Electrochemical DNA Biosensors Based on Labeling with Nanoparticles
Abstract
:1. Introduction
2. Synthesis and Modification of NPs for Labeling DNA Applications
2.1. Synthesis of AuNPs and AgNPs
2.2. Synthesis of QDs
2.3. Functionalization of Metal NPs and QDs with Biomolecules
3. Electrochemical Determination of NPs and QDs Labels
3.1. Voltammetric Determination of AuNPs Labels
Signal Enhancement Strategies Using AuNPs Labels
3.2. Voltammetric Determination of AgNPs Labels
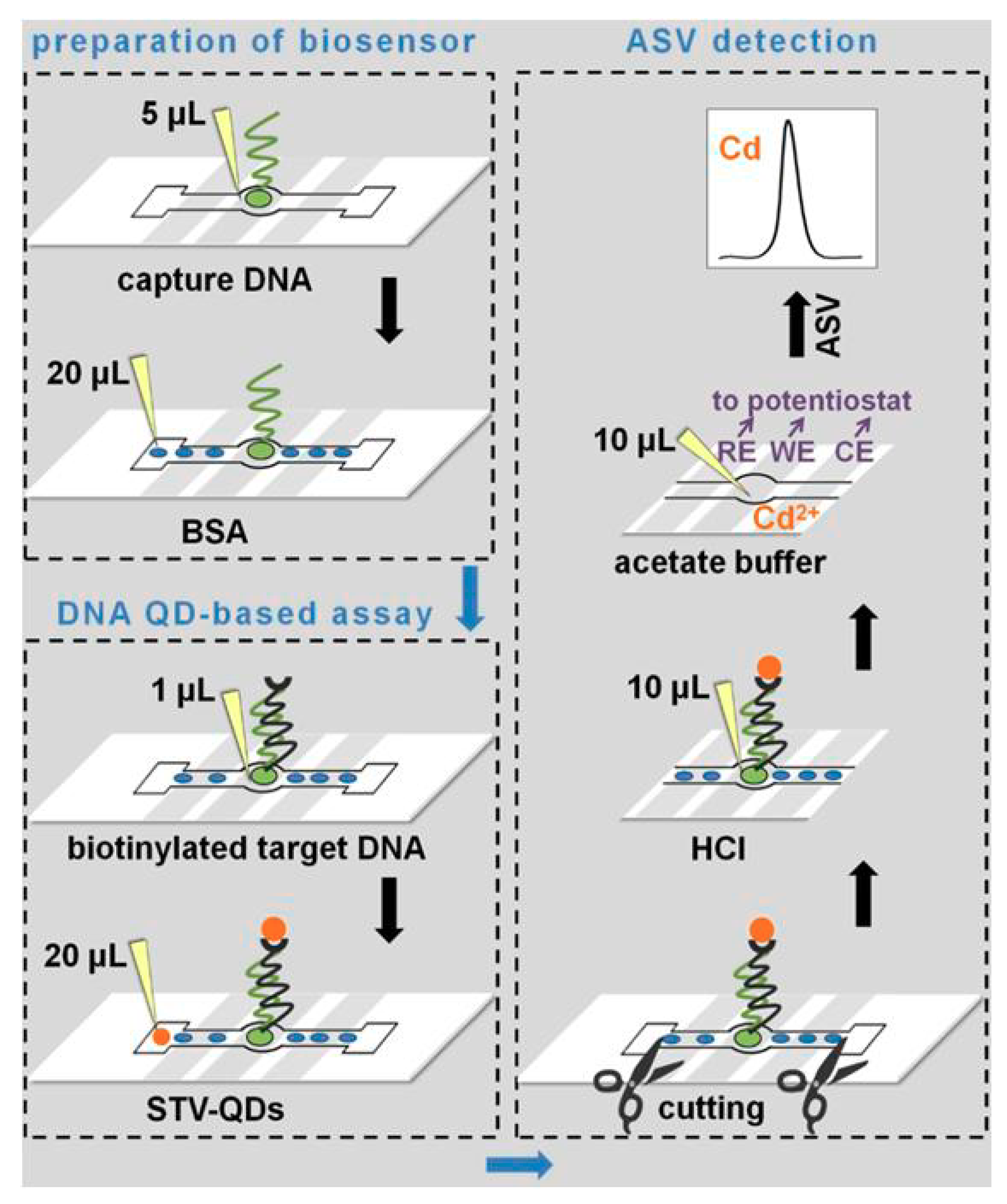
3.3. Voltammetric Determination of QDs Labels
3.3.1. Mercury-Based Sensors for QD-Based DNA Assay
3.3.2. Bismuth- and Tin-Based Sensors for QD-Based DNA Assay
3.3.3. Signal Enhancement Strategies Using QD Labels
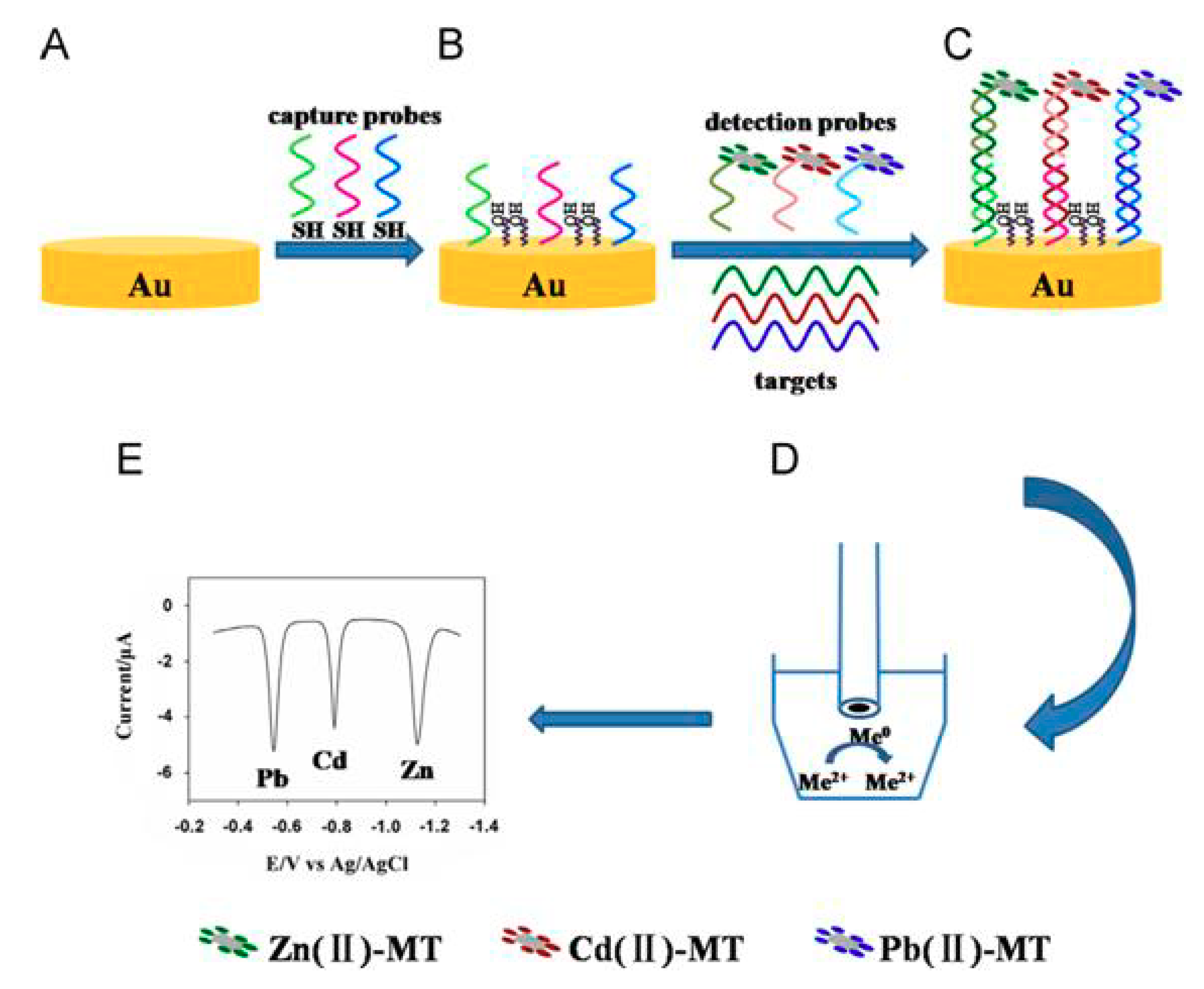
4. Multiplexed Detection of DNA Sequences Exploiting NPs as Labels
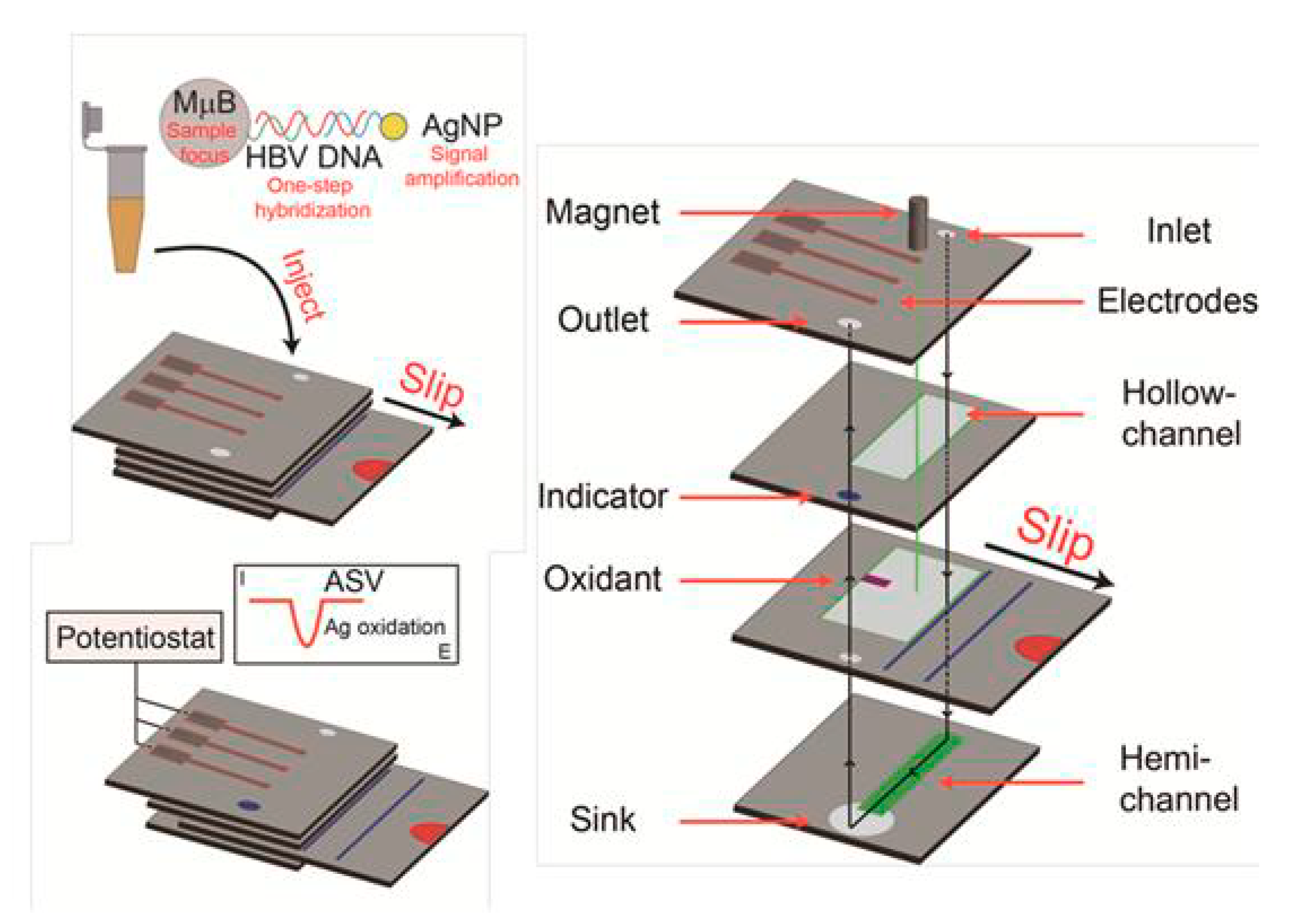
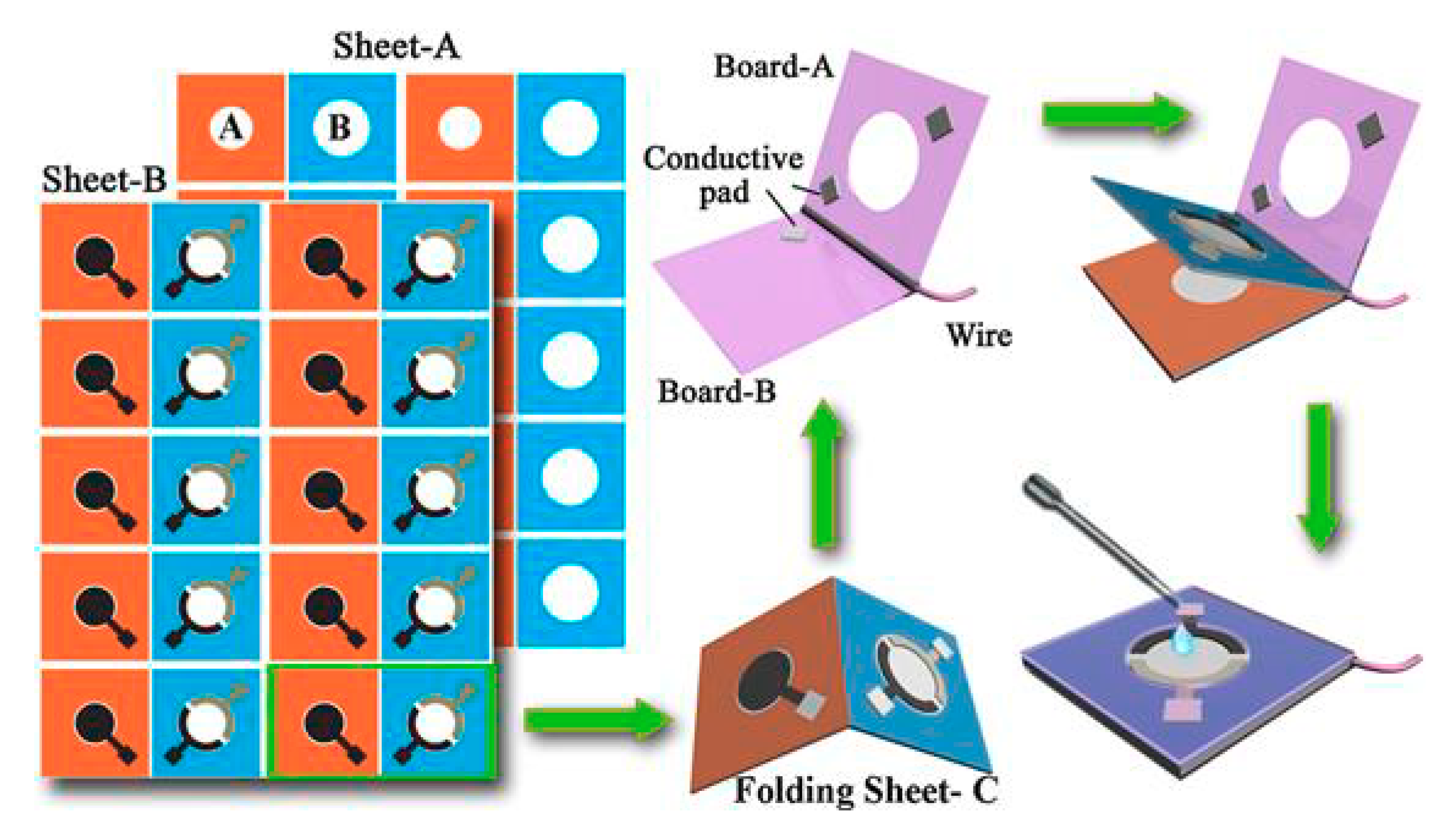
5. Paper-Based Devices for DNA Sensing Using NPs as Labels
6. Conclusions
Funding
Conflicts of Interest
References
- Blair, E.O.; Corrigan, D.K. A review of microfabricated electrochemical biosensors for DNA detection. Biosens. Bioelectron. 2019, 134, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Wang, K.; Weng, S.; Lei, Y.; Lin, L.; Chen, W.; Lin, X.; Chen, Y. Development of electrochemical DNA biosensors. TrAC Trends Anal. Chem. 2012, 37, 101–111. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, E.; Zhang, X.; Zhang, X.; Chen, J. Nanomaterials as signal amplification elements in DNA-based electrochemical sensing. Nano Today 2014, 9, 197–211. [Google Scholar] [CrossRef]
- Merkoci, A.; Aldavert, M.; Marın, S.; Alegret, S. New materials for electrochemical sensing V: Nanoparticles for DNA labeling. TrAC Trends Anal. Chem. 2005, 24, 341–349. [Google Scholar] [CrossRef]
- Rosario, R.; Mutharasan, R. Nucleic acid electrochemical and electromechanical biosensors: A review of techniques and developments. Rev. Anal. Chem. 2014, 33, 213–230. [Google Scholar] [CrossRef]
- Ermini, M.L.; Mariani, S.; Scarano, S.; Minunni, M. Bioanalytical approaches for the detection of single nucleotide polymorphisms by Surface Plasmon Resonance biosensors. Biosens. Bioelectron. 2014, 61, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yook, J.G. Recent research trends of radio-frequency biosensors for biomolecular detection. Biosens. Bioelectron. 2014, 61, 448–459. [Google Scholar] [CrossRef]
