A Polyol-Mediated Fluoride Ions Slow-Releasing Strategy for the Phase-Controlled Synthesis of Photofunctional Mesocrystals
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Chemicals and Materials
2.2. Synthesis
2.3. Characterization
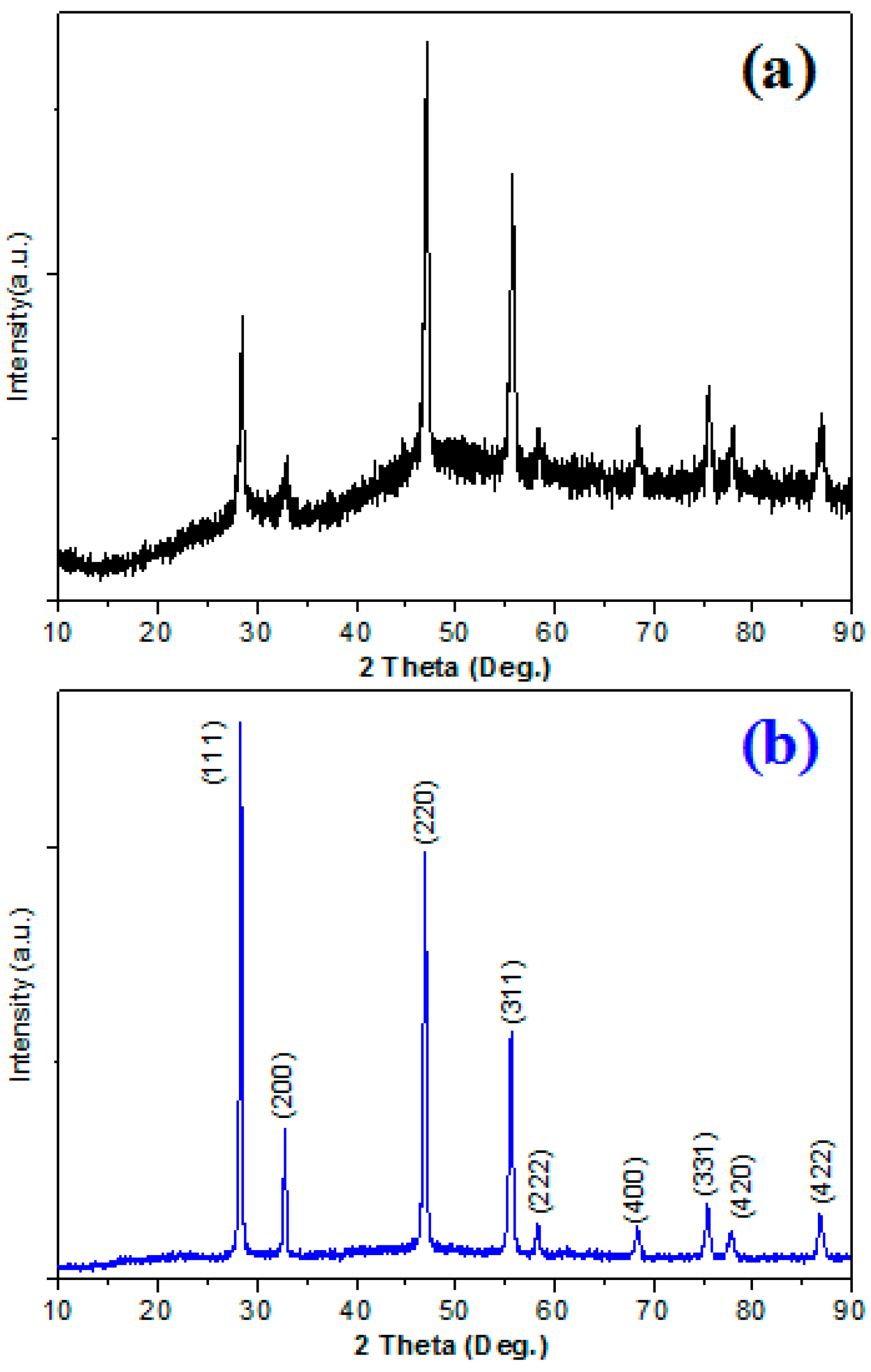
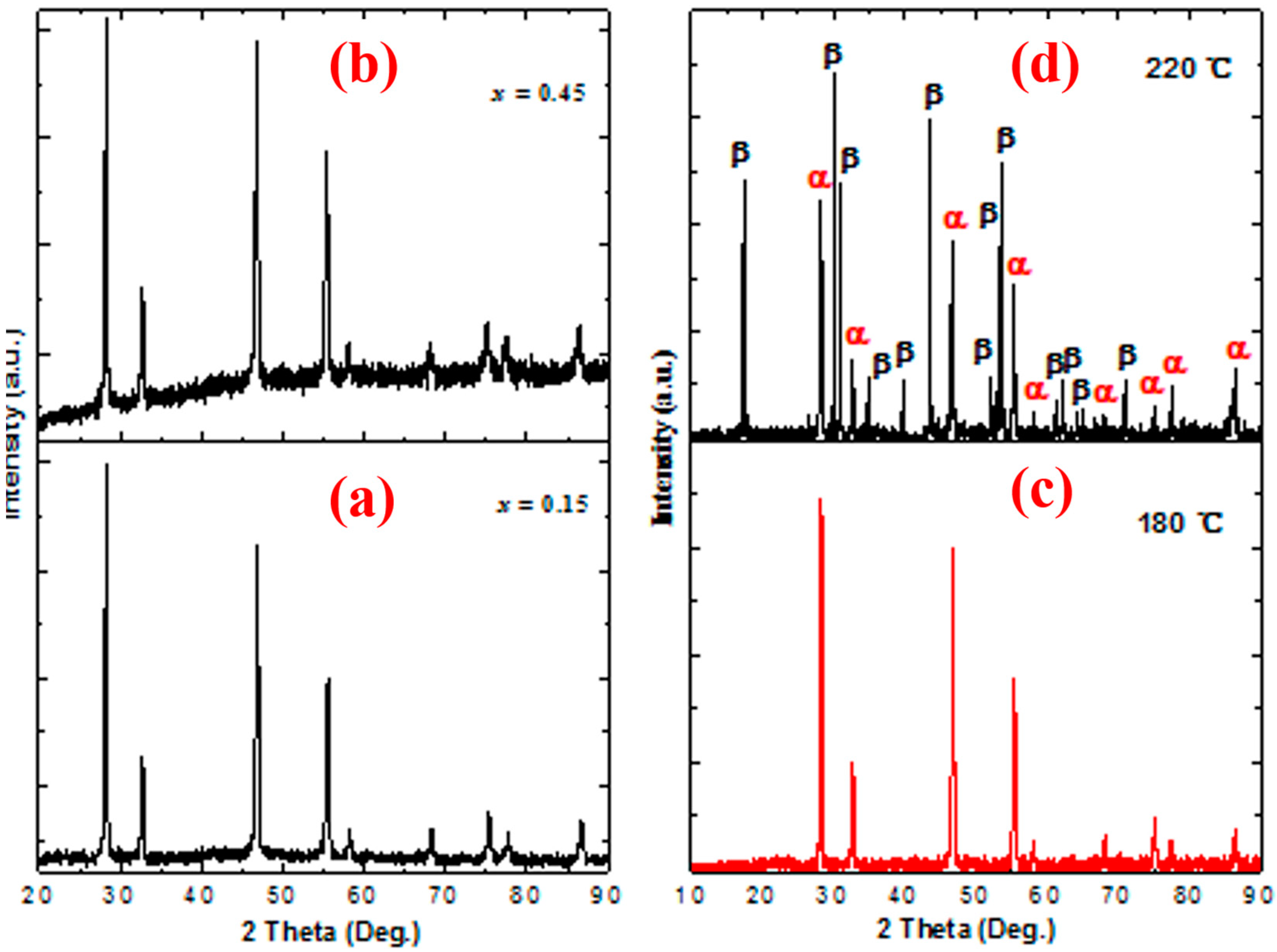
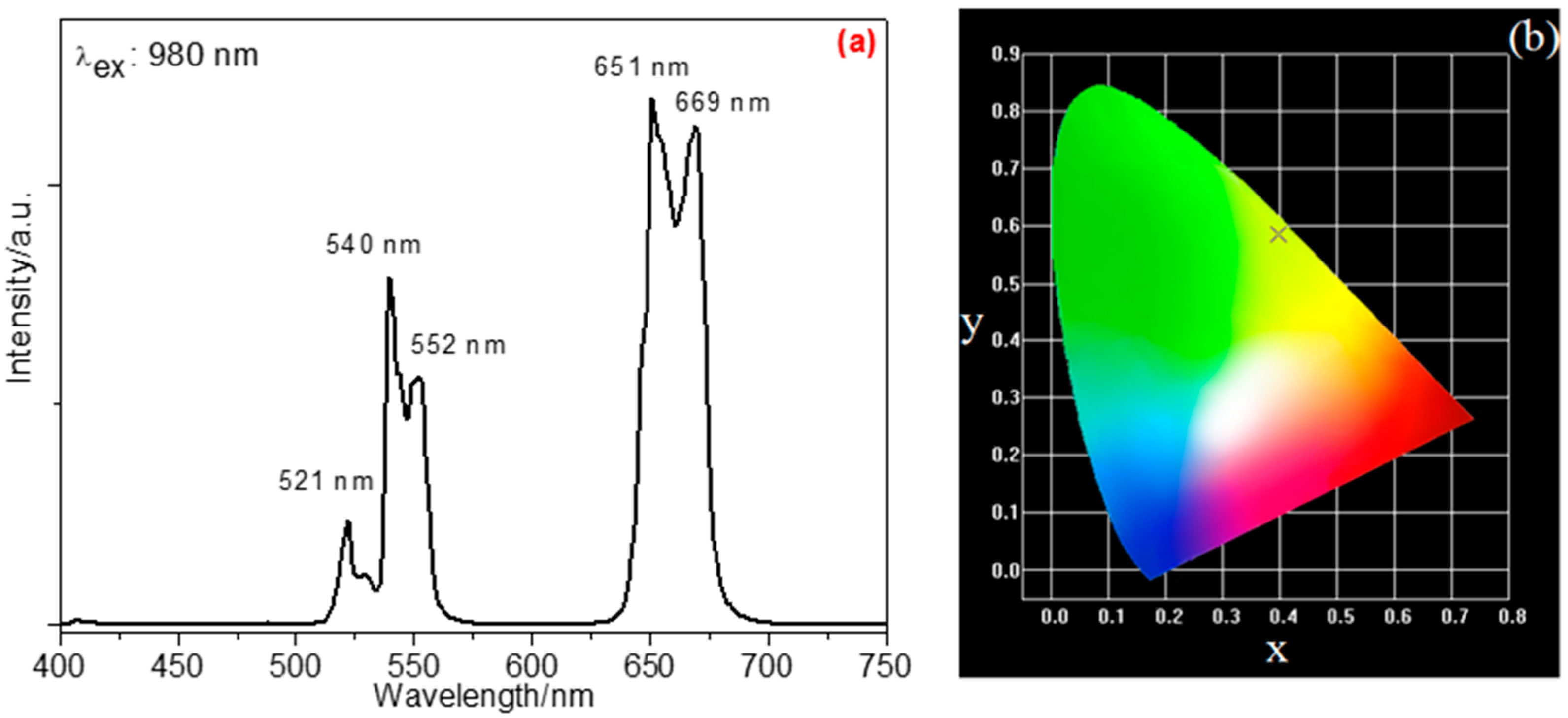
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.; Han, Y.; Lim, C.; Lu, Y.; Wang, J.; Xu, J.; Chen, H.; Zhang, C.; Hong, M.; Liu, X.G. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature 2010, 463, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Farvid, S.S.; Radovanovic, P.V. Phase Transformation of Colloidal In2O3 Nanocrystals Driven by the Interface Nucleation Mechanism: A Kinetic Study. J. Am. Chem. Soc. 2012, 134, 7015–7024. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kuang, Y.; Liu, J.; Yang, Q.; Luo, L.; Sun, X. Separation and phase transition investigation of Yb3+/Er3+ co-doped NaYF4 nanoparticles. Dalton Trans. 2013, 42, 13315–13318. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.Q.; Tao, K.; Tian, Q.; Sun, K. Interaction between Y3+ and Oleate Ions for the Cubic-to-Hexagonal Phase Transformation of NaYF4 Nanocrystals. J. Phys. Chem. C 2012, 116, 1732–1739. [Google Scholar] [CrossRef]
- Zhang, F.; Li, G.; Zhang, W.; Yan, Y.L. Phase-Dependent Enhancement of the Green-Emitting Upconversion Fluorescence in LaVO4:Yb3+,Er3+. Inorg. Chem. 2015, 54, 7325–7334. [Google Scholar] [CrossRef] [PubMed]
- Zhua, Y.; Chen, D.; Huang, L.; Liu, Y.; Brikd, M.G.; Zhong, J.; Wang, J. Phase-transition-induced giant enhancement of red emission in Mn4+-doped fluoride elpasolite phosphors. J. Mater. Chem. C 2018, 6, 3951–3960. [Google Scholar] [CrossRef]
- Thoma, R.E.; Hebert, G.M.; Insley, H.; Weaver, C.F. Phase Equilibria in the System Sodium Fluoride-Yttrium Fluoride. Inorg. Chem. 1963, 2, 1005–1012. [Google Scholar] [CrossRef]
