Porous Hybrid Nanofibers Comprising ZnSe/CoSe₂/Carbon with Uniformly Distributed Pores as Anodes for High-Performance Sodium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Techniques
2.3. Electrochemical Measurements
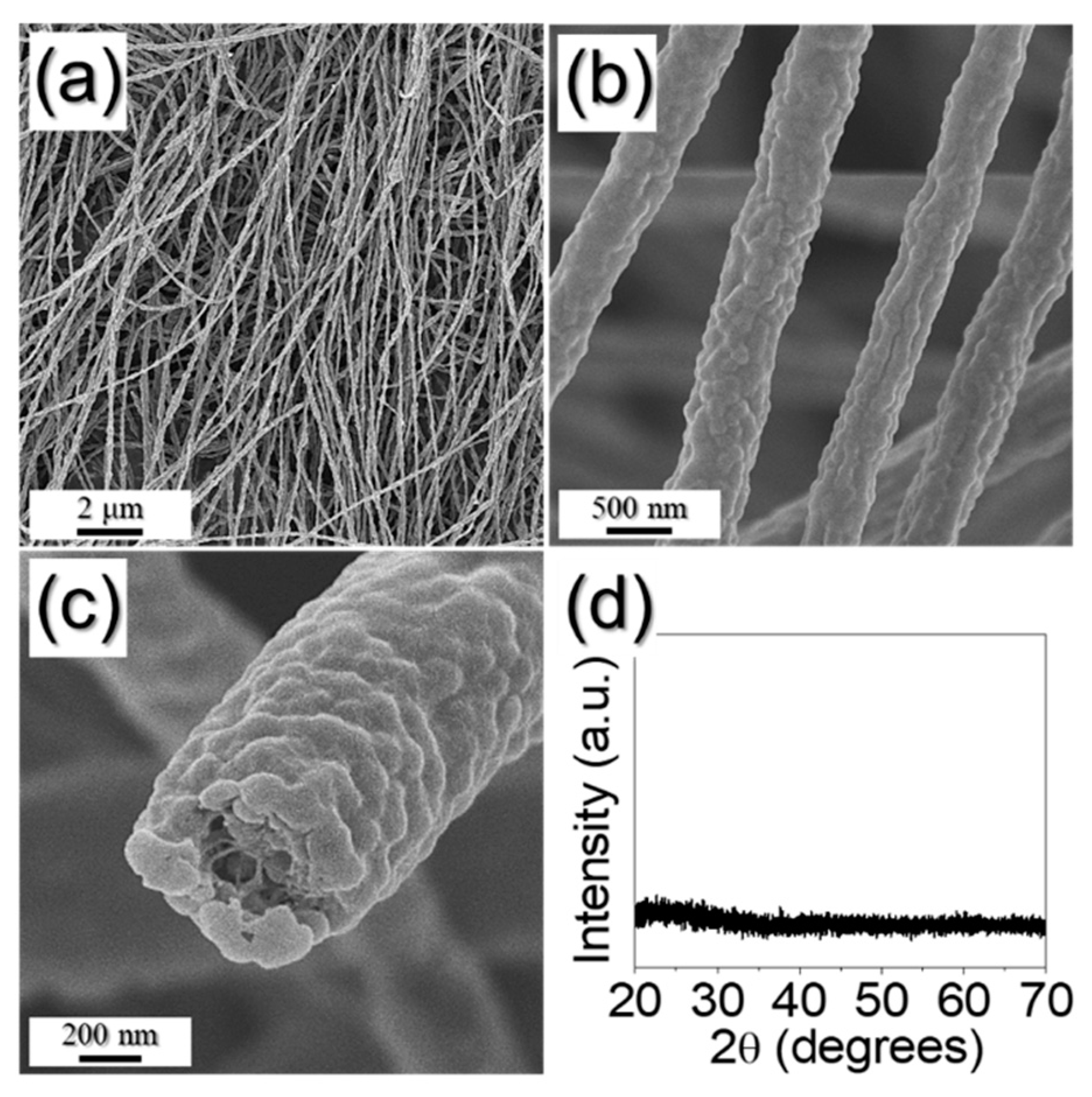
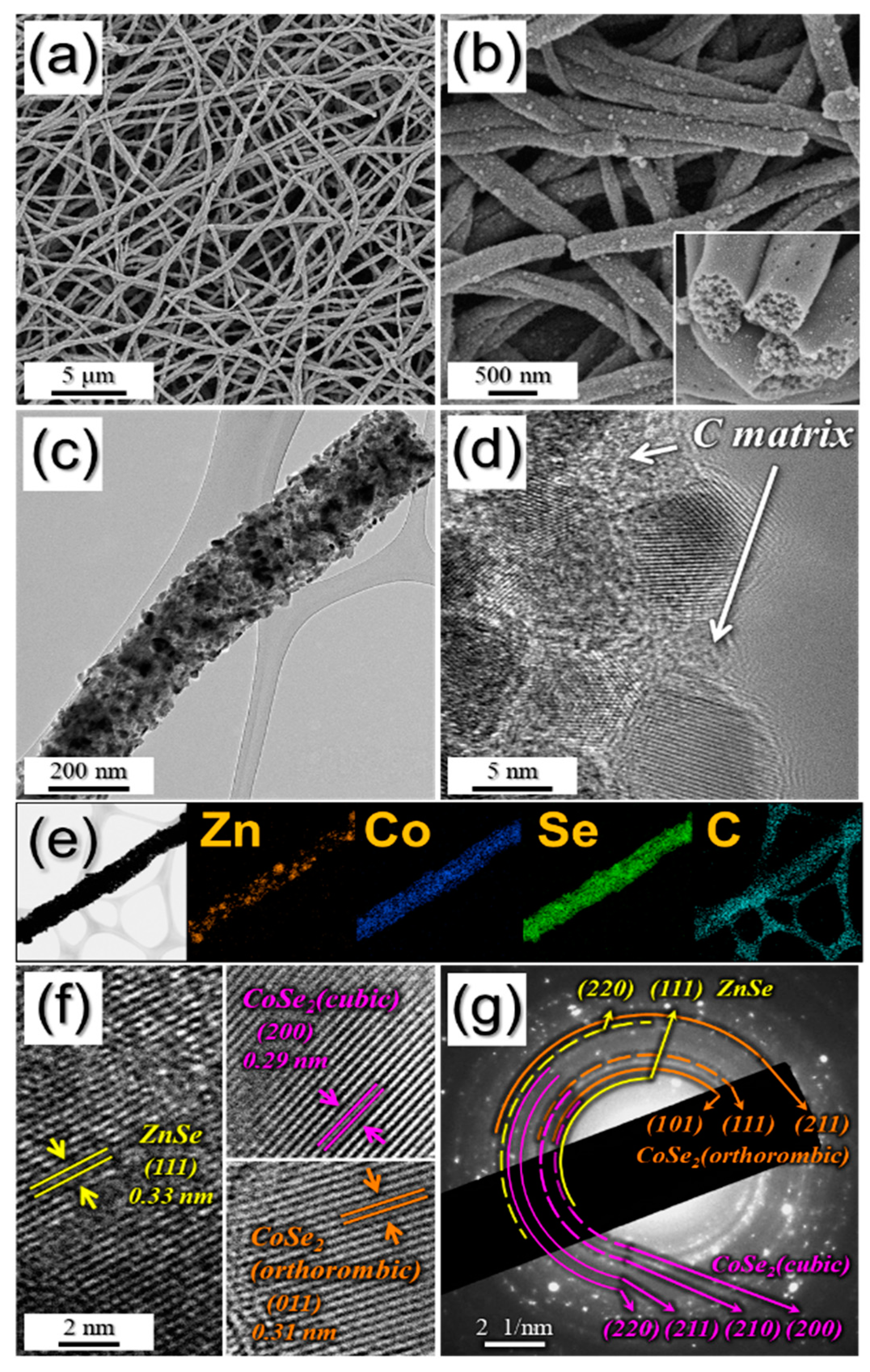
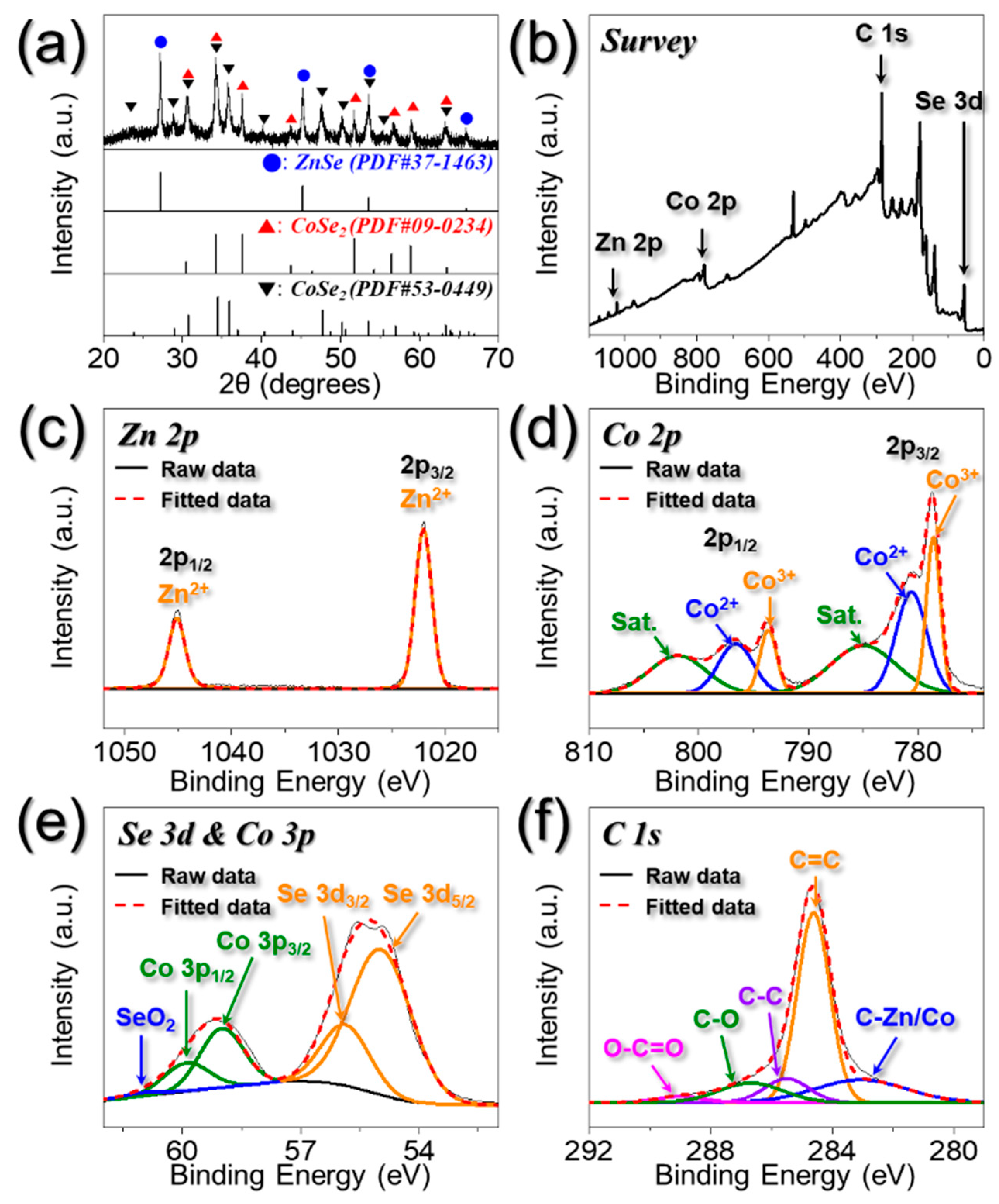
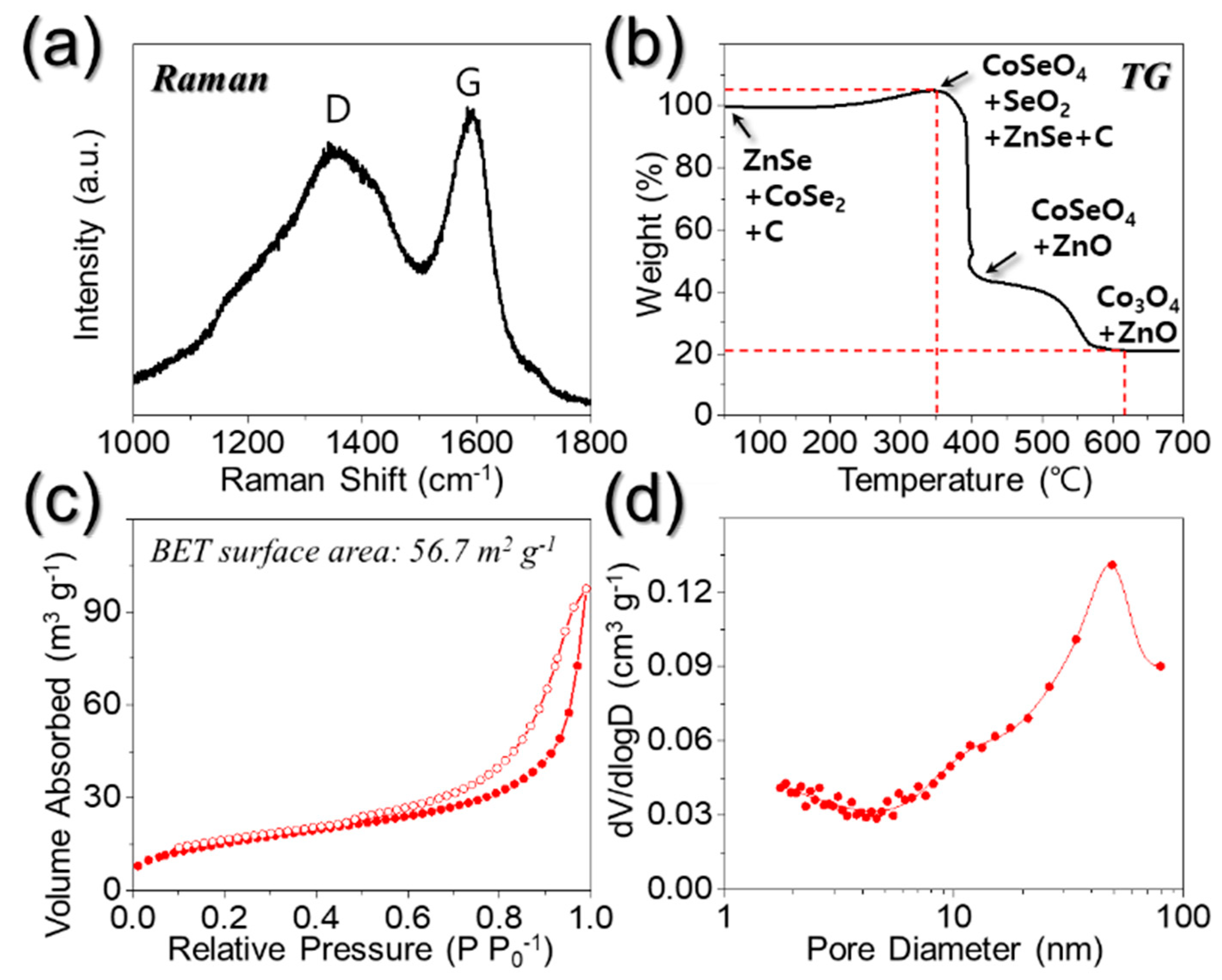
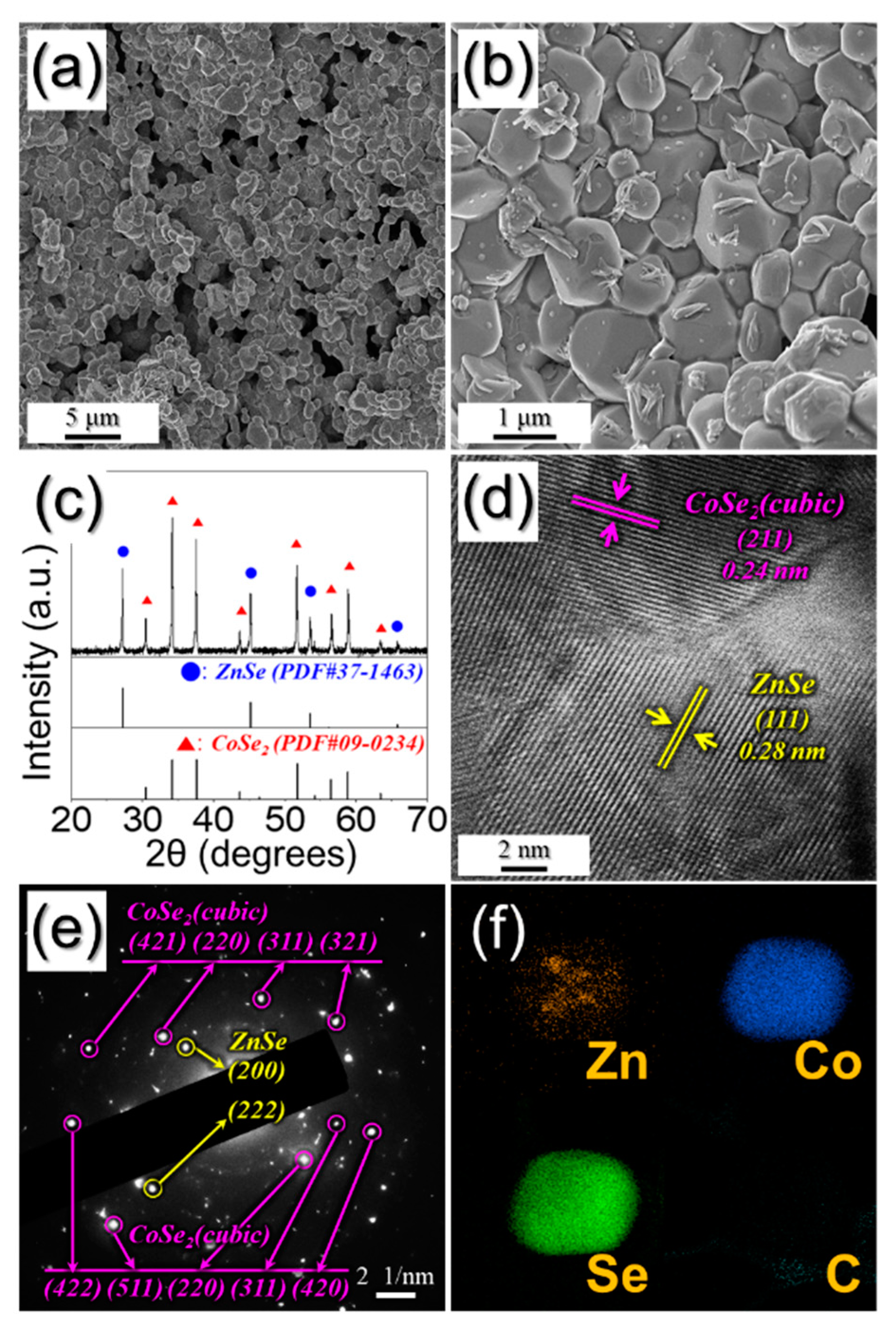
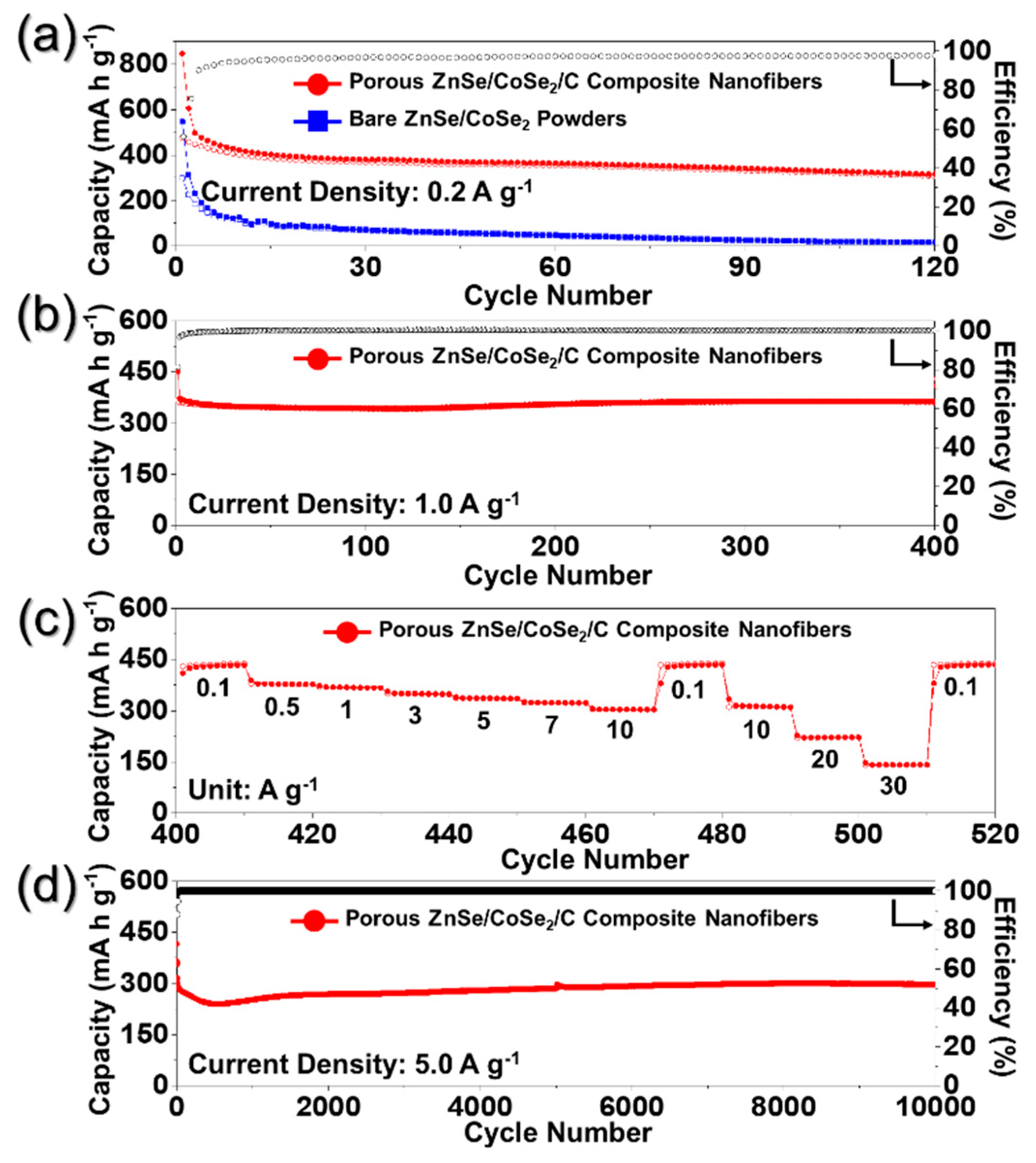
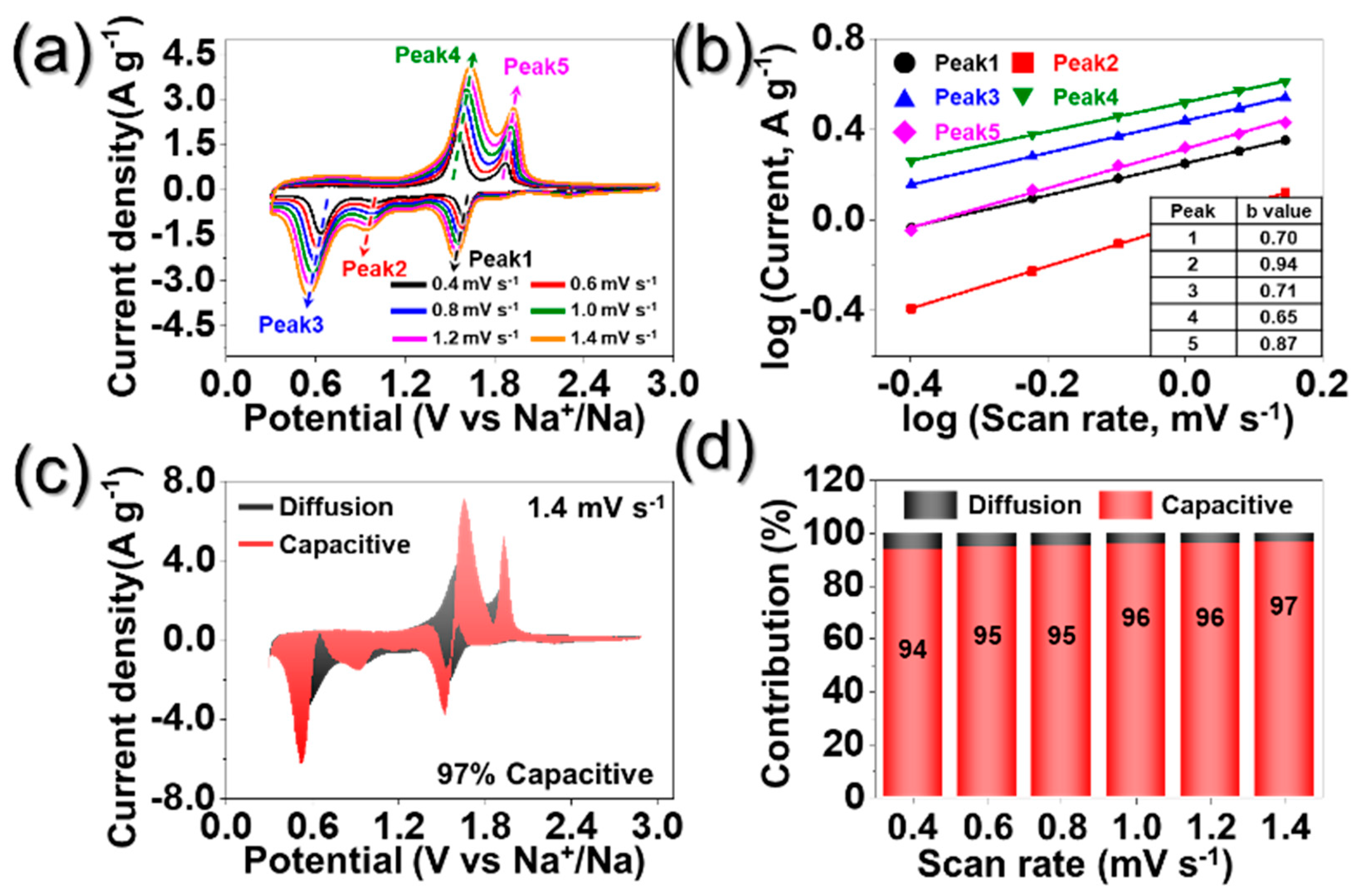
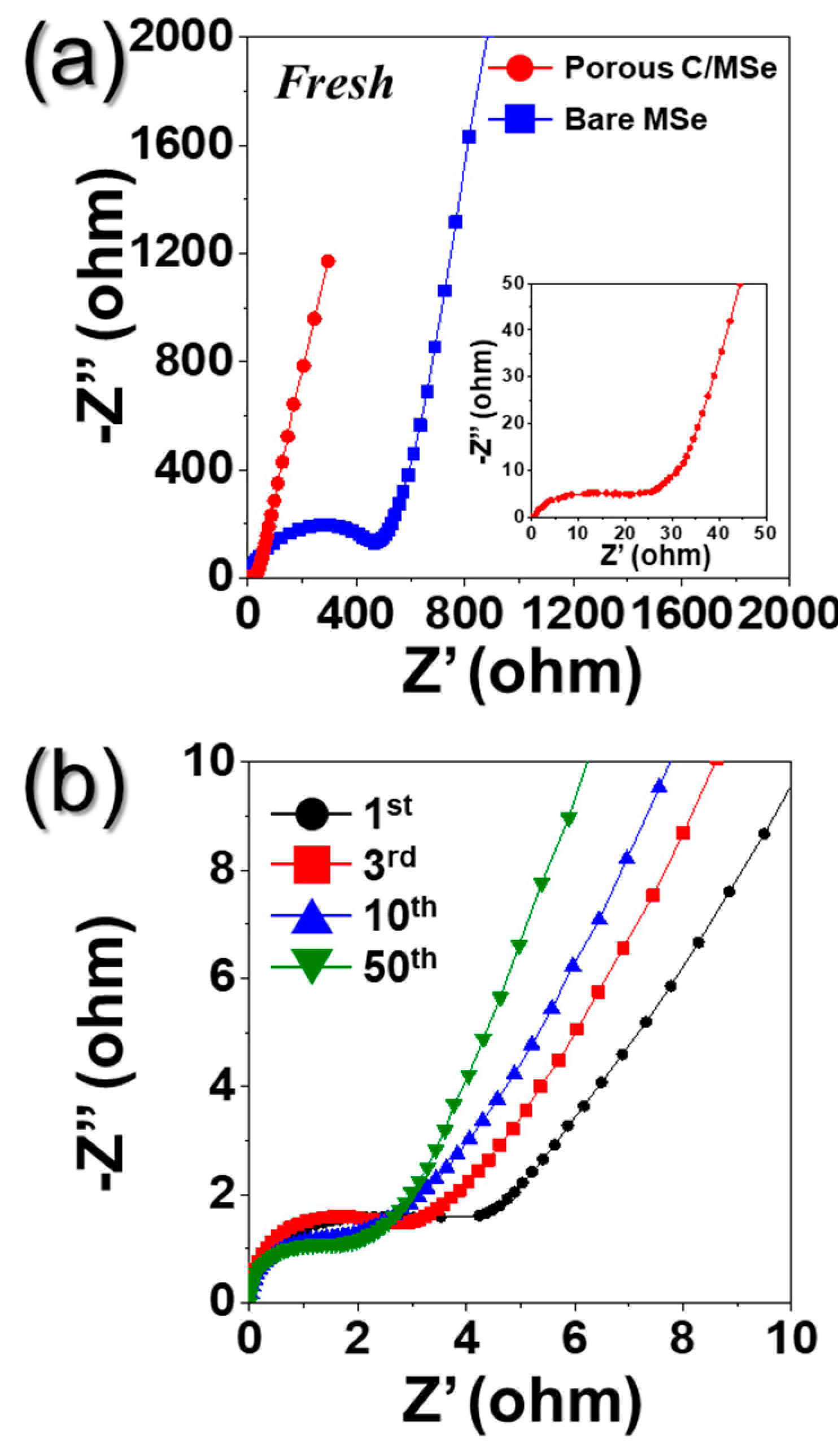
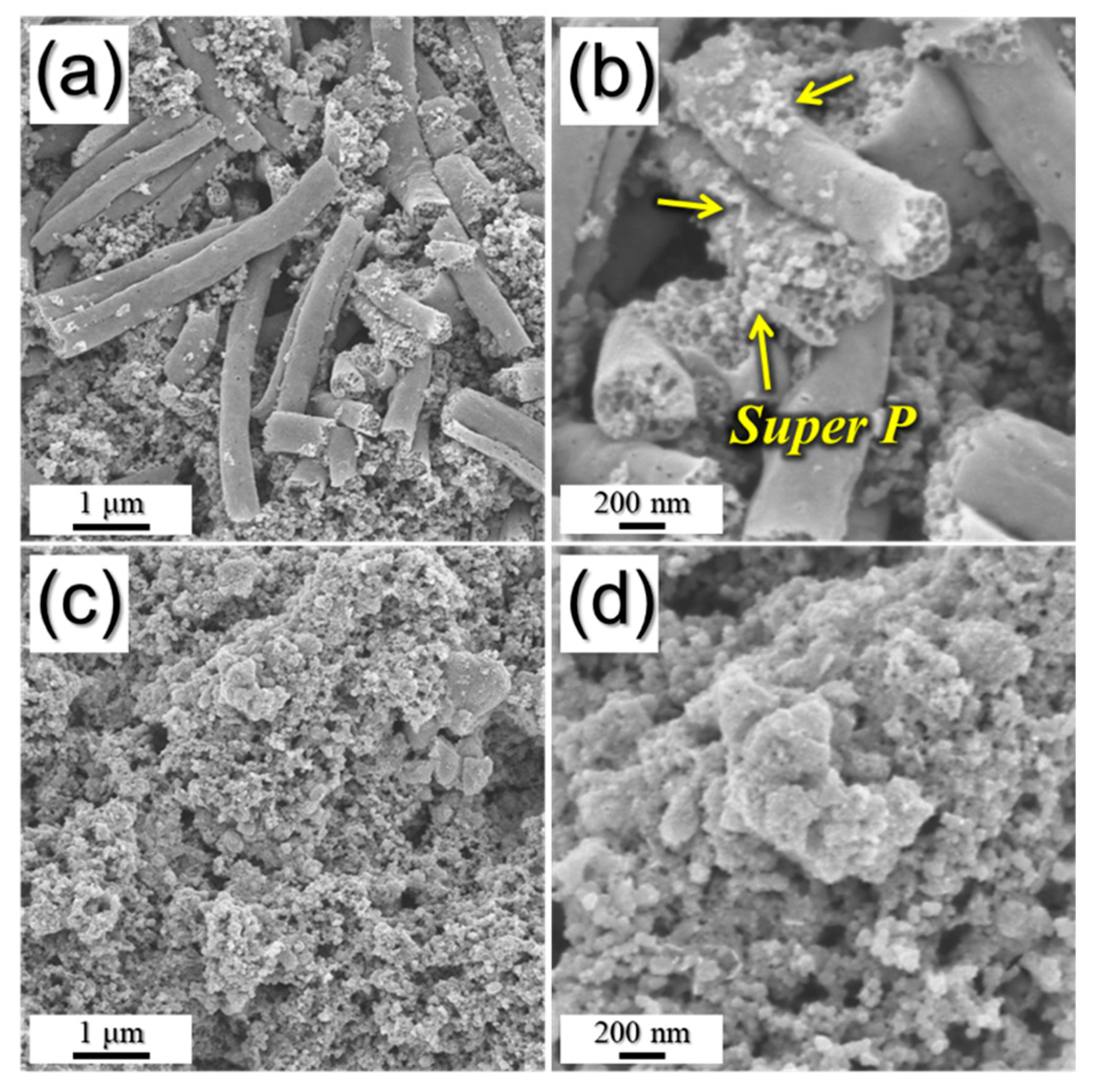
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Park, G.D.; Cho, J.S.; Kang, Y.C. Sodium-ion storage properties of nickel sulfide hollow nanospheres/reduced graphene oxide composite powders prepared by a spray drying process and the nanoscale Kirkendall effect. Nanoscale 2015, 7, 16781–16788. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Park, S.K.; Kang, Y.C. A Salt-templated strategy toward hollow iron selenides-graphitic carbon composite microspheres with interconnected multicavities as high-performance anode materials for sodium-ion batteries. Small 2019, 15, 1803043. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Zhang, C.; Hou, H.; Wu, B.; Zhou, L.; Li, S.; Wu, T.; Hu, J.; Mai, L.; Ji, X. Anions induced evolution of Co3X4 (X = O, S, Se) as sodium-ion anodes: The influences of electronic structure, morphology, electrochemical property. Nano Energy 2018, 48, 617–629. [Google Scholar] [CrossRef]
- Kim, J.K.; Jeong, S.Y.; Lim, S.H.; Oh, J.H.; Park, S.-K.; Cho, J.S.; Kang, Y.C. Recent advances on aerosol-assisted spray processes for design and fabrication of nanostructured metal chalcogenides for sodium ion batteries. Chem. Asian J. 2019, 14, 3127–3140. [Google Scholar] [CrossRef]
- Luo, M.; Yu, H.; Hu, F.; Liu, T.; Cheng, X.; Zheng, R.; Bai, Y.; Shui, M.; Shu, J. Metal selenides for high performance sodium ion batteries. Chem. Eng. J. 2019, 380, 122557. [Google Scholar] [CrossRef]
- Ali, Z.; Asif, M.; Huang, X.; Tang, T.; Hou, Y. Hierarchically porous Fe2CoSe4 binary-metal selenide for extraordinary rate performance and durable anode of sodium-ion batteries. Adv. Mater. 2018, 30, 1802745. [Google Scholar] [CrossRef]
- Fang, G.; Wu, Z.; Zhou, J.; Zhu, C.; Cao, X.; Lin, T.; Chen, Y.; Wang, C.; Pan, A.; Liang, S. Observation of pseudocapacitive effect and fast ion diffusion in bimetallic sulfides as an advanced sodium-ion battery anode. Adv. Energy Mater. 2018, 8, 1703155. [Google Scholar] [CrossRef]