- Huang, J.; Yang, X.; He, X.; Wang, K.; Liu, J.; Shi, H.; Wang, Q.; Guo, Q.; He, D. Design and bioanalytical applications of DNA hairpin-based fluorescent probes. TrAC Trends Anal. Chem. 2014, 53, 11–20. [Google Scholar] [CrossRef]
- Economou, A.; Kokkinos, C.; Prodromidis, M. Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing. Lab Chip 2018, 18, 1812–1830. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D.; Palleschi, G. How cutting-edge technologies impact the design of electrochemical (bio)sensors for environmental analysis. A review. Anal. Chim. Acta 2017, 9, 15–42. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Fenzl, C.; Hirsch, T.; Baeumner, A.J. Nanomaterials as versatile tools for signal amplification in(bio)analytical applications. TrAC Trends Anal. Chem. 2016, 79, 306–316. [Google Scholar] [CrossRef]
- Valera, E.; Hernandez-Albors, A.; Marco, M.P. Electrochemical coding strategies using metallic nanoprobes for biosensing applications. TrAC Trends Anal. Chem. 2016, 79, 9–22. [Google Scholar] [CrossRef]
- Wang, J. Nanomaterial-Based Amplified Transduction of Biomolecular Interactions. Small 2005, 1, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Siangproh, W.; Dungchai, W.; Rattanarat, P.; Chailapakul, O. Nanoparticle-based electrochemical detection in conventional and miniaturized systems and their bioanalytical applications: A review. Anal. Chim. Acta 2011, 690, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A. Emerging trends in biosensing using stripping voltammetric detection of metal-containing nanolabels-A review. Anal. Chim. Acta 2017, 961, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Delshadi-Jahromi, N.; Nazari-Vanani, R.; Yadegari, H.; Sattarahmady, N.; Hatam, G.R.; Heli, H. Label-free ultrasensitive electrochemical genosensing of Trichomonas vaginalis using anisotropic-shaped gold nanoparticles as a platform, a repeated sequence of the parasite DNA as a probe, and toluidine blue as a redox marker. Sens. Actuators B 2018, 273, 234–241. [Google Scholar] [CrossRef]
- Senel, M.; Dervisevic, M.; Kokkokoglu, F. Electrochemical DNA biosensors for label-free breast cancer gene marker detection. Anal. BioAnal. Chem. 2019, 411, 2925–2935. [Google Scholar] [CrossRef]
- Tahernejad-Javazmi, F.; Shabani-Nooshabadi, M.; Karimi-Maleh, H. Gold nanoparticles and reduced graphene oxide-amplified labelfree DNA biosensor for dasatinib detection. New J. Chem. 2018, 42, 16378–16383. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, Z.; Ju, H.; Yan, F. Triplex signal amplification for electrochemical DNA biosensing by coupling probe-gold nanoparticles–graphene modified electrode with enzyme functionalized carbon sphere as tracer. Biosens. Bioelectron. 2012, 33, 228–232. [Google Scholar] [CrossRef]