- Arnold, A.A.; Terskikh, V.; Li, Q.Y.; Naccache, R.; Marcotte, I.; Capobianco, J. Structure of NaYF4 Upconverting Nanoparticles: A Multinuclear Solid-State NMR and DFT Computational Study. J. Phys. Chem. C 2013, 117, 25733–25741. [Google Scholar] [CrossRef]
- Mai, H.X.; Zhang, Y.W.; Si, R.; Yan, Z.G.; Sun, L.D.; You, L.P.; Yan, C.H. High-Quality Sodium Rare-earth Fluoride Nanocrystals: Controlled Synthesis and Optical Properties. J. Am. Chem. Soc. 2006, 128, 6426–6436. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, F.; Zhang, X.; Chen, D. Synthesis of Oil-Dispersible Hexagonal-Phase and Hexagonal-Shaped NaYF4:Yb,Er Nanoplates. Chem. Mater. 2006, 18, 5733–5737. [Google Scholar] [CrossRef]
- Zhang, F.; Wan, Y.; Yu, T.; Zhang, F.; Shi, Y.; Xie, S.; Li, Y.; Xu, L.; Tu, B.; Zhao, D. Uniform Nanostructured Arrays of Sodium Rare-Earth Fluorides for Highly Efficient Multicolor Upconversion Luminescence. Angew. Chem. Int. Ed. 2007, 46, 7976–7979. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y. Controlled Synthesis and Luminescence of Lanthanide Doped NaYF4 Nanocrystals. Chem. Mater. 2007, 19, 727–734. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Synthesis of Hexagonal-Phase NaYF4:Yb,Er and NaYF4:Yb,Tm Nanocrystals with Efficient Up-Conversion Fluorescence. Adv. Funct. Mater. 2006, 16, 2324–2329. [Google Scholar] [CrossRef]
- Shan, J.; Ju, Y. A Single-Step Synthesis and the Kinetic Mechanism for Monodisperse and Hexagonal-Phase NaYF4:Yb,Er Upconversion Nanophosphors. Nanotechnology 2009, 20, 275603. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, C.; Hou, Z.; Wang, L.; Quan, Z.; Lian, H.; Lin, J. β-NaYF4 and β-NaYF4:Eu3+ Microstructures: Morphology Control and Tunable Luminescence Properties. J. Phys. Chem. C 2009, 113, 2332–2339. [Google Scholar] [CrossRef]
