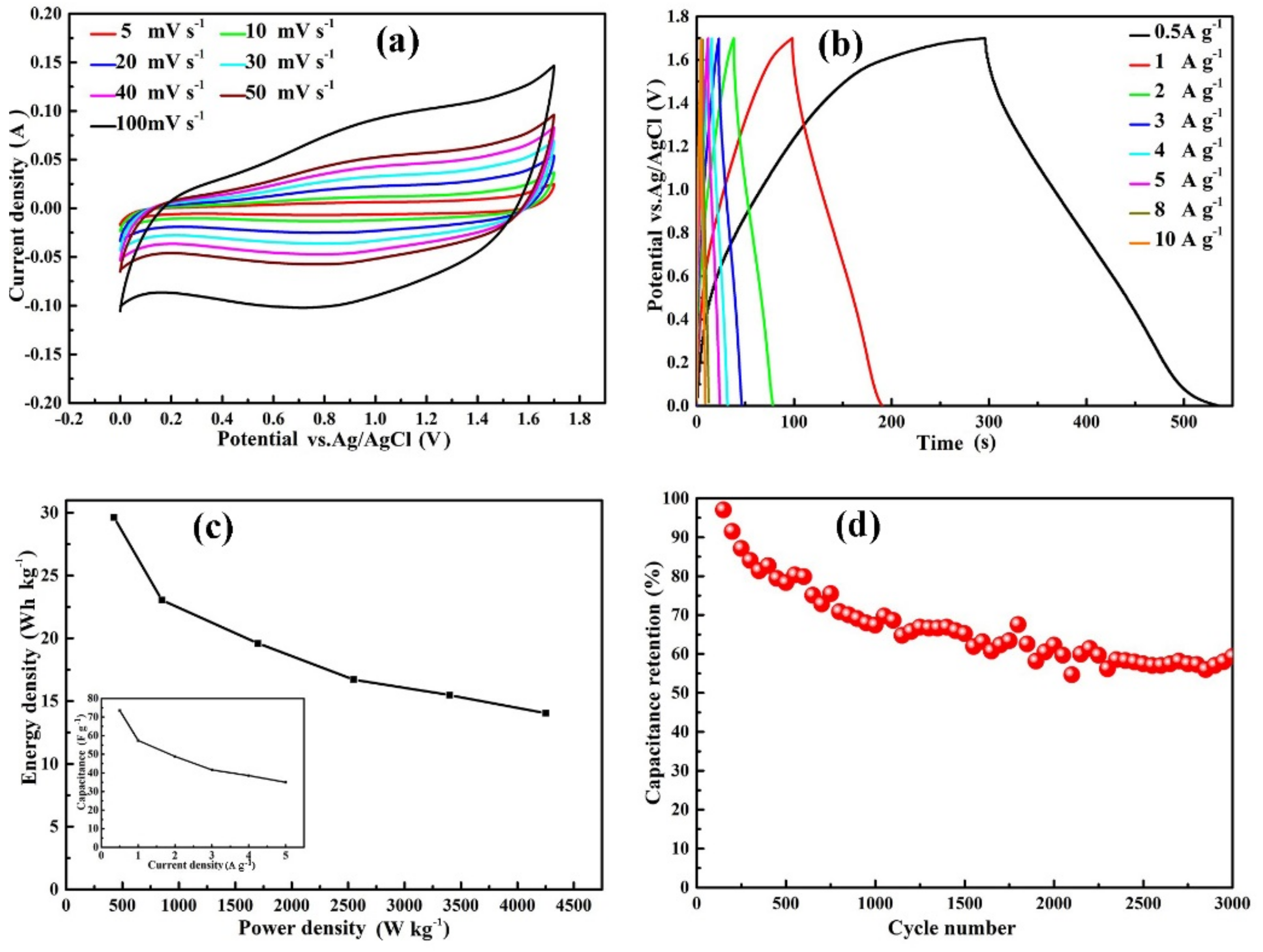
3.2. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kouchachvili, L.; Yaici, W.; Entchev, E. Hybrid battery/supercapacitor energy storage system for the electric vehicles. J. Power Sources 2018, 374, 237–248. [Google Scholar] [CrossRef]
- Lamiel, C.; Lee, Y.R.; Cho, M.H.; Yuma, D.; Shim, J.J. Enhanced electrochemical performance of nickel–cobalt–oxide@reduced graphene oxide//activated carbon asymmetric supercapacitors by the addition of a redox–active electrolyte. J. Colloid Interface Sci. 2017, 507, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ma, L.; Gan, M.Y.; Ye, M.H.; Li, X.R.; Zhai, Y.F.; Yan, F.B.; Cao, F.F. Monodisperse MnO2@NiCo2O4 core/shell nanospheres with highly opened structures as electrode materials for good–performance supercapacitor. Appl. Surf. Sci. 2018, 444, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, E.; He, W.; Deng, X.L.; Huang, J.; Ding, M.; Wei, X.; Liu, X.J.; Xu, X.J. NiCo2O4–based supercapacitor nanomaterials. Nanomaterials 2017, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.M.; Han, Z.; Wu, J.S.; Song, K.X.; Wu, J.; Gao, H.F.; Mi, Y.H. Synthesis and electrochemical performance of vertical carbon nanotubes on few–layer graphene as an anode material for Li–ion batteries. Mater. Chem. Phys. 2018, 205, 359–365. [Google Scholar] [CrossRef]
- Libich, J.; Maca, J.; Vondrak, J.; Cech, O.; Sedlarkova, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Kandhasamy, S.; Nallathamby, K.; Minakshi, M. Role of structural defect in olivine cathode. Prog. Solid State Chem. 2012, 40, 1–5. [Google Scholar] [CrossRef]
- Baskar, S.; Meyrick, D.; Ramakrishnan, K.S.; Minakshi, M. Facile and large scale combustion synthesis of α–CoMoO4: Mimics the redox behavior of a battery in aqueous hybrid device. Chem. Eng. J. 2014, 253, 502–507. [Google Scholar] [CrossRef]
- Yang, Y.F.; Cheng, D.; Chen, S.J.; Guan, Y.L.; Xiong, J. Construction of hierarchical NiCo2S4@Ni(OH)2 core–shell hybrid nanosheet arrays on Ni foam for high–performance aqueous hybrid supercapacitors. Electrochim. Acta 2016, 193, 116–127. [Google Scholar] [CrossRef]
- Yan, A.L.; Wang, X.C.; Cheng, J.P. Research progress of NiMn layered double hydroxides for supercapacitors: A review. Nanomaterials 2018, 8, 747. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Lin, J.X.; Zhang, Z.Q.; Zhang, D.; Yin, S.M.; Rong, Z.; Chen, Y.B.; Zhu, H.L.; Guo, S.Y. Cobalt molybdate nanoflake–assembling porous pillar array for high performance pseudocapacitor. Mater. Lett. 2016, 164, 260–263. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.P.; Wang, J.; Liu, F.; Zhang, X.B. The growth of nickel–manganese and cobalt–manganese layered double hydroxides on reduced graphene oxide for supercapacitor. Electrochim. Acta 2016, 206, 108–115. [Google Scholar] [CrossRef]
