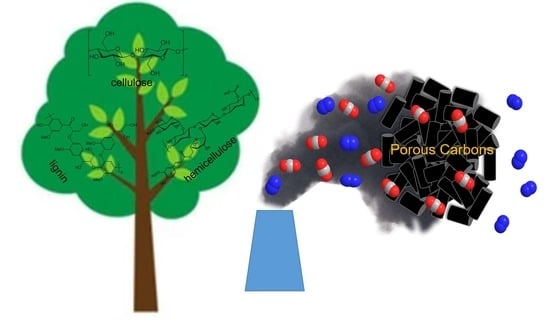
Sustainable Porous Carbon Materials Derived from Wood-Based Biopolymers for CO2 Capture
Abstract
1. Introduction
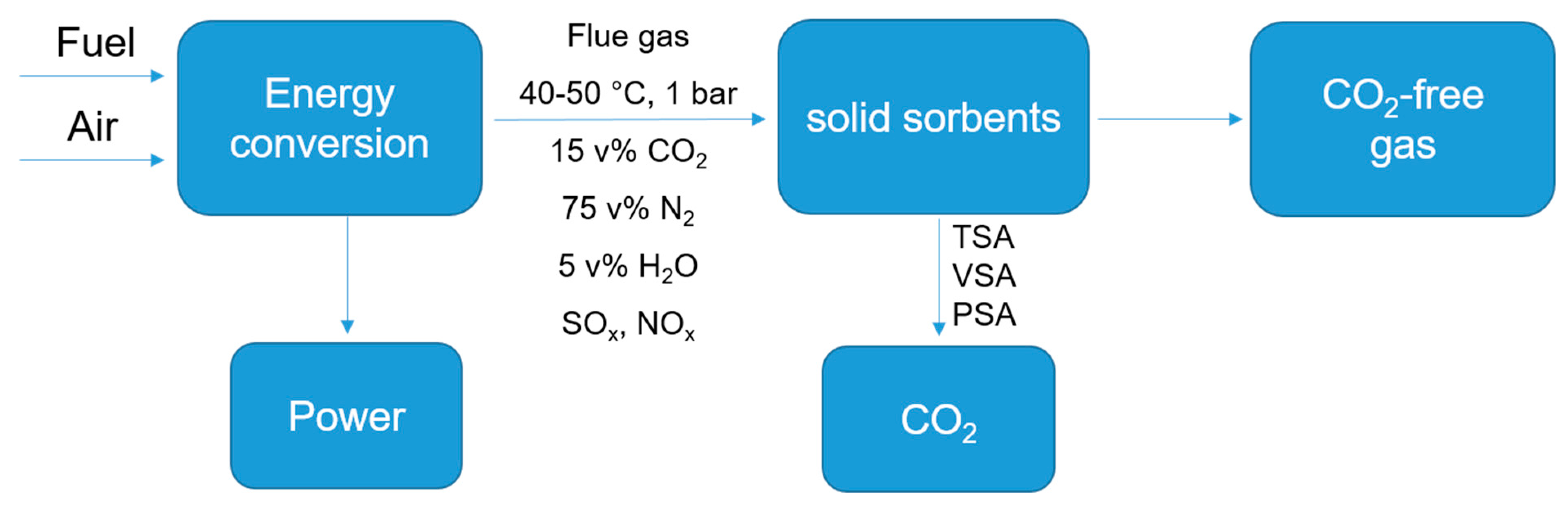
