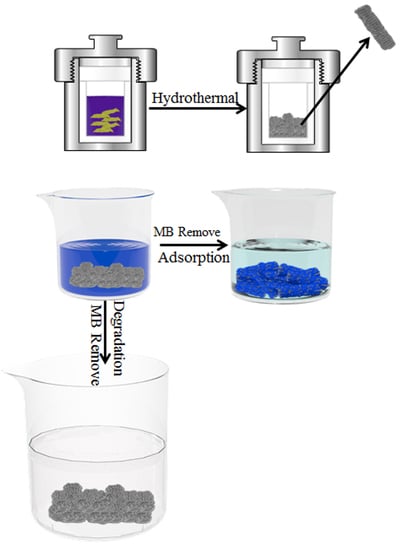
Facile Preparation of Rod-like MnO Nanomixtures via Hydrothermal Approach and Highly Efficient Removal of Methylene Blue for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of C3N4
2.3. Synthesis of MnO Nanomixtures
2.4. MB Adsorption and Degradation Experiments
2.5. Characterization
2.6. Kinetic, Adsorption and Degradation Isotherm Models
2.7. Thermodynamic Evaluation of the Adsorption Process
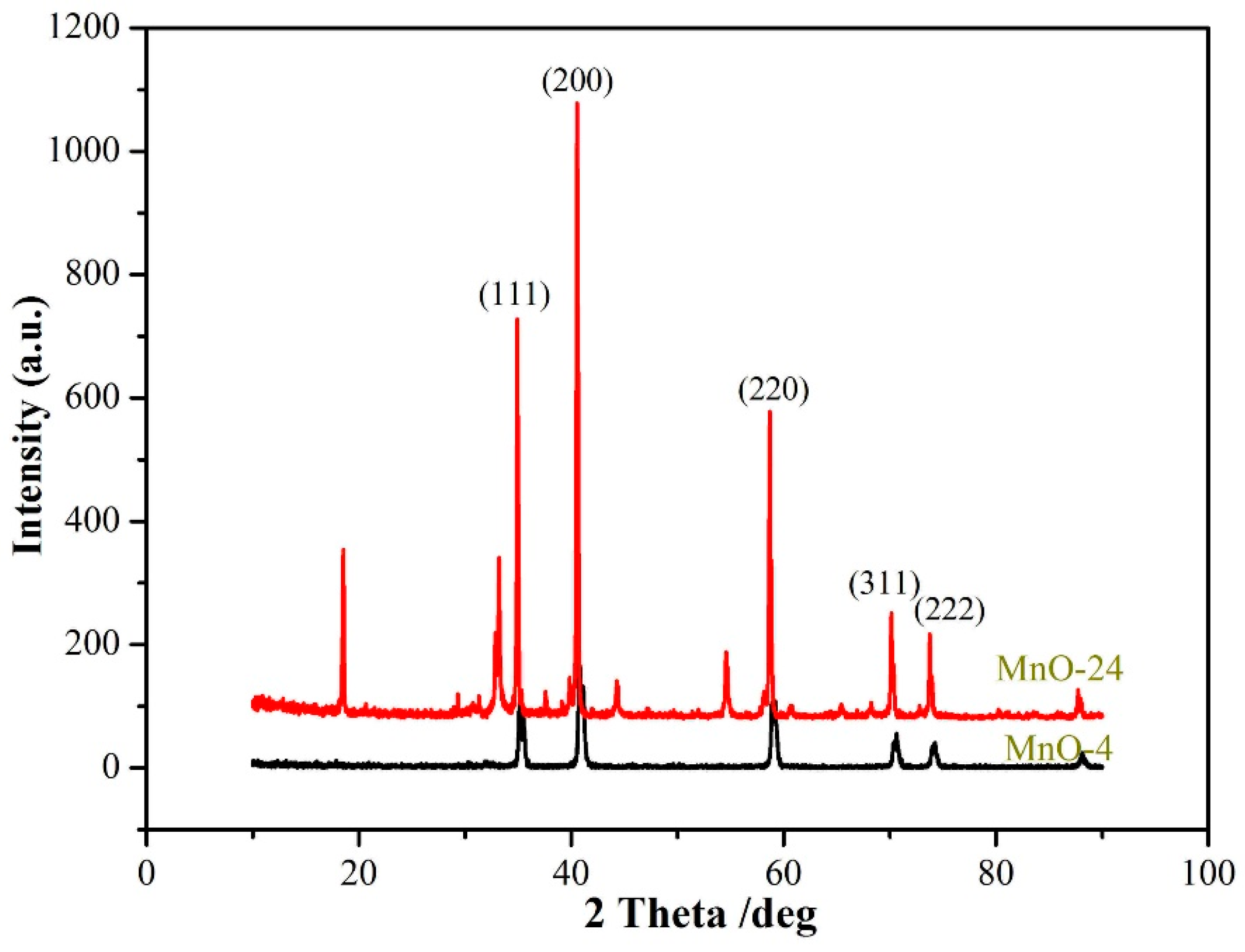
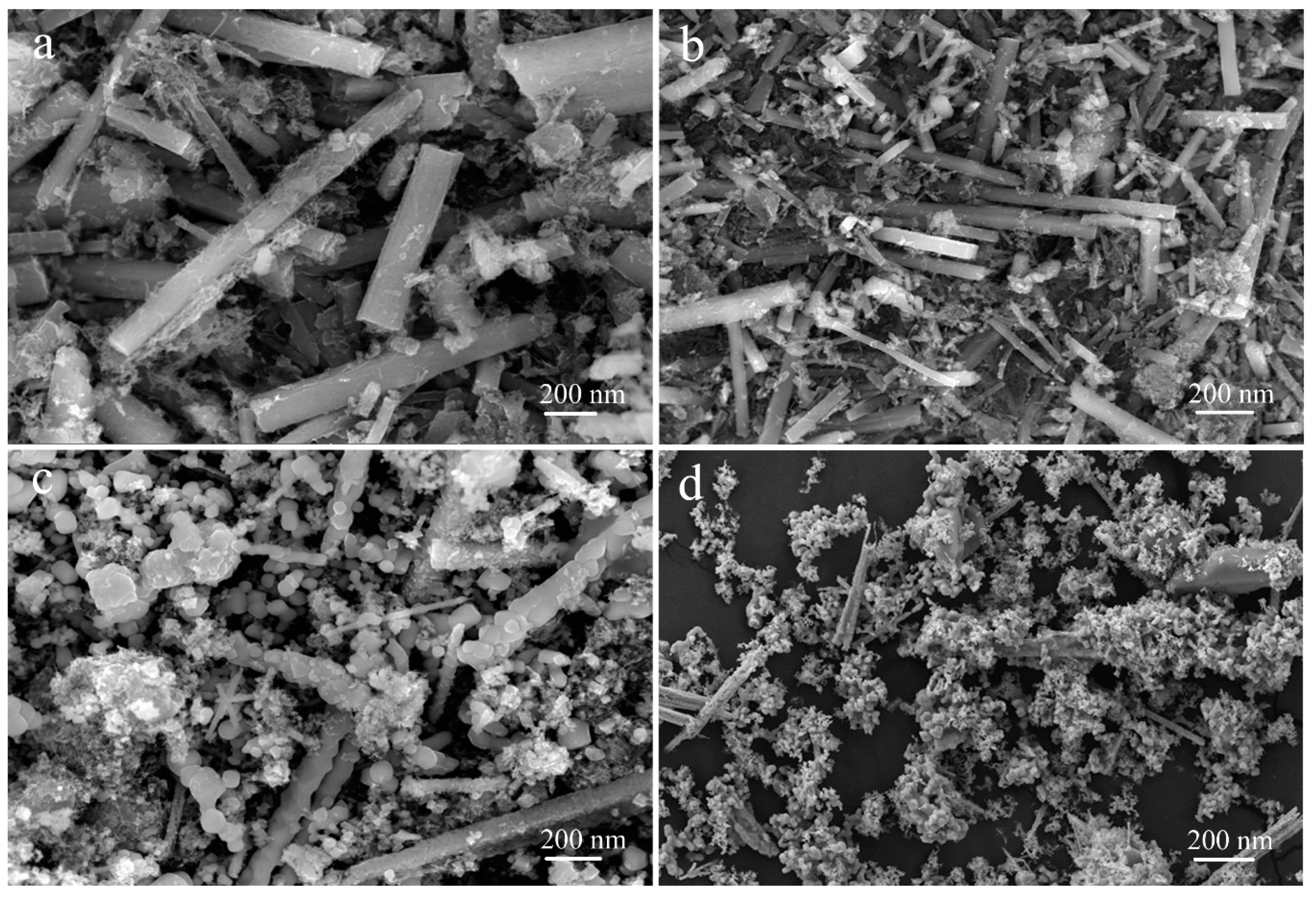
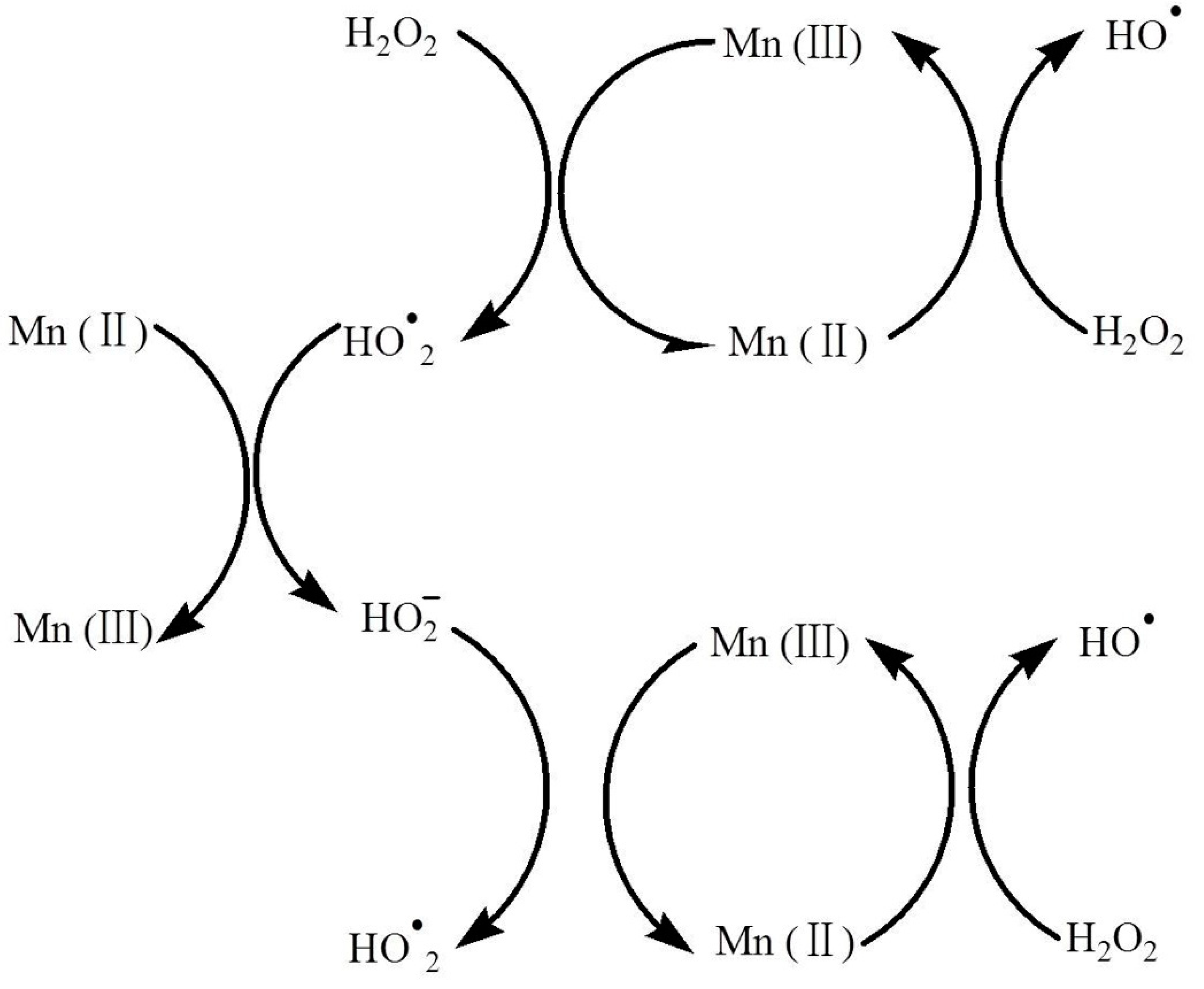
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marrakchi, F.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. Int. J. Biol. Macromol. 2017, 98, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Jiao, T.F.; Li, R.F.; Chen, Y.; Guo, W.C.; Zhang, L.X.; Zhou, J.X.; Zhang, Q.R.; Peng, Q.M. Sandwiched Fe3O4/carboxylate graphene oxide nanostructures constructed by layer-by-layer assembly for highly efficient and magnetically recyclable dye removal. ACS Sustain. Chem. Eng. 2018, 6, 1279–1288. [Google Scholar] [CrossRef]
- Vaz, M.G.; Pereira, A.G.B.; Fajardo, A.R.; Azevedo, A.C.N.; Rodrigues, F.H.A. Methylene Blue Adsorption on Chitosan-g-Poly(Acrylic Acid)/Rice Husk Ash Superabsorbent Composite: Kinetics, Equilibrium, and Thermodynamics. Water Air Soil Pollut. 2017, 228, 14. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, C.; Jiao, T.; Song, J.; Zhang, X.; Xing, R.; Zhou, J.; Zhang, L.; Peng, Q. Self-assembled AgNP-containing nanocomposites constructed by electrospinning as efficient dye photocatalyst materials for wastewater treatment. Nanomaterials 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- Zhao, X.N.; Jiao, T.F.; Ma, X.L.; Huang, H.; Hu, J.; Qu, Y.