- Huang, G.; Li, Q.; Yin, D.; Wang, L. Hierarchical porous Te@ZnCo2O4 nanofibers derived from Te@metal-organic frameworks for superior lithium storage capability. Adv. Funct. Mater. 2017, 27, 1604941. [Google Scholar] [CrossRef]
- Park, G.D.; Kang, Y.C. Multiroom-structured multicomponent metal selenide–graphitic carbon–carbon nanotube hybrid microspheres as efficient anode materials for sodium-ion batteries. Nanoscale 2018, 10, 8125–8132. [Google Scholar] [CrossRef]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Design and synthesis of bubble-nanorod-structured Fe2O3–carbon nanofibers as advanced anode material for Li-ion batteries. ACS Nano 2015, 9, 4026–4035. [Google Scholar] [CrossRef]
- Cho, J.S.; Park, J.-S.; Jeon, K.M.; Kang, Y.C. 1-D nanostructure comprising porous Fe2O3/Se composite nanorods with numerous nanovoids, and their electrochemical properties for use in lithium-ion batteries. J. Mater. Chem. A 2017, 5, 10632–10639. [Google Scholar] [CrossRef]
- Islam, M.S.; Fisher, C.A. Lithium and sodium battery cathode materials: Computational insights into voltage, diffusion and nanostructural properties. Chem. Soc. Rev. 2014, 43, 185–204. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.-Y.; Fang, Y.; Zhu, X.; Bao, J.; Zhou, X.; Lou, X.W.D. Confining SnS2 ultrathin nanosheets in hollow carbon nanostructures for efficient capacitive sodium storage. Joule 2018, 2, 725–735. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, S.H.; Jeong, S.Y.; Kang, Y.C.; Cho, J.S. Rattle-type porous Sn/C composite fibers with uniformly distributed nanovoids containing metallic Sn nanoparticles for high-performance anode materials in lithium-ion batteries. Nanoscale 2018, 10, 21483–21491. [Google Scholar] [CrossRef]
- Wan, Q.; Hu, S.; Dai, J.; Chen, C.; Li, W.-X. First-principles kinetic study for Ostwald ripening of late transition metals on TiO2(110). J. Phys. Chem. C 2018, 123, 1160–1169. [Google Scholar] [CrossRef]
- Shenasa, M.; Sainkar, S.; Lichtman, D. XPS study of some selected selenium compounds. J. Electron Spectrosc. Relat. Phenom. 1986, 40, 329–337. [Google Scholar] [CrossRef]
- Tang, C.; Wei, X.; Cai, X.; An, Q.; Hu, P.; Sheng, J.; Zhu, J.; Chou, S.; Wu, L.; Mai, L. ZnSe microsphere/multiwalled carbon nanotube composites as high-rate and long-life anodes for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 19626–19632. [Google Scholar] [CrossRef]