- Wang, Z.L.; Hao, J.; Chan, H.L.; Wong, W.T.; Wong, K.L. A strategy for simultaneously realizing the cubic-to-hexagonal phase transition and controlling the small size of NaYF4:Yb3+,Er3+ nanocrystals for in vitro cell imaging. Small 2012, 8, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, P.; Zhang, C.; Chen, F.; Wang, C.; Ma, J.; He, R.; Cui, D. A general strategy for the synthesis of upconversion rare earth fluoride nanocrystals via a novel OA/ionic liquid two-phase system. Chem. Commun. 2011, 47, 9510–9512. [Google Scholar] [CrossRef]
- He, M.; Huang, P.; Zhang, C.; Ma, J.; He, R.; Cui, D. Phase- and Size-Controllable Synthesis of Hexagonal Upconversion Rare-Earth Fluoride Nanocrystals through an Oleic Acid/Ionic Liquid Two-Phase System. Chem. Eur. J. 2012, 18, 5954–5969. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, B. Phase control of upconversion nanocrystals and new rare earth fluorides though a diffusion-controlled strategy in a hydrothermal system. Chem. Commun. 2011, 47, 5867–5869. [Google Scholar] [CrossRef]
- Chen, D.; Huang, P.; Yu, Y.; Huang, F.; Yang, A.; Wang, Y. Dopant-induced phase transition: A new strategy of synthesizing hexagonal upconversion NaYF4 at low temperature. Chem. Commun. 2011, 47, 5801–5803. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lu, F.; Zhang, X.; Chen, D. Polyol-mediated synthesis and luminescence of lanthanide-doped NaYF4 nanocrystal upconversion phosphors. J. Alloys Compd. 2008, 455, 376–384. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J. Facile EG/ionic liquid interfacial synthesis of uniform RE3+ doped NaYF4 nanocubes. Chem. Commun. 2010, 46, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, L.D.; Li, Z.X.; Li, L.L.; Zhang, J.; Zhang, Y.W.; Yan, C.H. Ionic Liquid-Based Route to Spherical NaYF4 Nanoclusters with the Assistance of Microwave Radiation and Their Multicolor Upconversion Luminescence. Langmuir 2010, 26, 8797–8803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, L. Controllable