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Facile fabrication of hierarchical diamond-based AuNPs-modified nanomixtures via layer-by-layer assembly with enhanced catalytic capacities. J. Taiwan Inst. Chem. Eng. 2017, 80, 614–623. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthik, N.; Karthikeyan, D.; Shanmugam, M.; RokLee, Y. Concurrent synthesis of nitrogen-doped carbon dots for cell imaging and ZnO@nitrogen-doped carbon sheets for photocatalytic degradation of methylene blue. J. Photochem. Photobiol. A Chem. 2018, 350, 75–85. [Google Scholar] [CrossRef]
- Tanhaei, M.; Mahjoub, A.R.; Safarifard, V. Sonochemical synthesis of amide-functionalized metal-organic framework/graphene oxide nanocomposite for the adsorption of methylene blue from aqueous solution. Ultrason. Sonochem. 2018, 41, 189–195. [Google Scholar] [CrossRef]
- More, A.T.; Vira, A.; Fogel, S. Biodegradation of trans-1, 2-dichloroethylene by methane-utilizing bacteria in an aquifer simulator. Environ. Sci. Technol. 1989, 23, 403–406. [Google Scholar] [CrossRef]
- Slokar, Y.M.; Marechal, A.M.L. Methods of decoloration of textile wastewaters. Dyes Pigment. 1998, 37, 335–356. [Google Scholar] [CrossRef]
- Tang, L.; Jia, C.T. Fabrication of compressible and recyclable macroscopic g-C3N4/GO aerogel hybrids for visible-light harvesting: A promising strategy for water remediation. Appl. Catal. B Environ. 2017, 219, 241–248. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.P. Enhanced visible-light-driven photocatalytic disinfection performance and organic pollutant degradation activity of porous g-C3N4 nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 27727–27735. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Cavallaro, G.; Gianguzza, A.; Lazzara, G.; Milioto, S.; Piazzese, D. Alginate gel beads filled with halloysite nanotubes. Appl. Clay Sci. 2013, 72, 132–137. [Google Scholar] [CrossRef]