- Pereira-Barros, M.A.; Barroso, M.F.; Martin-Pedraza, L.; Vargas, E.; Benede, S.; Villalba, M.; Rocha, J.M.; Campuzano, S.; Pingarron, J.M. Direct PCR-free electrochemical biosensing of plant-food derived nucleic acids in genomic DNA extracts. Application to the determination of the key allergen Sola l 7 in tomato seeds. Biosens. Bioelectron. 2019, 137, 171–177. [Google Scholar] [CrossRef]
- Shoaie, N.; Forouzandeh, M.; Omidfar, K. Voltammetric determination of the Escherichia coli DNA using a screen-printed carbon electrode modified with polyaniline and gold nanoparticles. Microchim. Acta 2018, 185, 217. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tian, M.; Liang, Z. DNA analysis based on the electrocatalytic amplification of gold nanoparticles. Electrochim. Acta 2013, 113, 186–193. [Google Scholar] [CrossRef]
- Cui, H.F.; Cheng, L.; Zhang, J.; Liu, R.; Zhang, C.; Fan, H. An electrochemical DNA sensor for sequence-specific DNA recognization in a homogeneous solution. Biosens. Bioelectron. 2014, 56, 124–128. [Google Scholar] [CrossRef]
- Daneshpour, M.; Syed moradi, L.; Izadi, P.; Omidfar, K. Femtomolar level detection of RASSF1A tumor suppressor gene methylation by electrochemical nano-genosensor based on Fe3O4/TMC/Au nanocomposite and PT-modified electrode. Biosens. Bioelectron. 2016, 77, 1095–1103. [Google Scholar] [CrossRef]
- Pinijsuwan, S.; Rijiravanich, P.; Somasundrum, M.; Surareungchai, W. Sub-Femtomolar Electrochemical Detection of DNA Hybridization Based on Latex/Gold Nanoparticle-Assisted Signal Amplification. Anal. Chem. 2008, 80, 6779–6784. [Google Scholar] [CrossRef]
- Ting, B.P.; Zhang, J.; Gao, Z.; Ying, J.Y. A DNA biosensor based on the detection of doxorubicin-conjugated Ag nanoparticle labels using solid-state voltammetry. Biosens. Bioelectron. 2009, 25, 282–287. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, W.; Kong, J.; Zhang, X. Ultrasensitive electrochemical DNA biosensor by exploiting hematin as efficient biomimetic catalyst toward in situ metallization. Biosens. Bioelectron. 2015, 63, 269–275. [Google Scholar] [CrossRef]
- Song, W.; Niu, Q.; Qiang, W.; Li, H.; Xu, D. Enzyme-free electrochemical aptasensor by using silver nanoparticles aggregates coupling with carbon nanotube inducing signal amplification through electrodeposition. J. ElectroAnal. Chem. 2016, 781, 62–69. [Google Scholar] [CrossRef]