Multicolor Upconversion Luminescence by Tuning the NaF Dosage. Chem. Asian J. 2014, 9, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gai, S.; Ma, P.; Wang, L.; Zhang, M.; Huang, S.; Yang, P. Highly Uniform α-NaYF4:Yb/Er Hollow Microspheres and Their Application as Drug Carrier. Inorg. Chem. 2013, 52, 9184–9191. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Di, W.; Liu, Z.; Zheng, K.; Qin, W. Synthesis of NaLuF4-based nanocrystals and large enhancement of upconversion luminescence of NaLuF4:Gd, Yb, Er by coating an active shell for bioimaging. Dalton Trans. 2014, 43, 14001–14008. [Google Scholar]
- Tian, G.; Gu, Z.; Zhou, L.; Yin, W.; Liu, X.; Yan, L.; Zhao, Y. Mn2+ Dopant-Controlled Synthesis of NaYF4:Yb/Er Upconversion Nanoparticles for in vivo Imaging and Drug Delivery. Adv. Mater. 2012, 24, 1226–1231. [Google Scholar] [CrossRef]
- Heer, S.; Kömpe, K.; Güdel, H.U.; Haase, M. Highly Efficient Multicolour Upconversion Emission in Transparent Colloids of Lanthanide-Doped NaYF4 Nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar] [CrossRef]
- Gao, L.; Ge, X.; Chai, Z.; Xu, G.; Wang, X.; Wang, C. Shape-controlled synthesis of octahedral α-NaYF4 and its rare earth doped submicrometer particles in acetic acid. Nano Res. 2010, 2, 565–574. [Google Scholar] [CrossRef]
- Liang, X.; Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. Synthesis of NaYF4 Nanocrystals with Predictable Phase and Shape. Adv. Funct. Mater. 2007, 17, 2757–2765. [Google Scholar] [CrossRef]
- Zhang, F.; Li, J.; Shan, J.; Xu, L.; Zhao, D. Shape, Size, and Phase-Controlled Rare-Earth Fluoride Nanocrystals with Optical Up-Conversion Properties. Chem. Eur. J. 2009, 15, 11010–11019. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Song, H.; Pan, G.; Zhao, H.; Ren, X.; Liu, L.; Bai, X.; Dai, Q.; Qu, X. Polyol-mediated synthesis of well-dispersed α-NaYF4 nanocubes. J. Cryst. Growth 2009, 311, 1559–1564. [Google Scholar] [CrossRef]