2. General Routes from Biomass to Porous Carbons
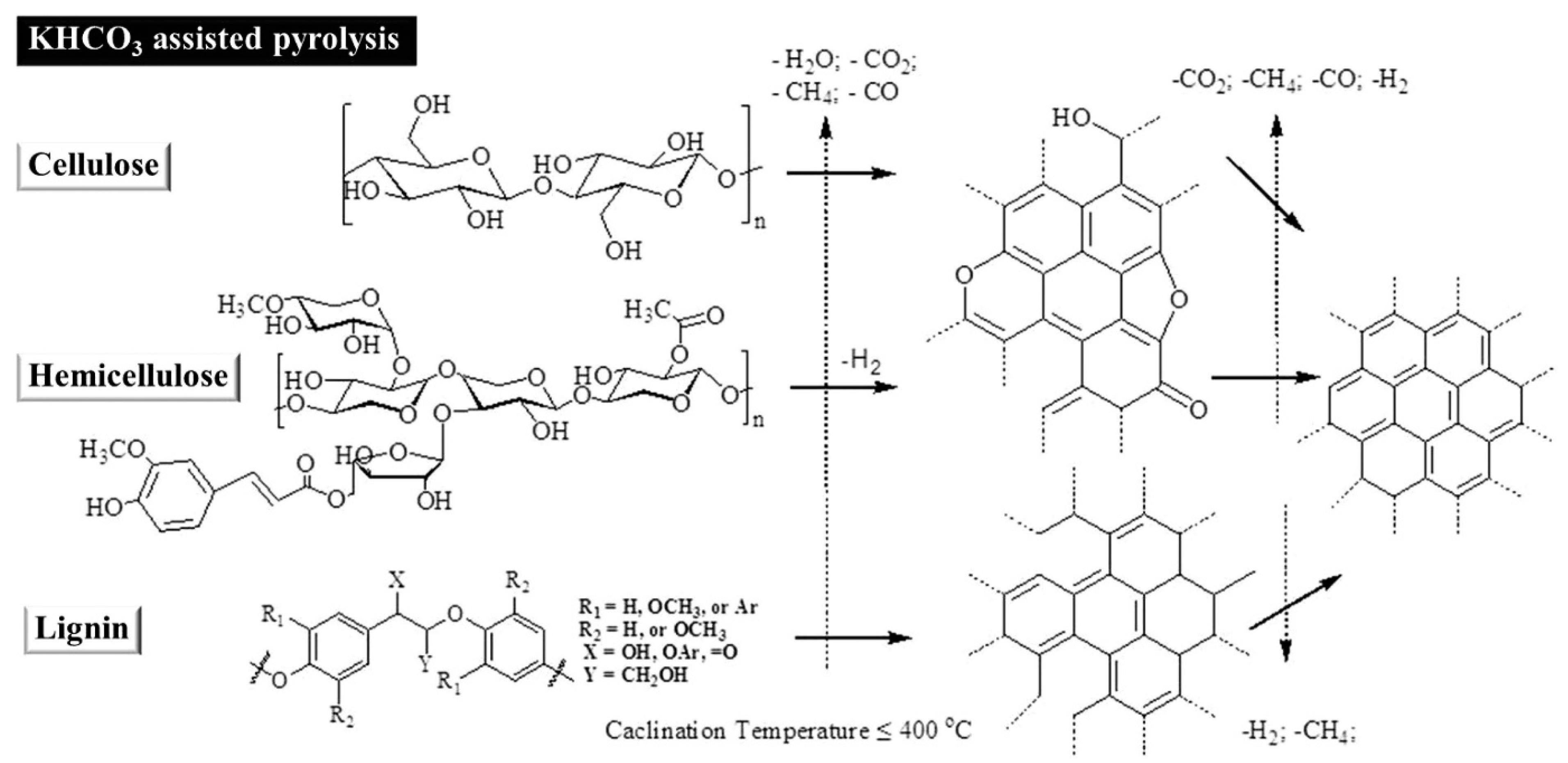
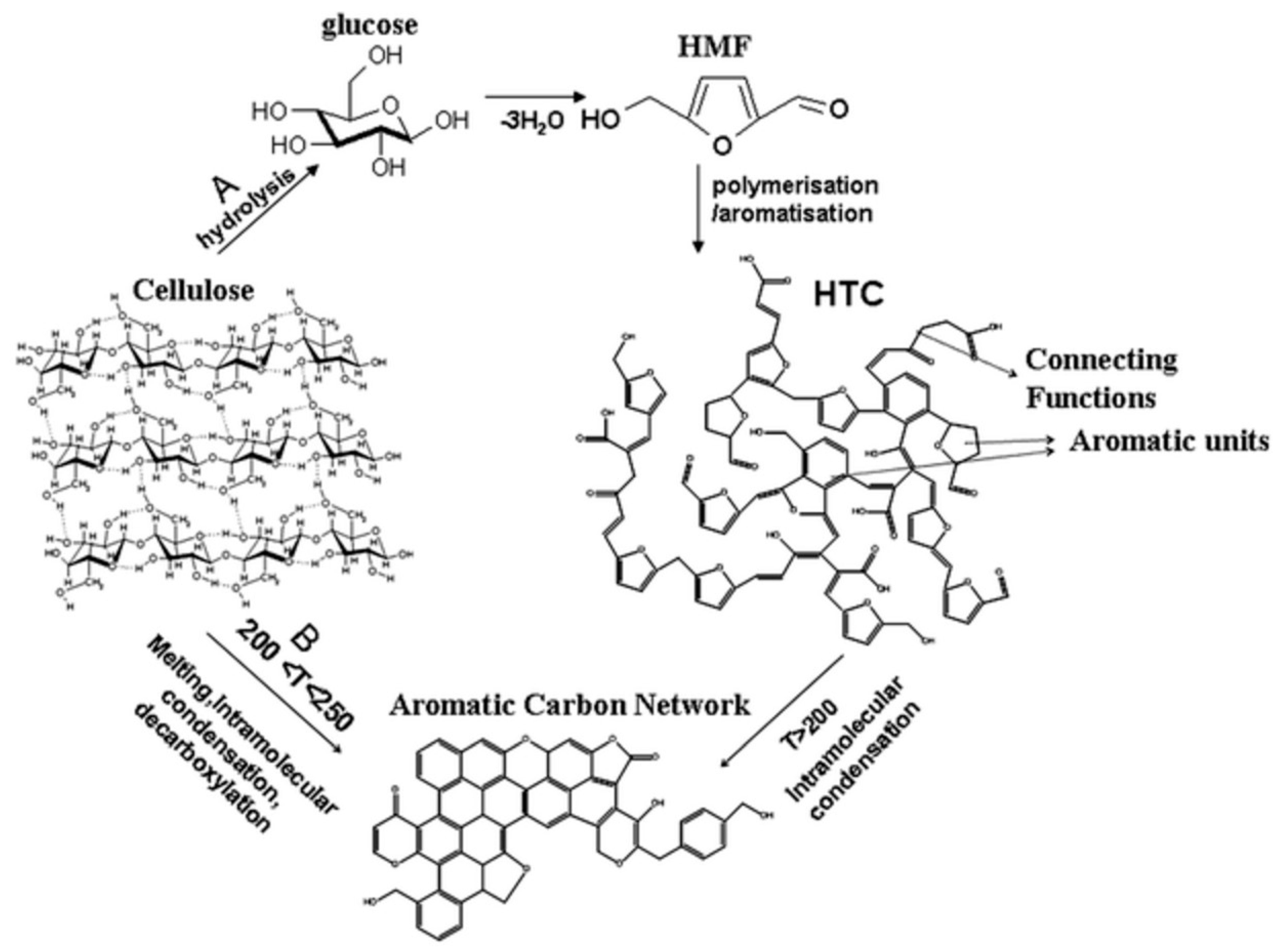
3. Synthesis of Porous Carbons from Wood-Based Biopolymers
4. CO2 Adsorption on Cellulose-, Hemicellulose- and Lignin-Derived Porous Carbons
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014.