- Cavallaro, G.; Grillo, I.; Gradzielski, M.; Lazzara, G. Structure of Hybrid Materials Based on Halloysite Nanotubes Filled with Anionic Surfactants. J. Phys. Chem. C 2016, 120, 13492–13502. [Google Scholar] [CrossRef]
- Li, T.T.; Wang, Z.H.; Liu, C.C.; Tang, C.M.; Wang, X.K.; Ding, G.S.; Ding, Y.C.; Yang, L.X. TiO2 Nanotubes/Ag/MoS2 Meshy Photoelectrode with Excellent Photoelectrocatalytic Degradation Activity for Tetracycline Hydrochloride. Nanomaterials 2018, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Riahi-Madvaar, R.; Taher, M.A.; Fazelirad, H. Synthesis and characterization of magnetic halloysite-iron oxide nanocomposite and its application for naphthol green B removal. Appl. Clay Sci. 2017, 137, 101–106. [Google Scholar] [CrossRef]
- Minero, C.; Lucchiari, M.; Vione, D.; Maurino, V. Fe(III)-Enhanced Sonochemical Degradation of Methylene Blue In Aqueous Solution. Environ. Sci. Technol. 2005, 39, 8936–8942. [Google Scholar] [CrossRef]
- Jiang, F.; Yan, T.T.; Chen, H.; Sun, A.; Xu, C.M.; Wang, X. A g-C3N4–CdS composite catalyst with high visible-light-driven catalytic activity and photostability for methylene blue degradation. Appl. Surf. Sci. 2014, 295, 164–172. [Google Scholar] [CrossRef]
- Xing, S.T.; Zhou, Z.C.; Ma, Z.C.; Wu, Y.S. Characterization and reactivity of Fe3O4/FeMnOx core/shell nanoparticles for methylene blue discoloration with H2O2. Appl. Catal. B Environ. 2011, 107, 386–392. [Google Scholar] [CrossRef]