- Oh, S.H.; Cho, J.S. Hierarchical (Ni, Co) Se2/CNT hybrid microspheres consisting of a porous yolk and embossed hollow thin shell for high-performance anodes in sodium-ion batteries. J. Alloy. Compd. 2019, 806, 1029–1038. [Google Scholar] [CrossRef]
- Zhang, K.; Park, M.; Zhou, L.; Lee, G.H.; Li, W.; Kang, Y.M.; Chen, J. Urchin-like CoSe2 as a high-performance anode material for sodium-ion batteries. Adv. Funct. Mater. 2016, 26, 6728–6735. [Google Scholar] [CrossRef]
- Park, S.-K.; Kim, J.K.; Kang, Y.C. Excellent sodium-ion storage performances of CoSe2 nanoparticles embedded within N-doped porous graphitic carbon nanocube/carbon nanotube composite. Chem. Eng. J. 2017, 328, 546–555. [Google Scholar] [CrossRef]
- Van der Heide, H.; Hemmel, R.; Van Bruggen, C.; Haas, C. X-Ray photoelectron spectra of 3D transition metal pyrites. J. Solid State Chem. 1980, 33, 17–25. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, S.-K.; Kang, Y.C.; Cho, J.S. One-dimensional nanostructure comprising MoSe2 nanosheets and carbon with uniformly defined nanovoids as an anode for high-performance sodium-ion batteries. Chem. Eng. J. 2018, 351, 559–568. [Google Scholar] [CrossRef]