- Guo, S.H.; Chen, W.Q.; Li, M.; Wang, J.; Liu, F.; Cheng, J.P. Effect of reaction temperature on the amorphous–crystalline transition of copper cobalt sulfide for supercapacitors. Electrochim. Acta 2018, 271, 498–506. [Google Scholar] [CrossRef]
- Shou, Q.L.; Cheng, J.P.; Zhang, L.; Nelson, B.J.; Zhang, X.B. Synthesis and characterization of a nanocomposite of goethite nanorods and reduced graphene oxide for electrochemical capacitor. J. Solid State Chem. 2012, 185, 191–197. [Google Scholar] [CrossRef]
- Minakshi, M.; Visbal, H.; Mitchell, D.R.G.; Fichtner, M. Bio–waste chicken eggshells to storage energy. Dalton Trans. 2018, 47, 16828–16834. [Google Scholar] [CrossRef]
- Minakshi, M.; Higley, S.; Baur, C.; Mitchell, D.R.G.; Jones, R.T.; Fichtner, M. Calcined chicken eggshell electrode for battery and supercapacitor applications. RSC Adv. 2019, 9, 26981–26995. [Google Scholar] [CrossRef]
- Eftekhari, A.; Li, L.; Yang, Y. Polyaniline supercapacitors. J. Power Sources 2017, 347, 86–107. [Google Scholar] [CrossRef]
- Lokhande, V.C.; Lokhande, A.C.; Lokhande, C.D.; Kim, J.H.; Ji, T. Supercapacitive composite metal oxide electrodes formed with carbon, metal oxides and conducting polymers. J. Alloys Compd. 2016, 682, 381–403. [Google Scholar] [CrossRef]
- Xie, J.L.; Zhan, Z.Y.; Zhang, S.W.; Li, G.; Xia, H.C.; Yang, Y.F.; Xiong, J. CoMoO4 nanoplates decorated CuCo2O4 nanowires as advanced electrodes for high–performance hybrid supercapacitors. Mater. Lett. 2018, 226, 30–33. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Lin, J.X.; Zhang, D.; Yin, S.M.; Zhao, Y.L.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Freestanding hierarchical NiO/MnO2 core–shell nanocomposites arrays for high–performance electrochemical energy storage. Electrochim. Acta 2017, 227, 303–309. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lu, Z.Y.; Li, T.; Sun, X.M.; Liu, J.F. Hierarchical construction of core–shell metal oxide nanoarrays with ultrahigh areal capacitance. Nano Energy 2014, 7, 170–178. [Google Scholar] [CrossRef]
- Mendoza-Sanchez, B.; Gogotsi, Y. Synthesis of two–dimensional materials for capacitive energy storage. Adv. Mater. 2016, 28, 6104–6135. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.P.; Tang, S.L.; Deng, M.S.; Du, Y.W. NiCo2O4@rGO hybrid nanostructures on Ni foam as high–performance supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 5912–5919. [Google Scholar] [CrossRef]