- Li, Y.Q.; Qu, J.Y.; Gao, F.; Lv, S.Y.; Shi, L.; He, C.X.; Sun, J.C. In situ fabrication of Mn3O4 decorated graphene oxide as a synergistic catalyst for degradation of methylene blue. Appl. Catal. B Environ. 2015, 162, 268–274. [Google Scholar] [CrossRef]
- Randorn, C.; Wongnawa, S.; Boonsin, P. Bleaching of Methylene Blue by Hydrated Titanium Dioxide. Sci. Asia 2004, 30, 149–156. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhao, H.Q.; Qi, H.B.; Liu, X.Y.; Liu, Y. Free radical behaviours during methylene blue degradation in the Fe2+/H2O2 system. Environ. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, G.K.; Sharma, A. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dyes Pigment. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- de Brito Benetoli, L.O.; Cadorin, B.M.; Baldissarelli, V.Z.; Geremias, R.; de Souza, I.G.; Debacher, N.A. Pyrite-enhanced methylene blue degradation in non-thermal plasma water treatment reactor. J. Hazard. Mater. 2012, 237, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, Y.M. Prussian-blue-modified iron oxide magnetic nanoparticles as effective peroxidase-like catalysts to degrade methylene blue with H2O2. J. Hazard. Mater. 2011, 191, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Wolski, L.; Ziolek, M. Insight into pathways of methylene blue degradation with H2O2 over mono and bimetallic Nb, Zn oxides. Appl. Catal. B Environ. 2018, 224, 634–647. [Google Scholar] [CrossRef]
- Prathap, M.U.A.; Kaur, B.; Srivastava, R. Hydrothermal synthesis of CuO micro-/nanostructures and their applications in the oxidative degradation of methylene blue and non-enzymatic sensing of glucose/H2O2. J. Colloid Interface Sci. 2012, 370, 144–154. [Google Scholar] [CrossRef]