- Nedfors, N.; Tengstrand, O.; Flink, A.; Andersson, A.; Eklund, P.; Hultman, L.; Jansson, U. Reactive sputtering of NbCx-based nanocomposite coatings: An up-scaling study. Surf. Coat. Technol. 2014, 253, 100–108. [Google Scholar] [CrossRef]
- Takahagi, T.; Ishitani, A. XPS studies by use of the digital difference spectrum technique of functional groups on the surface of carbon fiber. Carbon 1984, 22, 43–46. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Park, J.-S.; Jeong, S.Y.; Jeon, K.M.; Kang, Y.C.; Cho, J.S. Iron diselenide combined with hollow graphitic carbon nanospheres as a high-performance anode material for sodium-ion batteries. Chem. Eng. J. 2018, 339, 97–107. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, G.D.; Kim, J.H.; Park, S.K.; Kang, Y.C. Rational design and synthesis of extremely efficient macroporous CoSe2–CNT composite microspheres for hydrogen evolution reaction. Small 2017, 13, 1700068. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.; Ge, P.; Zhu, L.; Xie, L.; Hou, H.; Qiu, X.; Ji, X. Hollow-sphere ZnSe wrapped around carbon particles as a cycle-stable and high-rate anode material for reversible Li-ion batteries. New J. Chem. 2017, 41, 6693–6699. [Google Scholar] [CrossRef]