- Zhang, G.H.; Wang, T.H.; Yu, X.Z.; Zhang, H.N.; Duan, H.G.; Lu, B.A. Nanoforest of hierarchical Co3O4@NiCo2O4 nanowire arrays for high–performance supercapacitors. Nano Energy 2013, 2, 586–594. [Google Scholar] [CrossRef]
- Chen, S.M.; Yang, G.; Jia, Y.; Zheng, H.J. Three–dimensional NiCo2O4@NiWO4 core–shell nanowire arrays for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 1028–1034. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, J.; Chen, W.; Zhao, Y.; Mu, X.; Zhang, Z.; Pan, X.; Xie, E. Constructing highly–efficient electron transport channels in the 3D electrode materials for high–rate supercapacitors: The case of NiCo2O4@NiMoO4 hierarchical nanostructures. Chem. Eng. J. 2017, 307, 687–695. [Google Scholar] [CrossRef]
- Xu, K.; Li, W.; Liu, Q.; Liu, X.; An, L.; Chen, Z.; Zou, R.; Hu, J. Hierarchical mesoporous NiCo2O4@MnO2 core–shell nanowire arrays on nickel foam for aqueous asymmetric supercapacitors. J. Mater. Chem. A 2014, 2, 4795–4802. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, G.; Yuan, C.; Lou, X. Hierarchical NiCo2O4@MnO2 core–shell heterostructured nanowire arrays on Ni foam as high–performance supercapacitor electrodes. Chem. Commun. 2013, 49, 137–139. [Google Scholar] [CrossRef]
- Khalid, S.; Cao, C.; Wang, L.; Zhu, Y.; Wu, Y. A high performance solid state asymmetric supercapacitor device based on NiCo2O4 nanosheets//MnO2 microspheres. RSC Adv. 2016, 6, 70292–70302. [Google Scholar] [CrossRef]
- Bao, F.; Zhang, Z.; Guo, W.; Liu, X. facile synthesis of three dimensional NiCo2O4@MnO2 core–shell nanosheet arrays and its supercapacitive performance. Electrochim. Acta 2015, 157, 31–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Liu, F.; Cheng, J.; Zhang, X.; Zhang, L. Full synergistic contribution of electrodeposited three–dimensional NiCo2O4@MnO2 nanosheet networks electrode for asymmetric supercapacitors. Nano Energy 2016, 27, 627–637. [Google Scholar] [CrossRef]
- Garakani, M.A.; Abouali, S.; Xu, Z.Z.; Huang, J.; Huang, J.; Kim, J. Heterogeneous, mesoporous NiCo2O4–MnO2/graphene foam for asymmetric supercapacitors with ultrahigh specific energies. J. Mater. Chem. A 2017, 5, 3547–3557. [Google Scholar] [CrossRef]
- Xu, K.; Li, S.; Yang, J.; Xu, H.; Hu, J. Hierarchical MnO2 nanosheets on electrospun NiCo2O4 nanotubes as electrode materials for high rate capability and excellent cycling stability supercapacitors. J. Alloys Compd. 2016, 678, 120–125. [Google Scholar] [CrossRef]
- Chen, H.; Hsieh, C.K.; Yang, Y.; Liu, X.Y.; Lin, C.H.; Tsai, C.H.; Wen, Z.Q.; Dong, F.; Zhang, Y.X. Hierarchical nickel cobaltate/manganese dioxide core–shell nanowire arrays on graphene–decorated nickel foam for high–performance supercapacitors. ChemElectroChem 2017, 4, 2414–2422. [Google Scholar] [CrossRef]