- Saha, M.; Gayen, A.; Mukherjee, S. Microstructure, morphology, and methylene blue degradation over nano-CuFe2O4 synthesized by a modified complexometric method. J. Aust. Ceram. Soc. 2018, 54, 513–522. [Google Scholar] [CrossRef]
- Zhang, P.Q.; Zhan, Y.G.; Cai, B.X.; Hao, C.C.; Wang, J.; Liu, C.X.; Meng, Z.J.; Yin, Z.L.; Chen, Q.Y. Shape-Controlled Synthesis of Mn3O4 Nanocrystals and Their Catalysis of the Degradation of Methylene Blue. Nano Res. 2010, 3, 235–243. [Google Scholar] [CrossRef]
- Cai, X.G.; He, J.Y. A 2D-g-C3N4 nanosheet as an eco-friendly adsorbent for various environmental pollutants in water. Chemosphere 2017, 171, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Meng, Y.L. Pyrolysis Synthesized g-C3N4 for Photocatalytic Degradation of Methylene Blue. J. Chem. 2013, 2013, 187912. [Google Scholar] [CrossRef]
- Li, K.Y.; Fang, Z.L.; Xiong, S.; Luo, J. Novel graphitic-C3N4 nanosheets: Enhanced visible light photocatalytic activity and photoelectrochemical detection of methylene blue dye. Mater. Technol. 2017, 32, 391–398. [Google Scholar] [CrossRef]
- Chang, F.; Xie, Y.C.; Li, C.L.; Chen, J.; Luo, J.R.; Hu, X.F.; Shen, J.W. A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl. Surf. Sci. 2013, 280, 967–974. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, M.Z.; Li, G.; Dai, P.; Yu, X.X.; Bai, Z.M.; Chen, P. Photocatalytic degradation of methylene blue over boron-doped g-C3N4 together with nitrogenvacancies under visible light irradiation. React. Kinet. Mech. Catal. 2018, 125, 1179–1190. [Google Scholar] [CrossRef]