- Cui, C.; Wei, Z.; Zhou, G.; Wei, W.; Ma, J.; Chen, L.; Li, C. Quasi-reversible conversion reaction of CoSe2/nitrogen-doped carbon nanofibers towards long-lifetime anode materials for sodium-ion batteries. J. Mater. Chem. A 2018, 6, 7088–7098. [Google Scholar] [CrossRef]
- Cao, X.; Li, A.; Yang, Y.; Chen, J. ZnSe nanoparticles dispersed in reduced graphene oxides with enhanced electrochemical properties in lithium/sodium ion batteries. RSC Adv. 2018, 8, 25734–25744. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, Z.; Cheng, F.; Zhang, K.; Wang, J.; Chen, C.; Chen, J. Pyrite FeS2 for high-rate and long-life rechargeable sodium batteries. Energy Environ. Sci. 2015, 8, 1309–1316. [Google Scholar] [CrossRef]
- Kim, H.; Lim, K.; Yoon, G.; Park, J.H.; Ku, K.; Lim, H.D.; Sung, Y.E.; Kang, K. Exploiting lithium–ether co-intercalation in graphite for high-power lithium-ion batteries. Adv. Energy Mater. 2017, 7, 1700418. [Google Scholar] [CrossRef]
- Su, D.; Kretschmer, K.; Wang, G. Improved electrochemical performance of Na-ion batteries in ether-based electrolytes: A case study of ZnS nanospheres. Adv. Energy Mater. 2016, 6, 1501785. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.-K.; Park, J.