- Li, X.; Scida, K.; Crooks, R.M. Detection of Hepatitis B Virus DNA with a Paper Electrochemical Sensor. Anal. Chem. 2015, 87, 9009–9015. [Google Scholar] [CrossRef]
- Liu, H.; Lou, Y.; Zhou, F.; Zhu, H.; Abdel-Halim, E.S.; Zhu, J.J. An amplified electrochemical strategy using DNA-QDs dendrimer superstructure for the detection of thymine DNA glycosylase activity. Biosens. Bioelectron. 2015, 71, 249–255. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, X.; He, S.; Gao, Z.; Di, Y.; Lu, K.; Li, K.; Wang, J. Aptamer-DNA concatamer-quantum dots based electrochemical biosensing strategy for green and ultrasensitive detection of tumor cells via mercury free anodic stripping voltammetry. Biosens. Bioelectron. 2019, 126, 261–268. [Google Scholar] [CrossRef]
- Yang, T.; Huang, D.; Li, Q.; Zhang, W.; Jiao, K. Synergistic Effect of MWNTs/CeO2/CHIT Film for Detection of CdSe Nanoparticle Labeled Sequence-Specific of 35S Promoter of Cauliflower Mosaic Virus Gene. Electroanalysis 2012, 24, 392–397. [Google Scholar] [CrossRef]
- Jie, G.; Zhang, J.; Jie, G.; Wang, L. A novel quantum dot nanocluster as versatile probe for electrochemiluminescence and electrochemical assays of DNA and cancer cells. Biosens. Bioelectron. 2014, 52, 69–75. [Google Scholar] [CrossRef]
- Kokkinos, C.; Prodromidis, M.; Economou, A.; Petrou, P.; Kakabakos, S. Quantum dot-based electrochemical DNA biosensor using a screen-printed graphite surface with embedded bismuth precursor. Electrochem. Commun. 2015, 60, 47–51. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Merkoci, A. Electrochemical Coding Technology for Simultaneous Detection of MultipleDNA Targets. J. Am. Chem. Soc. 2003, 125, 3214–3215. [Google Scholar] [CrossRef]
- Sun, A.L.; Zhang, Y.F.; Wang, X.N. Sensitive voltammetric determination of DNA via a target-induced strand-displacement reaction using quantum dot-labeled probe DNA. Microchim. Acta 2015, 182, 1403–1410. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Jan, M.R.; Zhu, Q. Electrochemical detection of DNA hybridization based on carbon-nanotubes loaded with CdS tags. Electrochem. Commun. 2003, 5, 1000–1004. [Google Scholar] [CrossRef]
- Baek, S.; Won, B.Y.; Park, K.S.; Park, H.G. An electrochemical one-step system for assaying methyltransferase activity based on transport of a quantum dot signaling tracer. Biosens. Bioelectron. 2013, 49, 542–546. [Google Scholar] [CrossRef]