- Deng, F.; Tie, J.; Lan, B.; Sun, M.; Peng, S.; Deng, S.; Li, B.; Sun, W.; Yu, L. NiCo2O4/MnO2 heterostructured nanosheet: Influence of preparation conditions on its electrochemical properties. Electrochim. Acta 2015, 176, 359–368. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Li, H.; Cai, S.; Hu, H.; Fang, C.; Shi, L.; Zhang, D. Rational design and in situ fabrication of MnO2@NiCo2O4 nanowire arrays on Ni foam as high–performance monolith de–NOx catalysts. J. Mater. Chem. A 2015, 3, 11543–11553. [Google Scholar] [CrossRef]
- Sun, J.F.; Wu, C.; Sun, X.F.; Hu, H.; Zhi, C.Y.; Hou, L.R.; Yuan, C.Z. Recent progresses in high–energy–density all pseudocapacitive–electrode–materials–based asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 9443–9464. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Han, Y.; Tong, Y.; Lu, X.; Yang, S. Recent progress in the development of anodes for asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 4634–4658. [Google Scholar] [CrossRef]
- Watcharatharapong, T.; Sundaram, M.M.; Chakraborty, S.; Li, D.; Shafiullah, G.M.; Aughterson, R.D.; Ahuja, R. Effect of transition metal cations on stability enhancement for molybdate–based hybrid supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 17977–17991. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Wu, J.; Yi, T.; Xie, Y. Mesoporous NiCo2O4 nanoneedles@MnO2 nanoparticles grown on nickel foam for electrode used in high–performance supercapacitors. J. Energy Chem. 2019, 31, 167–177. [Google Scholar] [CrossRef]
- Biswal, A.; Tripathy, B.C.; Sanjay, K.; Subbaiah, T.; Minakshi, M. Electrolytic manganese dioxide (EMD): A perspective on worldwide production, reserves and its role in electrochemistry. RSC Adv. 2015, 5, 58255–58283. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Ma, K.Y.; Li, M.; Guo, S.H.; Liu, F.; Cheng, J.P. Hierarchical NiCo2O4@Co–Fe LDH core–shell nanowire arrays for high–performance supercapacitor. Appl. Surf. Sci. 2018, 451, 280–288. [Google Scholar] [CrossRef]
- Gong, X.F.; Cheng, J.P.; Liu, F.; Zhang, L.; Zhang, X.B. Nickel–cobalt hydroxide microspheres electrodepositioned on nickel cobaltite nanowires grown on Ni foam for high–performance pseudocapacitors. J. Power Sources 2014, 267, 610–616. [Google Scholar] [CrossRef]
- Chou, S.; Chen, F.; Chen, J. Electrodeposition synthesis and electrochemical properties of nanostructured γ–MnO2 films. J. Power Sources 2006, 162, 727–734. [Google Scholar] [CrossRef]