- He, L.; Zou, X.; He, X.; Lei, F.; Jiang, N.; Zheng, Q.; Xu, C.; Liu, Y.; Lin, D. Reducing Grain Size and Enhancing Luminescence of NaYF4:Yb3+, Er3+ Upconversion Materials. Cryst. Growth Des. 2018, 18, 808–817. [Google Scholar] [CrossRef]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M.; Cölfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef] [PubMed]
- Bergström, L.; Sturm (née Rosseeva), E.V.; German, S.; Cölfen, H. Mesocrystals in Biominerals and Colloidal Arrays. Acc. Chem. Res. 2015, 48, 1391–1402. [Google Scholar] [CrossRef]
- Zhou, L.; O’Brien, P. Mesocrystals-Properties and Applications. J. Phys. Chem. Lett. 2012, 3, 620–628. [Google Scholar] [CrossRef]
- He, X.; Yan, B. Yttrium hydroxide fluoride based monodisperse mesocrystals: Additive-free synthesis, enhanced fluorescent properties, and potential application in temperature sensing. CrystEngComm 2015, 17, 621–627. [Google Scholar] [CrossRef]
- He, X.; Yan, B. Double role of the hydroxy group for water dispersibility and luminescence of REF3 (RE = Yb, Er, Tm) based mesocrystals. New J. Chem. 2015, 39, 6730–6733. [Google Scholar] [CrossRef]
- Zhuang, J.; Yang, X.; Fu, J.; Liang, C.; Wu, M.; Wang, J.; Su, Q. Monodispersed β-NaYF4 Mesocrystals: In Situ Ion Exchange and Multicolor Up-and Down-Conversions. Cryst. Growth Des. 2013, 13, 2292–2297. [Google Scholar] [CrossRef]
- Zhong, S.L.; Lu, Y.; Gao, M.R.; Liu, S.J.; Peng, J.; Zhang, L.C.; Yu, S.H. Monodisperse Mesocrystals of YF3 and Ce3+/Ln3+ (Ln=Tb, Eu) Co-Activated YF3: Shape Control Synthesis, Luminescent Properties, and Biocompatibility. Chem. Eur. J. 2012, 18, 5222–5231. [Google Scholar] [CrossRef] [PubMed]
- Lausser, C.; Kumke, M.U.; Antonietti, M.; Cölfen, H. Fabrication of EuF3-Mesocrystals in a Gel Matrix. Z. Anorg. Allg. Chem. 2010, 636, 1925–1930. [Google Scholar] [CrossRef]