- Li, C.C.; Hu, J.; Lu, M.; Zhang, C.Y. Quantum dot-based electrochemical biosensor for stripping voltammetric detection of telomerase at the single-cell level. Biosens. Bioelectron. 2018, 122, 51–57. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Tan, Y.; Zhou, J.; Zhu, J.J. Quantum dot-based DNA hybridization by electrochemiluminescence and anodic stripping voltammetry. Analyst 2010, 135, 1773–1778. [Google Scholar] [CrossRef]
- Zhang, C.; Lou, J.; Tu, W.; Bao, J.; Dai, Z. Ultrasensitive electrochemical biosensing for DNA using quantum dots combined with restriction endonuclease. Analyst 2015, 140, 506–511. [Google Scholar] [CrossRef]
- Tang, S.; Lu, W.; Gu, F.; Tong, P.; Yan, Z.; Zhang, L. A novel electrochemical sensor for lead ion based on cascade DNA and quantum dots amplification. Electrochim. Acta 2014, 134, 1–7. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Kawde, A.N.; Polsky, R. Metal Nanoparticle-Based Electrochemical Stripping Potentiometric Detection of DNA Hybridization. Anal. Chem. 2001, 73, 5576–5581. [Google Scholar] [CrossRef]
- Liao, K.T.; Cheng, J.T.; Li, C.L.; Liu, R.T.; Huang, H.J. Ultra-sensitive detection of mutated papillary thyroid carcinoma DNA using square wave stripping voltammetry method and amplified gold nanoparticle biomarkers. Biosens. Bioelectron. 2009, 24, 1899–1904. [Google Scholar] [CrossRef]
- Low, K.F.; Rijiravanich, P.; Singh, K.K.B.; Surareungchai, W.; Yean, C.Y. An Electrochemical Genosensing Assay Based on Magnetic Beads and Gold Nanoparticle-Loaded Latex Microspheres for Vibrio cholerae Detection. J. Biomed. Nanotechnol. 2015, 11, 702–710. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Wang, J.; Wang, Z.; Yi, X.; Wang, J.; Wang, N. Voltammetric determination of the Alzheimer’s disease-related ApoE 4 gene from unamplified genomic DNA extracts by ferrocene-capped gold nanoparticles. Microchim. Acta 2018, 185, 549. [Google Scholar] [CrossRef]
- Wang, J.; Polsky, R.; Xu, D. Silver-Enhanced Colloidal Gold Electrochemical Stripping Detection of DNA Hybridization. Langmuir 2001, 17, 5739–5741. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Polsky, R. Magnetically-Induced Solid-State Electrochemical Detection of DNA Hybridization. J. Am. Chem. Soc. 2002, 124, 4208–4209. [Google Scholar] [CrossRef]