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Xu, C.; Hedin, N. Microporous adsorbents for CO2 capture—A case for microporous polymers? Mater. Today 2014, 17, 397–403. [Google Scholar] [CrossRef]
- Slater, A.G.; Cooper, A.I. Function-led design of new porous materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- Hedin, N.; Andersson, L.; Bergström, L.; Yan, J. Adsorbents for the post-combustion capture of CO2 using rapid temperature swing or vacuum swing adsorption. Appl. Energy 2013, 104, 418–433. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P. Adsorption of CO2, N2, and O2 on Natural Zeolites. Energy Fules 2003, 17, 571–576. [Google Scholar] [CrossRef]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal-organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030–3040. [Google Scholar] [CrossRef]
- Hao, G.-P.; Li, W.-C.; Qian, D.; Lu, A.-H. Rapid Synthesis of Nitrogen-Doped Porous Carbon Monolith for CO2 Capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Valle-Vigón, P.; Fuertes, A.B. N-Doped Polypyrrole-Based Porous Carbons for CO2 Capture. Adv. Funct. Mater. 2011, 21, 2781–2787. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Berglund Lars, A.; Burgert, I. Bioinspired Wood Nanotechnology for Functional Materials. Adv. Mater. 2018, 30, 1704285. [Google Scholar] [CrossRef]
- Jiang, F.; Li, T.; Li, Y.; Zhang, Y.; Gong, A.; Dai, J.; Hitz, E.; Luo, W.; Hu, L. Wood-Based Nanotechnologies toward Sustainability. Adv. Mater. 2018, 30, 1703453. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Lin, J.-C.M. Development of a high yield and low cycle time biomass char production system. Fuel Process. Technol. 2006, 87, 487–495. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.-M.; White, R.J.; Brun, N.; Budarin, V.L.; Su, D.S.; del Monte, F.; Clark, J.H.; MacLachlan, M.J. Sustainable carbon materials. Chem. Soc. Rev. 2015, 44, 250–290. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.-H.; Antonietti, M.; Titirici, M.-M. Engineering Carbon Materials from the Hydrothermal Carbonization Process of Biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, N.; Liu, D.; Xu, J.; Sha, J.; Yin, J.; Tan, X.; Yang, H.; Lu, H.; Lin, H. Direct carbonization of rice husk to prepare porous carbon for supercapacitor applications. Energy 2017, 128, 618–625. [Google Scholar] [CrossRef]
- Hayashi, J.I.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, H.; Chen, J.; Li, G.-D.; Zhang, Y.; Chen, J.-S. Preparation and gas storage of high surface area microporous carbon derived from biomass source cornstalks. Bioresour. Technol. 2008, 99, 4803–4808. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400–1410. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, E.; Cordero, T.; Rodriguez-Mirasol, J.; Cotoruelo, L.; Rodriguez, J.J. Removal of water pollutants with activated carbons prepared from H3PO4 activation of lignin from kraft black liquors. Water Res. 2004, 38, 3043–3050. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.H.; Rio, S.; Faur, C.; Le Coq, L.; Le Cloirec, P.; Nguyen, T.H. Production of fibrous activated carbons from natural cellulose (jute, coconut) fibers for water treatment applications. Carbon 2006, 44, 2569–2577. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Zou, L.; Wang, F.; Wang, X.; Zhang, Y.; Yao, X. Nanocellulose-derived highly porous carbon aerogels for supercapacitors. Carbon 2016, 99, 203–211. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Maciá-Agulló, J.A.; Moore, B.C.; Cazorla-Amorós, D.; Linares-Solano, A. Activation of coal tar pitch carbon fibres: Physical activation vs. chemical activation. Carbon 2004, 42, 1367–1370. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-Depth Investigation of Biomass Pyrolysis Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Fules 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Kwon, E.E.; Jeon, E.-C.; Castaldi, M.J.; Jeon, Y.J. Effect of Carbon Dioxide on the Thermal Degradation of Lignocellulosic Biomass. Environ. Sci. Technol. 2013, 47, 10541–10547. [Google Scholar] [CrossRef]
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of Cellulose, Hemicellulose, and Lignin on the Structure and Morphology of Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756. [Google Scholar] [CrossRef]
- Bommier, C.; Xu, R.; Wang, W.; Wang, X.; Wen, D.; Lu, J.; Ji, X. Self-activation of cellulose: A new preparation methodology for activated carbon electrodes in electrochemical capacitors. Nano Energy 2015, 13, 709–717. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Baccile, N.; Laurent, G.; Babonneau, F.; Fayon, F.; Titirici, M.-M.; Antonietti, M. Structural Characterization of Hydrothermal Carbon Spheres by Advanced Solid-State MAS 13C NMR Investigations. J. Phys. Chem. C 2009, 113, 9644–9654. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Park, S.-J. A role of steam activation on CO2 capture and separation of narrow microporous carbons produced from cellulose fibers. Energy 2015, 91, 142–150. [Google Scholar] [CrossRef]
- Xu, C.; Ruan, C.-Q.; Li, Y.; Lindh, J.; Strømme, M. High-Performance Activated Carbons Synthesized from Nanocellulose for CO2 Capture and Extremely Selective Removal of Volatile Organic Compounds. Adv. Sustain. Syst. 2018, 2, 1700147. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable hierarchical porous carbon aerogel from cellulose for high-performance supercapacitor and CO2 capture. Ind. Crop. Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Li, M.; Zhao, Z. Hydrothermal preparation of highly porous carbon spheres from hemp (Cannabis sativa L.) stem hemicellulose for use in energy-related applications. Ind. Crop. Prod. 2015, 65, 216–226. [Google Scholar] [CrossRef]
- Hao, W.; Björnerbäck, F.; Trushkina, Y.; Oregui Bengoechea, M.; Salazar-Alvarez, G.; Barth, T.; Hedin, N. High-Performance Magnetic Activated Carbon from Solid Waste from Lignin Conversion Processes. 1. Their Use As Adsorbents for CO2. ACS Sustain. Chem. Eng. 2017, 5, 3087–3095. [Google Scholar] [CrossRef]
- Sangchoom, W.; Mokaya, R. Valorization of Lignin Waste: Carbons from Hydrothermal Carbonization of Renewable Lignin as Superior Sorbents for CO2 and Hydrogen Storage. ACS Sustain. Chem. Eng. 2015, 3, 1658–1667. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized Mechanochemical Synthesis of Biomass-Derived Sustainable Carbons for High Performance CO2 Storage. Adv. Energy Mater. 2015, 5, 1500867. [Google Scholar] [CrossRef]