- Wang, J.; Liu, B.Q.; Huang, G.; Zhang, Z.J.; Zhao, J.T. Monodisperse NaxY(OH)yF3+x-y mesocrystals with tunable morphology and chemical composition: pH-mediated ion-exchange. Cryst. Growth Des. 2017, 17, 711–718. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Zhou, Y.; Li, D. Ionic liquid-based “all-in-one” synthesis and photoluminescence properties of lanthanide fluorides. J. Phys. Chem. C 2008, 112, 10083–10088. [Google Scholar] [CrossRef]
- Zhao, Q.; You, H.; Lü, W.; Guo, N.; Jia, Y.; Lv, W.; Jiao, M. Dendritic Y4O(OH)9NO3:Eu3+/Y2O3:Eu3+ hierarchical structures: Controlled synthesis, growth mechanism, and luminescence properties. CrystEngComm 2013, 15, 4844–4851. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Ionic liquids are not always green: Hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate. Green Chem. 2003, 5, 361–363. [Google Scholar] [CrossRef]
- Xie, N.; Luan, W. Ionic-liquid-induced microfluidic reaction for water-soluble Ce1−xTbxF3 nanocrystal synthesis. Nanotechnology 2011, 22, 265609. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Q. Systematic studies for the novel synthesis of nano-structured lanthanide fluorides. Chem. Eng. J. 2014, 250, 190–197. [Google Scholar] [CrossRef]
- Klier, D.T.; Kumke, M.U. Upconversion Luminescence Properties of NaYF4:Yb:Er Nanoparticles Codoped with Gd3+. J. Phys. Chem. C 2015, 119, 3363–3373. [Google Scholar] [CrossRef]
- He, M.; Huang, P.; Zhang, C.; Hu, H.; Bao, C.; Gao, G.; He, R.; Cui, D. Dual Phase-Controlled Synthesis of Uniform Lanthanide-Doped NaGdF4 Upconversion Nanocrystals Via an OA/Ionic Liquid Two-Phase System for In Vivo Dual-Modality Imaging. Adv. Funct. Mater. 2011, 21, 4470–4477. [Google Scholar] [CrossRef]
- Ghosh, P.; Patra, A. Tuning of crystal phase and luminescence properties of Eu3+ doped sodium yttrium fluoride nanocrystals. J. Phys. Chem. C 2008, 112, 3223–3231. [Google Scholar] [CrossRef]