- Marin, S.; Merkoci, A. Direct electrochemical stripping detection of cystic-fibrosis-related DNA linked through cadmium sulfide quantum dots. Nanotechnology 2009, 20, 055101. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Speliotis, T.; Petrou, P.; Kakabakos, S. Flexible Microfabricated Film Sensors for the in Situ Quantum Dot-Based Voltammetric Detection of DNA Hybridization in Microwells. Anal. Chem. 2015, 87, 853–857. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Petrou, P.; Kakabakos, S. Microfabricated Tin−Film Electrodes for Protein and DNA Sensing Based on Stripping Voltammetric Detection of Cd(II) Released from Quantum Dots Labels. Anal. Chem. 2013, 85, 10686–10691. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A. Stripping analysis at bismuth-based electrodes. Curr. Anal. Chem. 2008, 4, 183–190. [Google Scholar] [CrossRef]
- Serrano, N.; Diaz-Cruz, J.M.; Arino, C.; Esteban, M. Antimony-based electrodes for analytical determinations. TrAC Trends Anal. Chem. 2016, 77, 203–213. [Google Scholar] [CrossRef]
- Zhu, W.W.; Li, N.B.; Luo, H.Q. Simultaneous determination of chromium(III) and cadmium(II) by differential pulse anodic stripping voltammetry on a stannum film electrode. Talanta 2007, 72, 1733–1737. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Speliotis, T. Tin-film mini-sensors fabricated by a thin-layer microelectronic approach for stripping voltammetric determination of trace metals. Electrochem. Commun. 2014, 38, 96–99. [Google Scholar] [CrossRef]
- Wang, C.; Qian, J.; An, K.; Huang, X.; Zhao, L.; Liu, Q.; Hao, N.; Wang, K. Magneto-controlled aptasensor for simultaneous electrochemical detection of dual mycotoxins in maize using metal sulfide quantum dots coated silica as labels. Biosens. Bioelectron. 2017, 89, 802–809. [Google Scholar] [CrossRef]
- Dong, H.; Yan, F.; Ji, H.; Wong, D.K.Y.; Ju, H. Quantum-Dot-Functionalized Poly(styrene-co-acrylic acid) Microbeads: Step-Wise Self-Assembly, Characterization, and Applications for Sub-femtomolar Electrochemical Detection of DNA Hybridization. Adv. Funct. Mater. 2010, 20, 1173–1179. [Google Scholar] [CrossRef]
- Chen, X.; Ge, L.; Guo, B.; Yan, M.; Hao, N.; Xu, L. Homogeneously ultrasensitive electrochemical detection of adenosine triphosphate based on multiple signal amplification strategy. Biosens. Bioelectron. 2014, 58, 48–56. [Google Scholar] [CrossRef]
- Kokkinos, C.T.; Giokas, D.L.; Economou, A.S.; Petrou, P.S.; Kakabakos, S.E. Paper-Based Microfluidic Device with Integrated Sputtered Electrodes for Stripping Voltammetric Determination of DNA via Quantum Dot Labeling. Anal. Chem. 2018, 90, 1092–1097. [Google Scholar] [CrossRef]