- Hu, Y.; Tong, X.; Zhuo, H.; Zhong, L.; Peng, X.; Wang, S.; Sun, R. 3D hierarchical porous N-doped carbon aerogel from renewable cellulose: An attractive carbon for high-performance supercapacitor electrodes and CO2 adsorption. RSC Adv. 2016, 6, 15788–15795. [Google Scholar] [CrossRef]
- Demir, M.; Tessema, T.-D.; Farghaly, A.A.; Nyankson, E.; Saraswat, S.K.; Aksoy, B.; Islamoglu, T.; Collinson, M.M.; El-Kaderi, H.M.; Gupta, R.B. Lignin-derived heteroatom-doped porous carbons for supercapacitor and CO2 capture applications. Int. J. Energy Res. 2018, 42, 2686–2700. [Google Scholar] [CrossRef]
- Saha, D.; Van Bramer, S.E.; Orkoulas, G.; Ho, H.-C.; Chen, J.; Henley, D.K. CO2 capture in lignin-derived and nitrogen-doped hierarchical porous carbons. Carbon 2017, 121, 257–266. [Google Scholar] [CrossRef]
- Meng, Q.B.; Weber, J. Lignin-based Microporous Materials as Selective Adsorbents for Carbon Dioxide Separation. ChemSusChem 2014, 7, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Parra, J.B.; Fuertes, A.B. Assessment of the Role of Micropore Size and N-Doping in CO2 Capture by Porous Carbons. ACS Appl. Mater. Interfaces 2013, 5, 6360–6368. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Plaza, M.G.; García, S.; Rubiera, F.; Pis, J.J.; Pevida, C. Post-combustion CO2 capture with a commercial activated carbon: Comparison of different regeneration strategies. Chem. Eng. J. 2010, 163, 41–47. [Google Scholar] [CrossRef]
- Xu, C.; Hedin, N. Synthesis of microporous organic polymers with high CO2-over-N2 selectivity and CO2 adsorption. J. Mater. Chem. A 2013, 1, 3406–3414. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Li, Y.; Yao, K.; Zhu, Y.; Deng, Z.; Yang, F.; Zhou, X.; Li, G.; Wu, H.; et al. Enhanced Binding Affinity, Remarkable Selectivity, and High Capacity of CO2 by Dual Functionalization of a rht-Type Metal–Organic Framework. Angew. Chem. Int. Ed. 2012, 51, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Chue, K.T.; Kim, J.N.; Yoo, Y.J.; Cho, S.H. Comparison of Activated Carbon and Zeolite 13X for CO2 Recovery from Flue Gas by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 1995, 34, 591–598. [Google Scholar] [CrossRef]

| Sample | Biopolymer | Activating Agent | SBET (m2/g) | Vmicropores (cm3/g) a | CO2 Uptake (mmol/g) b | S c | Qst (kJ/mol) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| 1 bar | 0.15 bar | ||||||||
| CA800 | Cellulose | N2 | 496 | 0.17 | 3.56 | - | - | - | [54] |
| CF-700 | Cellulose | N2 | 499 | 0.193 | 2.846 | - | 28.7 | 27.2 | [47] |
| CF-750 | Cellulose | N2 | 494 | 0.193 | 3.271 | - | 26.4 | 24.5 | [47] |
| CF-800 | Cellulose | N2 | 540 | 0.209 | 3.664 | - | 27.6 | 25.1 | [47] |
| CF-850 | Cellulose | N2 | 452 | 0.174 | 3.189 | - | 27.9 | 25.7 | [47] |
| CF-700-act | Cellulose | CO2 | 599 | 0.229 | 3.395 | - | 35.1 | 29.5 | [47] |
| CF-750-act | Cellulose | CO2 | 696 | 0.267 | 3.792 | - | 36.7 | 31.0 | [47] |
| CF-800-act | Cellulose | CO2 | 863 | 0.334 | 4.192 | - | 47.1 | 37.8 | [47] |
| CF-850-act | Cellulose | CO2 | 1018 | 0.393 | 4.416 | - | 39.2 | 33.4 | [47] |