-S.; Kang, Y.C. Uniquely structured composite microspheres of metal sulfides and carbon with cubic nanorooms for highly efficient anode materials for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 2636–2645. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.Y.; Cho, J.S. Porous Hybrid Nanofibers Comprising ZnSe/CoSe₂/Carbon with Uniformly Distributed Pores as Anodes for High-Performance Sodium-Ion Batteries. Nanomaterials 2019, 9, 1362. https://doi.org/10.3390/nano9101362
Jeong SY, Cho JS. Porous Hybrid Nanofibers Comprising ZnSe/CoSe₂/Carbon with Uniformly Distributed Pores as Anodes for High-Performance Sodium-Ion Batteries. Nanomaterials. 2019; 9(10):1362. https://doi.org/10.3390/nano9101362
Chicago/Turabian StyleJeong, Sun Young, and Jung Sang Cho. 2019. "Porous Hybrid Nanofibers Comprising ZnSe/CoSe₂/Carbon with Uniformly Distributed Pores as Anodes for High-Performance Sodium-Ion Batteries" Nanomaterials 9, no. 10: 1362. https://doi.org/10.3390/nano9101362
APA StyleJeong, S. Y., & Cho, J. S. (2019). Porous Hybrid Nanofibers Comprising ZnSe/CoSe₂/Carbon with Uniformly Distributed Pores as Anodes for High-Performance Sodium-Ion Batteries. Nanomaterials, 9(10), 1362. https://doi.org/10.3390/nano9101362