- Su, J.; Zhang, H.; Jiang, B.; Zheng, H.; Chai, Y.; Yuan, R.; Xiang, Y. Dual signal amplification for highly sensitive electrochemical detection of uropathogens via enzyme-based catalytic target recycling. Biosens. Bioelectron. 2011, 29, 184–188. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, H.; Jiang, B.; Chai, Y.; Yuan, R. Quantum Dot Layer-by-Layer Assemblies as Signal Amplification Labels for Ultrasensitive Electronic Detection of Uropathogens. Anal. Chem. 2011, 83, 4302–4306. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Kumar, R.; Dilbaghi, N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef]
- Vijian, D.; Chinni, S.V.; Yin, L.S.; Lertanantawong, B.; Surareungchai, W. Non-protein coding RNA-based genosensor with quantum dots as electrochemical labels for attomolar detection of multiple pathogens. Biosens. Bioelectron. 2016, 77, 805–811. [Google Scholar] [CrossRef]
- Krejcova, L.; Huska, D.; Hynek, D.; Kopel, P.; Adam, V.; Hubalek, J.; Trnkova, L.; Kizek, R. Using of Paramagnetic Microparticles and Quantum Dots for Isolation and Electrochemical Detection of Influenza Viruses’ Specific Nucleic Acids. Int. J. Electrochem. Sci. 2013, 8, 689–702. [Google Scholar]
- Zheng, L.; Li, X.; Liu, P.; Wu, G.; Lu, X.; Liu, X. Simultaneous detection of multiple DNA targets based on encoding metal ions. Biosens. Bioelectron. 2014, 52, 354–359. [Google Scholar] [CrossRef]
- Zhang, D.; Huarng, M.C.; Alocilja, E.C. A multiplex nanoparticle-based bio-barcoded DNA sensor for the simultaneous detection of multiple pathogens. Biosens. Bioelectron. 2010, 26, 1736–1742. [Google Scholar] [CrossRef]
- Leng, C.; Lai, G.; Yan, F.; Ju, H. Gold nanoparticle as an electrochemical label for inherently crosstalk-free multiplexed immunoassay on a disposable chip. Anal. Chim. Acta 2010, 666, 97–101. [Google Scholar] [CrossRef]
- Lai, G.; Wang, L.; Wu, J.; Jua, H.; Yan, F. Electrochemical stripping analysis of nanogold label-induced silver deposition for ultrasensitive multiplexed detection of tumor markers. Anal. Chim. Acta 2012, 721, 1–6. [Google Scholar] [CrossRef]
- Lu, J.; Ge, S.; Ge, L.; Yan, M.; Yu, J. Electrochemical DNA sensor based on three-dimensional folding paper device for specific and sensitive point-of-care testing. Electrochim. Acta 2012, 80, 334–341. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zhao, L.; Lei, W.; Wen, W.; Wang, J.Y.; Bao, T.; Xiong, H.Y.; Zhang, X.H.; Wang, S.F. A high-sensitivity electrochemical aptasensor of carcinoembryonic antigen based on graphene quantum dots-ionic liquid-nafion nanomatrix and DNAzyme-assisted signal amplification strategy. Biosens. Bioelectron. 2018, 99, 28–33. [Google Scholar] [CrossRef]