| LCN-1 | Lignin | KOH | 2922 | 1.22 | 8.64 | 3.2 | - | 40.0 | [56] |
| LCN-2 | Lignin | KOH | 2779 | 1.10 | 8.56 | 3.03 | - | 32.5 | [56] |
| LCN-3 | Lignin | KOH | 1631 | 0.60 | 4.92 | 1.88 | - | 41.0 | [56] |
| L2600 | Lignin | KOH | 1277 | 0.59 | 5.3 | 1.3 | - | - | [53] |
| L2600P | Lignin | KOH | 2224 | 0.91 | 7.3 | 2.3 | - | - | [53] |
| MAC-E-7 | Lignin | KOH | 1674 | 0.60 | 6.0 | 1.8 | 15.0 | 30.0 | [51] |
| MAC-E-8 | Lignin | KOH | 2875 | - | 3.7 | 0.9 | 16.0 | - | [51] |
| MAC-S-7 | Lignin | KOH | 1380 | 0.45 | 3.8 | 1.1 | 11.0 | - | [51] |
| MAC-S-8 | Lignin | KOH | 1706 | - | 2.1 | 0.5 | 11.0 | - | [51] |
| HACS-1 | Hemicellulose | KOH | 1276 | 0.49 | 3.75 | 0.68 | - | - | [50] |
| HACS-2 | Hemicellulose | KOH | 1397 | 0.54 | 5.31 | 1.44 | - | - | [50] |
| HACS-3 | Hemicellulose | KOH | 1764 | 0.49 | 5.00 | 1.37 | - | - | [50] |
| HACS-4 | Hemicellulose | KOH | 2431 | 0.83 | 5.63 | 1.16 | - | - | [50] |
| HACS-5 | Hemicellulose | KOH | 3062 | 0.83 | 4.78 | 0.85 | - | - | [50] |
| LHPC-700 | Lignin | KOH | 1788 | 0.49 | 8.2 | 2.48 | 21.8 | 28.6 | [55] |
| LHPC-850 | Lignin | KOH | 2957 | 0.56 | 7.6 | 2.26 | 15.6 | 28.4 | [55] |
| LHPC-1000 | Lignin | KOH | 1075 | 0.21 | 6.5 | 2.07 | 13.5 | 27.3 | [55] |
| PPC-850 | Lignin | KOH | 2396 | 0.79 | 6.7 | 1.97 | 10.8 | 27.6 | [55] |
| Cell-N2 | Cellulose | N2 | 859 | 0.32 | 3.00 | 0.82 | - | - | [49] |
| Cell-CO2 | Cellulose | CO2 | 1364 | 0.37 | 3.42 | 1.02 | - | - | [49] |
| AC-4-700 | Cellulose | KOH | 2370 | 0.96 (0.37) | 5.80 | 1.48 | 5.4 | 20.0 | [16] |
| LAC2600 | Lignin | KOH | 1157 | 0.54 | 4.4 | - | 24.5 | - | [52] |
| LAC2700 | Lignin | KOH | 1551 | 0.72 | 7.4 | - | 25.0 | - | [52] |
| LAC2800 | Lignin | KOH | 1924 | 0.87 | 6.5 | - | 20.5 | - | [52] |
| CC-AC-N2 | cellulose | N2 | 500 | 0.15 | 2.64 | 1.30 | 32.6 | 31.5 | [48] |
| DAC-AC-N2 | Dialdehyde cellulose | N2 | 455 | 0.15 (0.11) | 3.21 | 1.66 | 40.5 | 26.8 | [48] |
| CLC-AC-N2 | Cross-linked cellulose | N2 | 393 | 0.13 (0.11) | 3.39 | 1.82 | 41.8 | 29.9 | [48] |
| DAC-AC-CO2 | Dialdehyde cellulose | CO2 | 1241 | 0.40 (0.29) | 5.52 | 1.96 | 28.4 | 32.1 | [48] |
| CLC-AC-CO2 | Cross-linked cellulose | CO2 | 832 | 0.29 (0.24) | 4.97 | 2.29 | 32.9 | 28.5 | [48] |
| MOP A-B1 | 378 | 0.11 | 2.68 | 1.20 | 68 | 29.0 | [61] | ||
| Cu-TDPAT | 1938 | 0.93 | 10.1 | 2.60 | 300 | 42.2 | [62] | ||
| Zeolite 13 d | 616 | 0.34 | 4.80 | 3.50 | - | - | [63] | ||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Strømme, M. Sustainable Porous Carbon Materials Derived from Wood-Based Biopolymers for CO2 Capture. Nanomaterials 2019, 9, 103. https://doi.org/10.3390/nano9010103
Xu C, Strømme M. Sustainable Porous Carbon Materials Derived from Wood-Based Biopolymers for CO2 Capture. Nanomaterials. 2019; 9(1):103. https://doi.org/10.3390/nano9010103
Chicago/Turabian StyleXu, Chao, and Maria Strømme. 2019. "Sustainable Porous Carbon Materials Derived from Wood-Based Biopolymers for CO2 Capture" Nanomaterials 9, no. 1: 103. https://doi.org/10.3390/nano9010103
APA StyleXu, C., & Strømme, M. (2019). Sustainable Porous Carbon Materials Derived from Wood-Based Biopolymers for CO2 Capture. Nanomaterials, 9(1), 103. https://doi.org/10.3390/nano9010103