- Xue, X.; Duan, Z.; Suzuki, T.; Tiwari, R.N.; Yoshimura, M.; Ohishi, Y. Luminescence Properties of α-NaYF4:Nd3+ Nanocrystals Dispersed in Liquid: Local Field Effect Investigation. J. Phys. Chem. C 2012, 116, 22545–22551. [Google Scholar] [CrossRef]
- Chaumont, A.; Wipff, G. Solvation of M3+ lanthanide cations in room-temperature ionic liquids. A molecular dynamics investigation. Phys. Chem. Chem. Phys. 2003, 5, 3481–3488. [Google Scholar] [CrossRef]
- Yasui, K.; Kato, K. Oriented Attachment of Cubic or Spherical BaTiO3 Nanocrystals by van der Waals Torque. J. Phys. Chem. C 2015, 119, 24597–24605. [Google Scholar] [CrossRef]
- Ye, J.; Liu, W.; Cai, J.; Chen, S.; Zhao, X.; Zhou, H.; Qi, L. Nanoporous anatase TiO2 mesocrystals: Additive-free synthesis, remarkable crystalline-phase stability, and improved lithium insertion behavior. J. Am. Chem. Soc. 2011, 133, 933–940. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Zhang, Y.; Fu, Y.; Lian, N.; Li, Z. A Polyol-Mediated Fluoride Ions Slow-Releasing Strategy for the Phase-Controlled Synthesis of Photofunctional Mesocrystals. Nanomaterials 2019, 9, 28. https://doi.org/10.3390/nano9010028
He X, Zhang Y, Fu Y, Lian N, Li Z. A Polyol-Mediated Fluoride Ions Slow-Releasing Strategy for the Phase-Controlled Synthesis of Photofunctional Mesocrystals. Nanomaterials. 2019; 9(1):28. https://doi.org/10.3390/nano9010028
Chicago/Turabian StyleHe, Xianghong, Yaheng Zhang, Yu Fu, Ning Lian, and Zhongchun Li. 2019. "A Polyol-Mediated Fluoride Ions Slow-Releasing Strategy for the Phase-Controlled Synthesis of Photofunctional Mesocrystals" Nanomaterials 9, no. 1: 28. https://doi.org/10.3390/nano9010028
APA StyleHe, X., Zhang, Y., Fu, Y., Lian, N., & Li, Z. (2019). A Polyol-Mediated Fluoride Ions Slow-Releasing Strategy for the Phase-Controlled Synthesis of Photofunctional Mesocrystals. Nanomaterials, 9(1), 28. https://doi.org/10.3390/nano9010028