- Campuzano, S.; Sedeno, P.Y.; Pingarron, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef]
- Delaney, J.L.; Doeven, E.H.; Harsant, A.J.; Hogan, C.F. Use of a mobile phone for potentiostatic control with low cost paper-based microfluidic sensors. Anal. Chim. Acta 2013, 790, 56–60. [Google Scholar] [CrossRef]
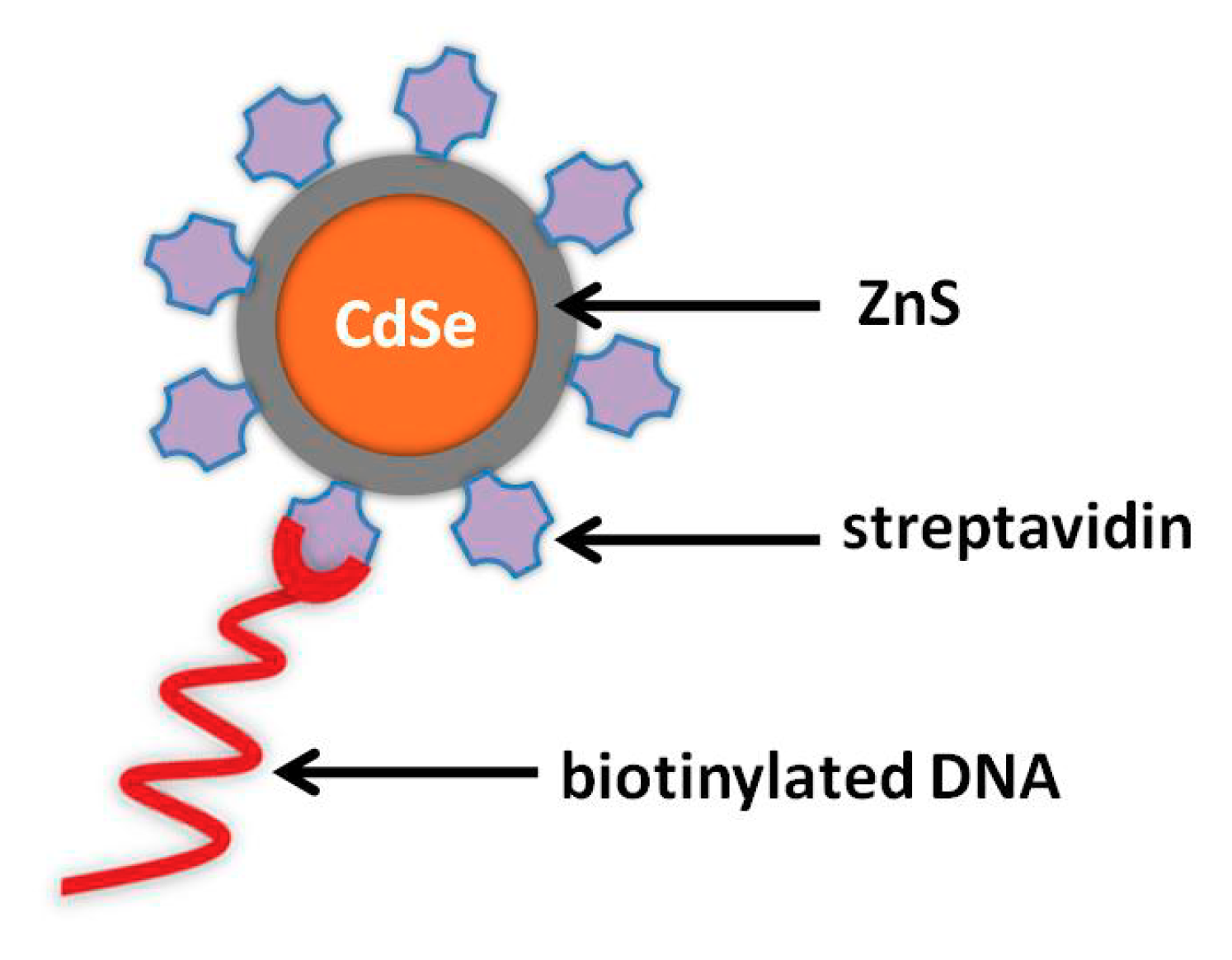
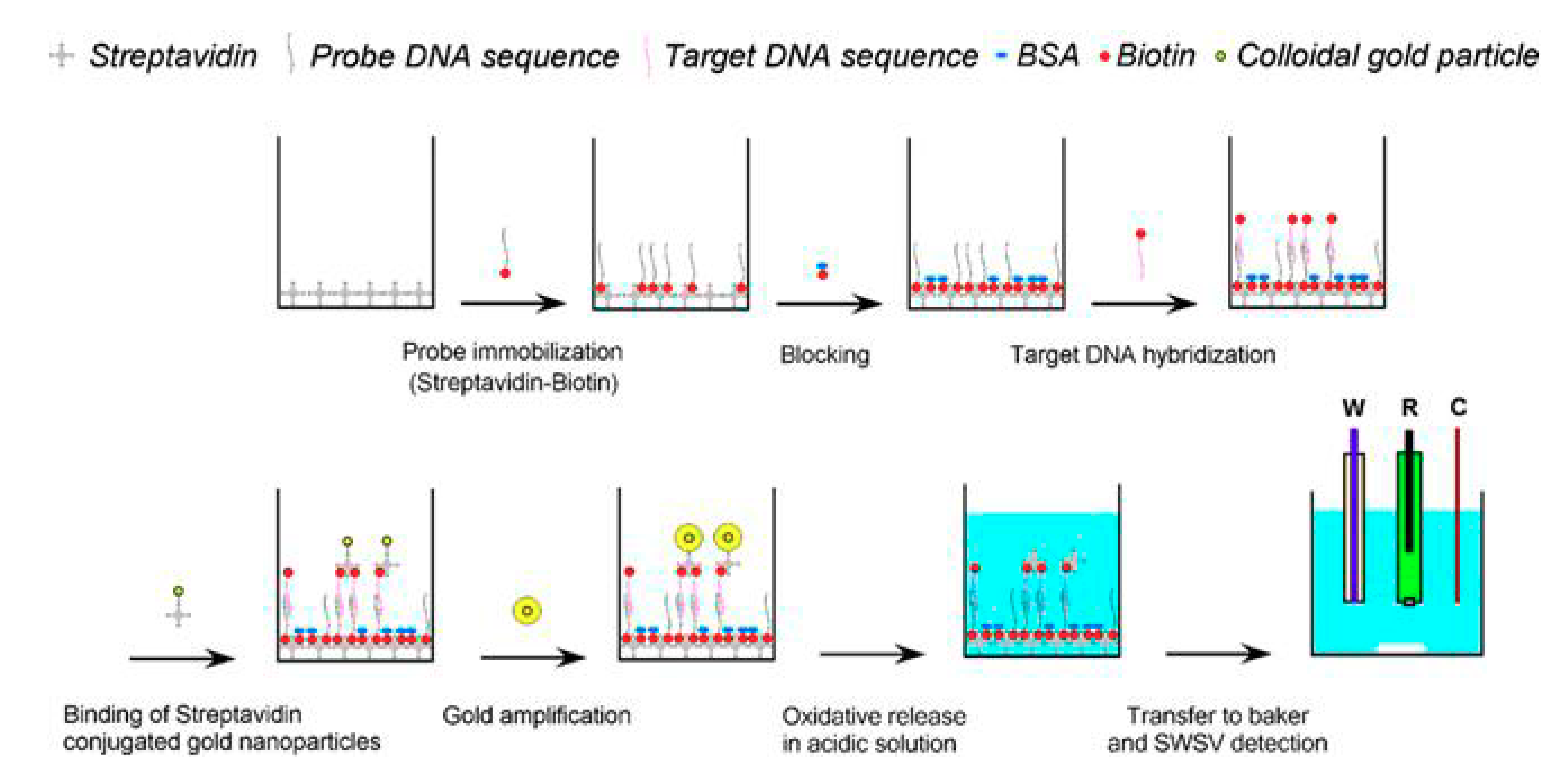
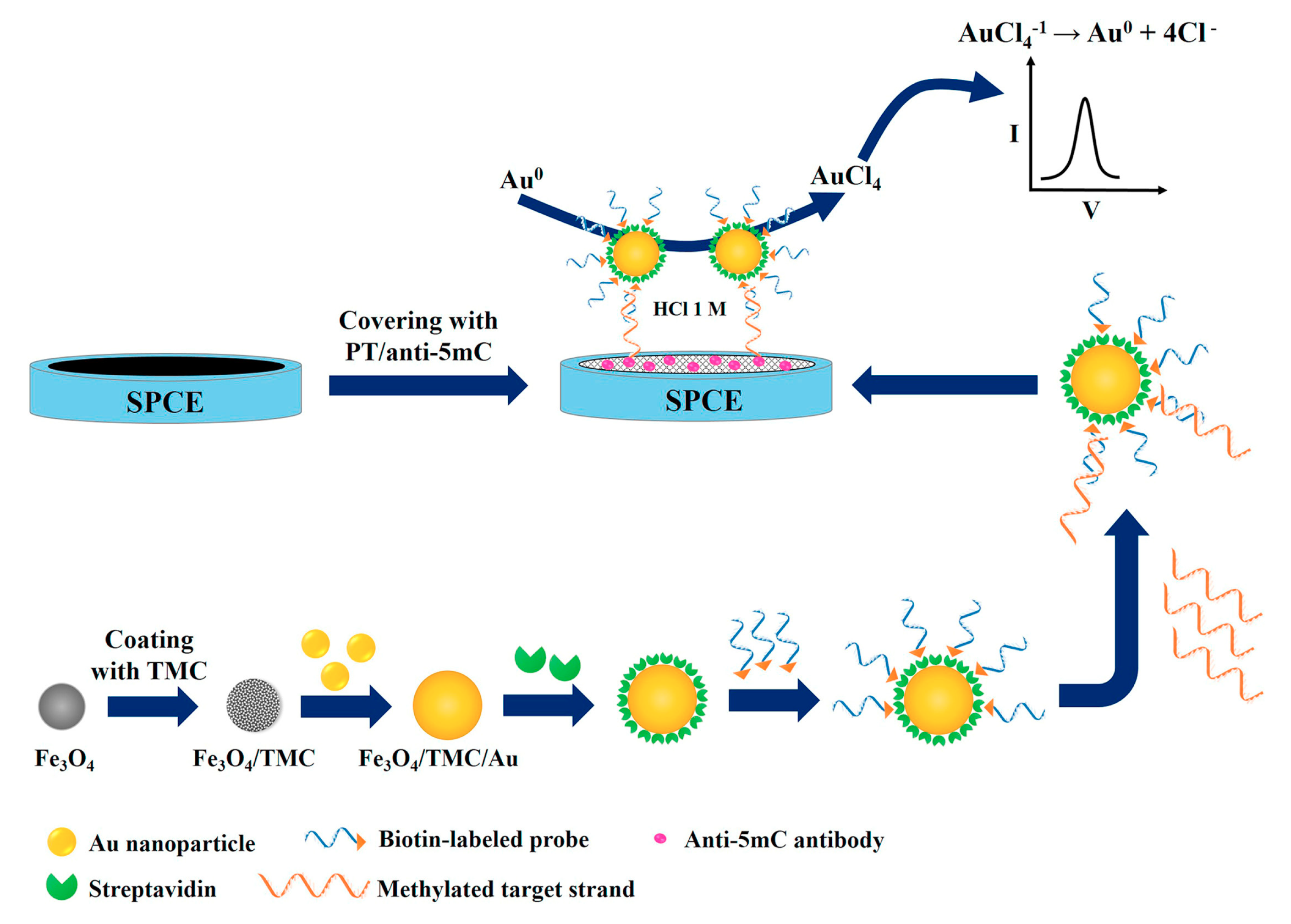
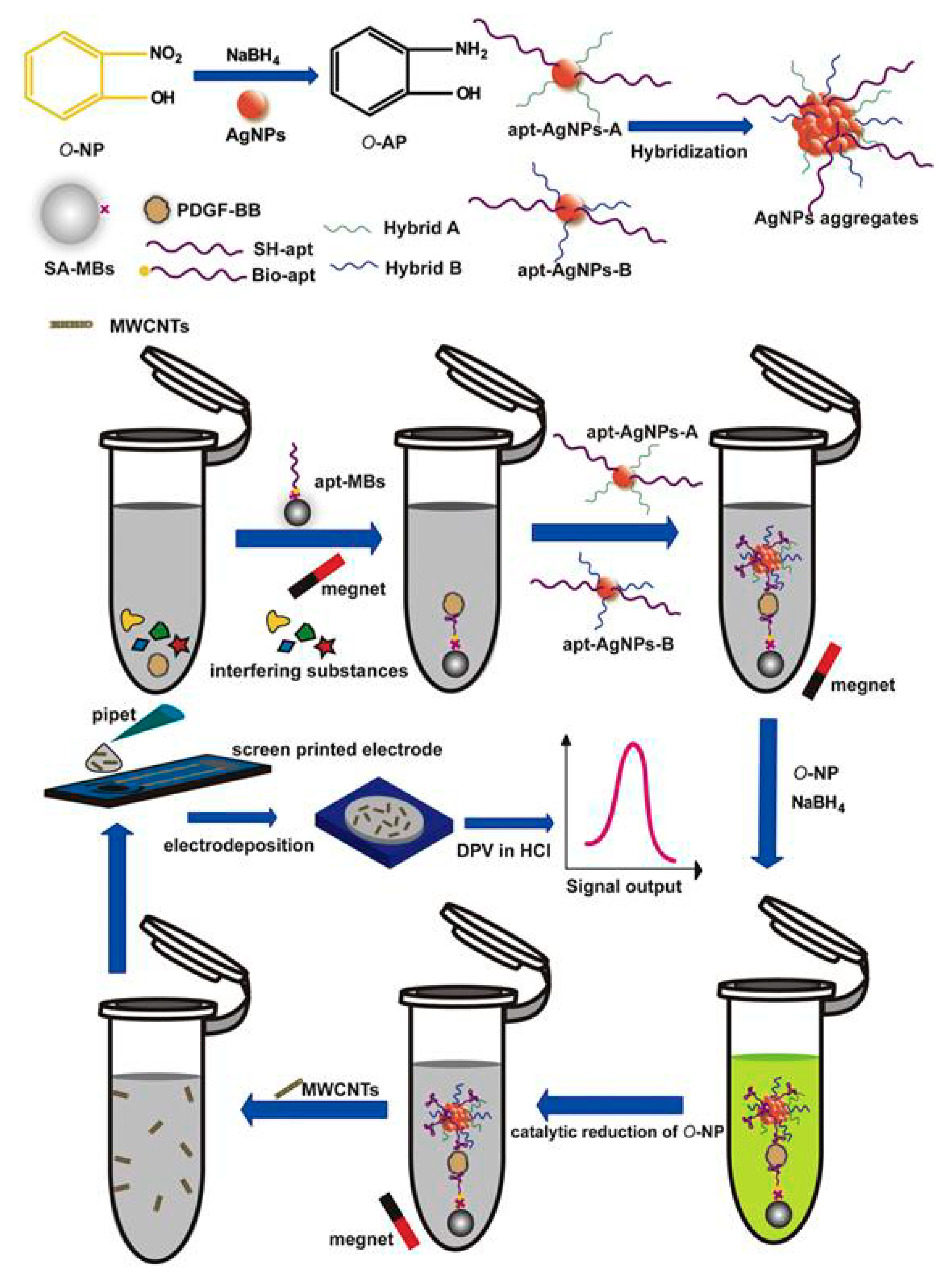
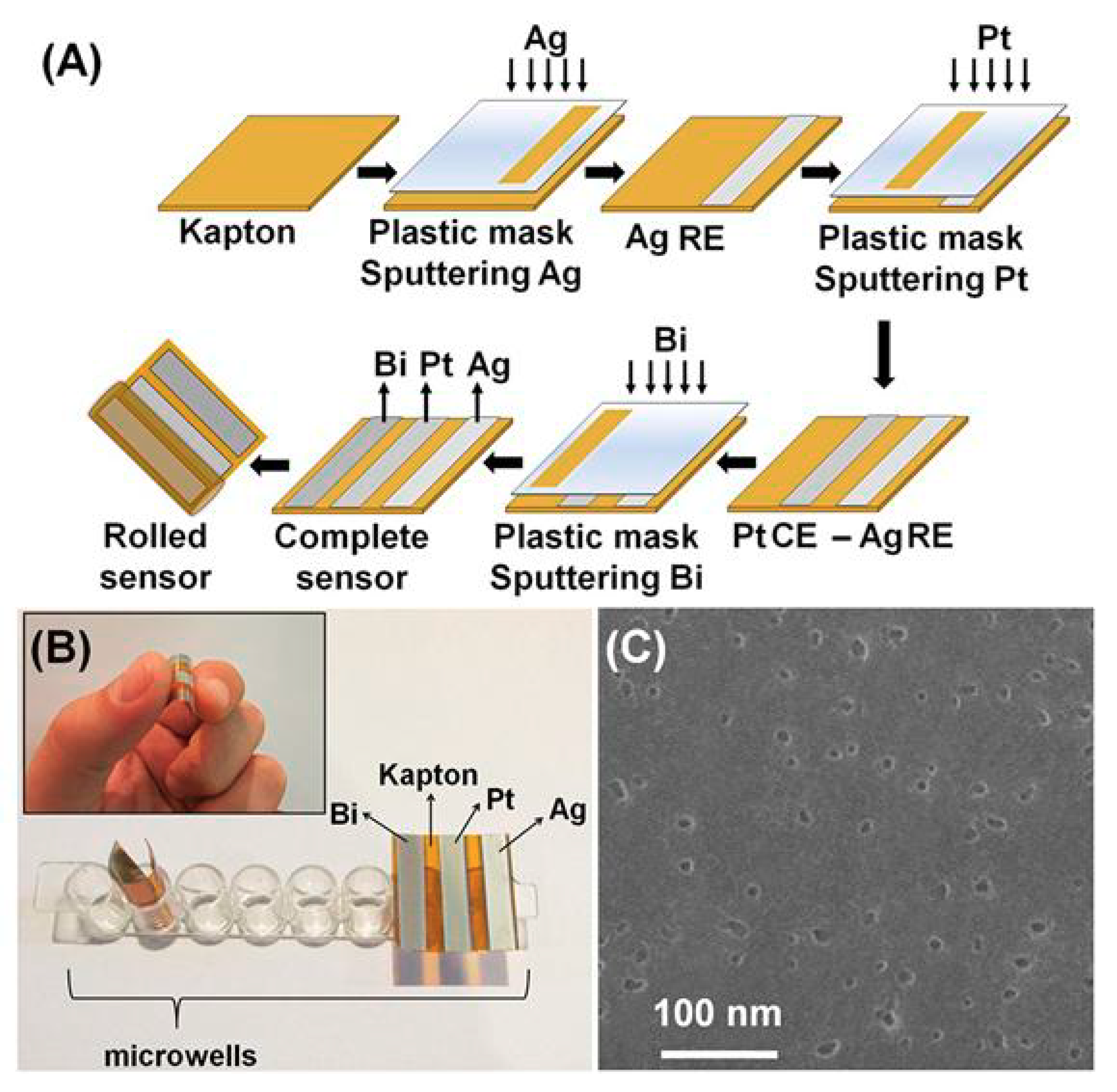
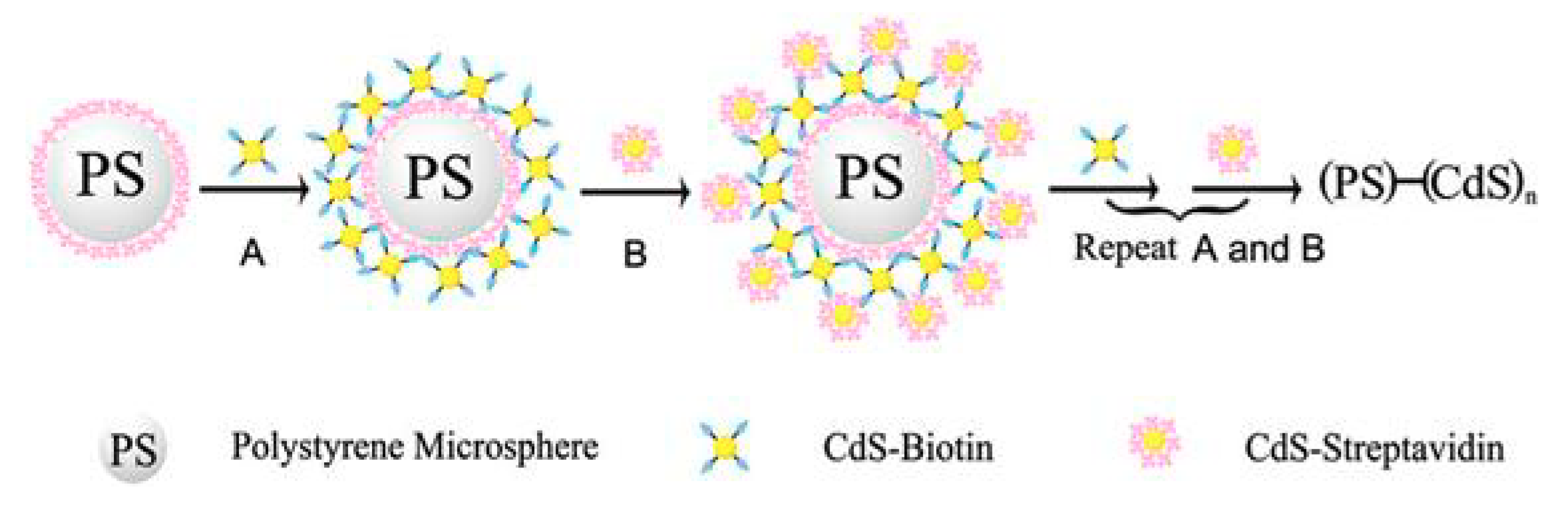
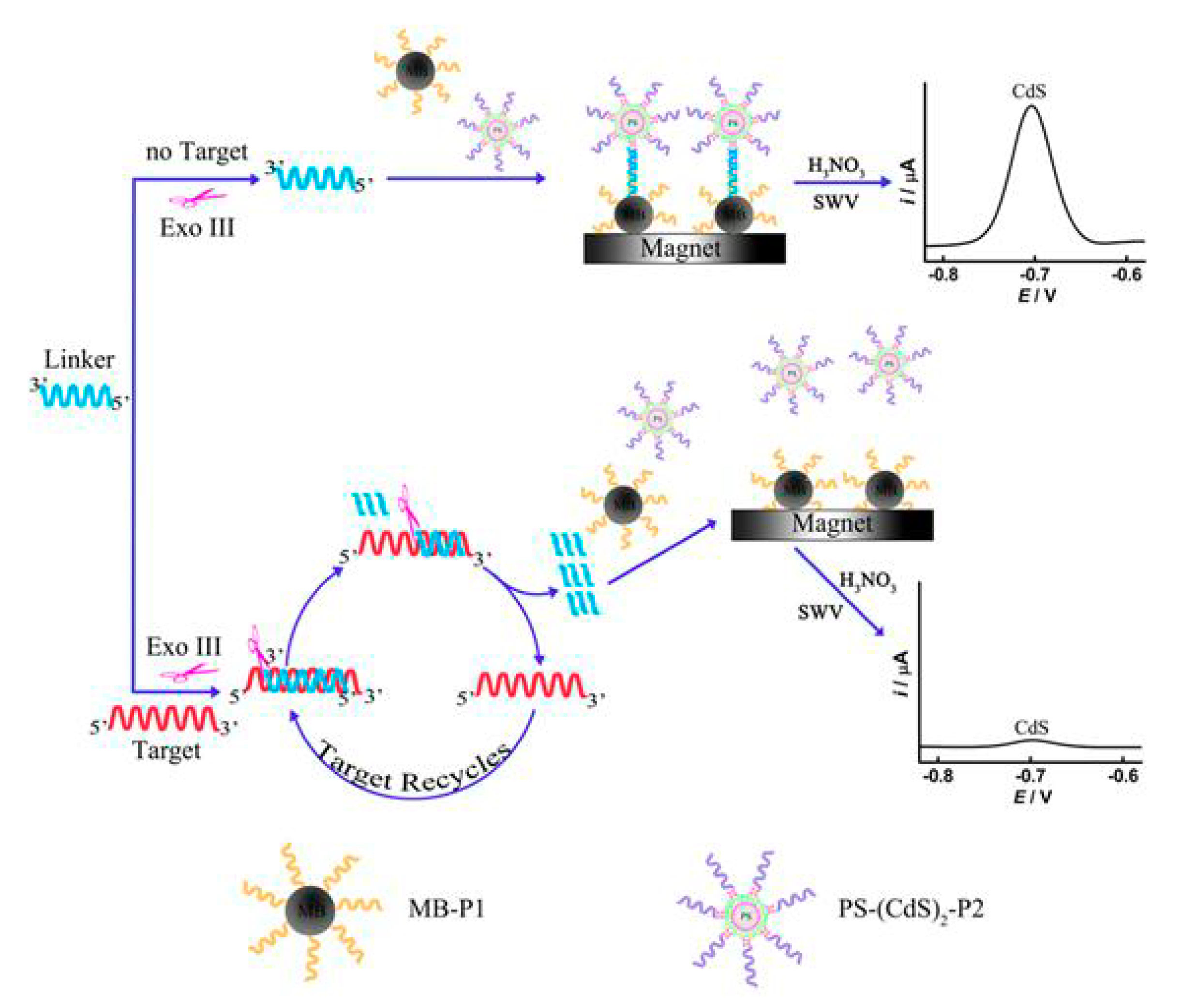
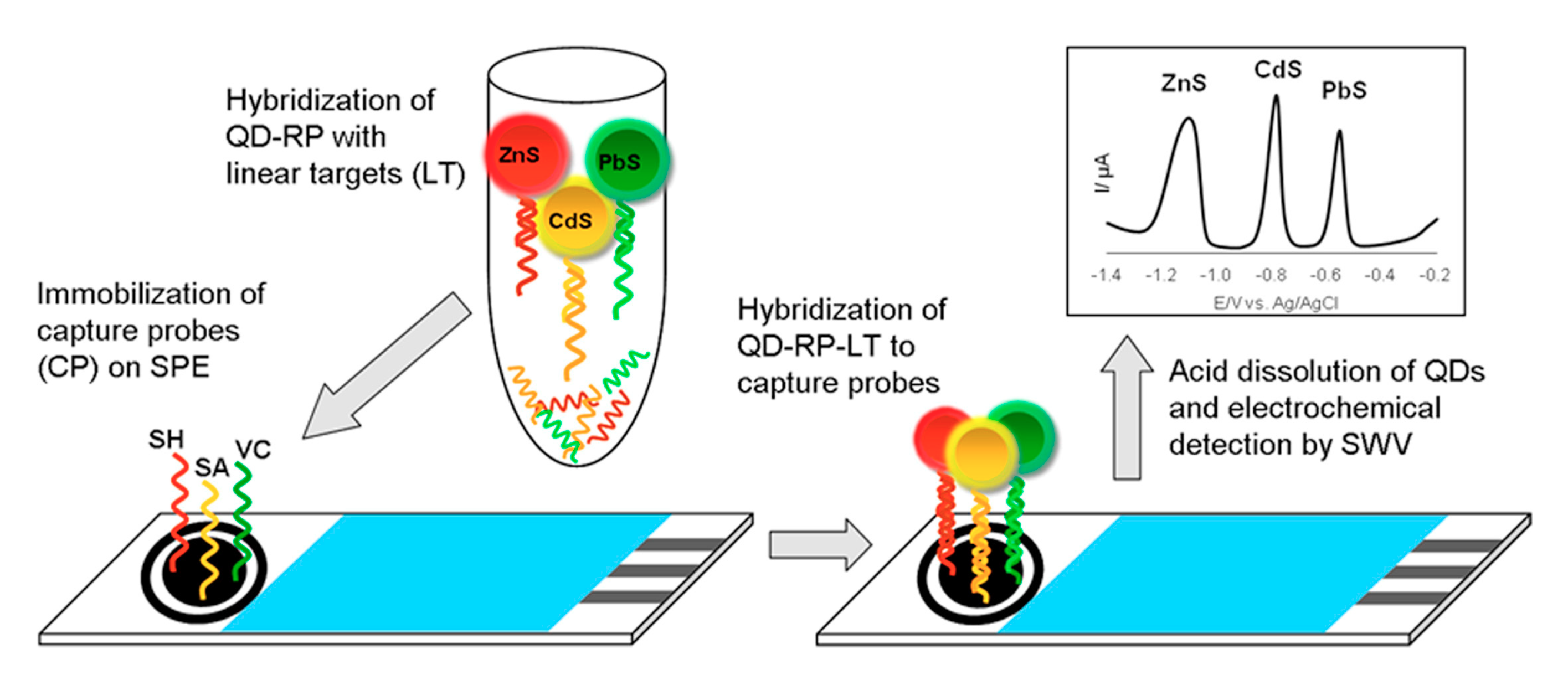
| Electrode | Analyte | Signal Amplification | QDs | Reference |
|---|---|---|---|---|
| MFE plated in situ on GCE | 35S promoter of cauliflower mosaic virus | CNTs | CdSe | [35] |
| MFE plated in situ on GCE | Single DNA target | CNTs/AuNPs/MBs | CdSe–CdS | [36] |
| MFE plated in situ on GCE | Multiple DNA targets | MBs | ZnS, PbS, CdS, | [38] |
| MFE plated in situ on GCE | Single DNA target | CNTs | CdS | [40] |
| Carbon SPCE | Cystic-fibrosis-related DNA sequence | MBs | CdS | [53] |
| BiFE plated in situ on GCE | Ochratoxin A and fumonisin B1 in maize | MBs | CdTe, PbS | [60] |
| MFE plated in situ on GCE | HPV-16 | PS | CdTe | [61] |
| MFE plated in situ on GCE | Single DNA target | PS/MBs | CdS | [64] |
| MFE plated in situ on GCE | Escherichia coli uropathogens | PS/MBs | CdS | [65] |
| BiFE plated in situ on SPCE | Sequences of Vibrio cholerae, Salmonella sp, Shigella sp., | - | PbS, CdS, ZnS | [67] |
| HMDE | Virus of H5N1 chains | MBs | PbS, CdS, ZnS | [68] |
| BiFE plated in situ on GCE | Multiple DNA targets | - | MT–Pb, MT–Cd, MT–Zn | [69] |
| BiFE plated in situ on SPCE | Gene of Bacillus anthracis and gene of Salmonella enteritidis | MBs | PbS, CdS | [70] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinos, C. Electrochemical DNA Biosensors Based on Labeling with Nanoparticles. Nanomaterials 2019, 9, 1361. https://doi.org/10.3390/nano9101361
Kokkinos C. Electrochemical DNA Biosensors Based on Labeling with Nanoparticles. Nanomaterials. 2019; 9(10):1361. https://doi.org/10.3390/nano9101361
Chicago/Turabian StyleKokkinos, Christos. 2019. "Electrochemical DNA Biosensors Based on Labeling with Nanoparticles" Nanomaterials 9, no. 10: 1361. https://doi.org/10.3390/nano9101361
APA StyleKokkinos, C. (2019). Electrochemical DNA Biosensors Based on Labeling with Nanoparticles. Nanomaterials, 9(10), 1361. https://doi.org/10.3390/nano9101361