- Yang, J.; Lian, L.F.; Ruan, H.C.; Xie, F.Y.; Wei, M.D. Nanostructured porous MnO2 on Ni foam substrate with a high mass loading via a CV electrodeposition route for supercapacitor application. Electrochim. Acta 2014, 136, 189–194. [Google Scholar] [CrossRef]
- Bahloul, A.; Nessark, B.; Briot, E.; Groult, H.; Mauger, A.; Zaghib, K.; Julien, C.M. Polypyrrole–covered MnO2 as electrode material for supercapacitor. J. Power Sources 2013, 240, 267–272. [Google Scholar] [CrossRef]
- Ma, K.Y.; Liu, F.; Zhang, M.B.; Zhang, X.B.; Cheng, J.P. Core/shell microrod arrays of NiO/Co–Fe layered double hydroxides deposited on nickel foam for energy storage and conversion. Electrochim. Acta 2017, 225, 425–434. [Google Scholar] [CrossRef]
- Zhang, W.F.; Ma, C.; Fang, J.H.; Cheng, J.P.; Zhang, X.B.; Dong, S.R.; Zhang, L. Asymmetric electrochemical capacitors with high energy and power density based on graphene/CoAl–LDH and activated carbon electrodes. RSC Adv. 2013, 3, 2483–2490. [Google Scholar] [CrossRef]


| Two Electrodes of Hybrid SCs | Energy Density/Wh·kg−1 | Power Density/W·kg−1 | Work Voltage/V | Specific Capacitance/F·g−1 | Current Density/A·g−1 | Reference |
|---|---|---|---|---|---|---|
| NiCo2O4@MnO2 nanospheres//AC | 26.6 | 800 | 1.6 | 75 | 1 | [3] |
| MnO2@NiCo2O4 nanowires//AC | 35 | 163 | 1.5 | 112 | 0.83 | [28] |
| MnO2@NiCo2O4 nanosheet networks//AC | 37.5 | 187.5 | 1.5 | 120.9 | 0.25 | [32] |
| MnO2@NiCo2O4 on graphene//CNTs and graphene | 55.1 | 187.5 | 1.5 | 146.2 | 0.5 | [33] |
| MnO2@NiCo2O4 on graphene//activated graphene | 27.8 | 400.3 | 1.6 | 78.1 | 0.5 | [35] |
| MnO2@NiCo2O4 nanowires//AC | 29.6 | 425 | 1.7 | 73.5 | 0.5 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, A.-L.; Wang, W.-D.; Chen, W.-Q.; Wang, X.-C.; Liu, F.; Cheng, J.-P. The Synthesis of NiCo2O4–MnO2 Core–Shell Nanowires by Electrodeposition and Its Supercapacitive Properties. Nanomaterials 2019, 9, 1398. https://doi.org/10.3390/nano9101398
Yan A-L, Wang W-D, Chen W-Q, Wang X-C, Liu F, Cheng J-P. The Synthesis of NiCo2O4–MnO2 Core–Shell Nanowires by Electrodeposition and Its Supercapacitive Properties. Nanomaterials. 2019; 9(10):1398. https://doi.org/10.3390/nano9101398
Chicago/Turabian StyleYan, Ai-Lan, Wei-Dong Wang, Wen-Qiang Chen, Xin-Chang Wang, Fu Liu, and Ji-Peng Cheng. 2019. "The Synthesis of NiCo2O4–MnO2 Core–Shell Nanowires by Electrodeposition and Its Supercapacitive Properties" Nanomaterials 9, no. 10: 1398. https://doi.org/10.3390/nano9101398
APA StyleYan, A.-L., Wang, W.-D., Chen, W.-Q., Wang, X.-C., Liu, F., & Cheng, J.-P. (2019). The Synthesis of NiCo2O4–MnO2 Core–Shell Nanowires by Electrodeposition and Its Supercapacitive Properties. Nanomaterials, 9(10), 1398. https://doi.org/10.3390/nano9101398