- Chu, Y.T.; Guo, L.Y.; Xi, B.J.; Feng, Z.Y.; Wu, F.F.; Lin, Y.; Liu, J.C.; Sun, D.; Feng, J.K.; Qian, Y.T.; et al. Embedding MnO@Mn3O4 Nanoparticles in an N-Doped-Carbon Framework Derived from Mn-Organic Clusters for Effcient Lithium Storage. Adv. Mater. 2018, 30, 1704244. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.H.; Zhao, K. Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chem. Commun. 2012, 48, 6178–6180. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Xu, Y.L.; Zhang, L.H.; Liu, Z.F. Carbon-doped graphitic carbon nitride as environment-benign adsorbent for methylene blue adsorption: Kinetics, isotherm and thermodynamics study. J. Taiwan Inst. Chem. Eng. 2018, 88, 114–120. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Xie, J.; Li, C.J. Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water. Fuel 2013, 103, 480–485. [Google Scholar] [CrossRef]
- Saini, J.; Garg, V.K.; Gupta, R.K. Removal of Methylene Blue from aqueous solution by Fe3O4@Ag/SiO2 nanospheres: Synthesis, characterization and adsorption performance. J. Mol. Liq. 2018, 250, 413–422. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Qin, M.; Zhao, M.J.; Zhang, Y.S. Green one-pot preparation of α-Fe2O3@carboxyl-functionalized yeast composite with high adsorption and catalysis properties for removal of methylene blue. Surf. Interface Anal. 2017, 50, 311–320. [Google Scholar] [CrossRef]
- Chen, B.L.; Yang, Z.X.; Ma, G.P.; Kong, D.L.; Xiong, W.; Wang, J.B.; Zhu, Y.Q.; Xia, Y.D. Heteroatom-doped porous carbons with enhanced carbon dioxide uptake and excellent methylene blue adsorption capacities. Microporous Mesoporous Mater. 2018, 257, 1–8. [Google Scholar] [CrossRef]
- Mounia, L.; Belkhir, L.I.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Xing, R.; Wang, W.; Jiao, T.; Ma, K.; Zhang, Q.; Hong, W.; Qiu, H.; Zhou, J.; Zhang, L.; Peng, Q. Bioinspired polydopamine sheathed nanofibers containing carboxylate graphene oxide nanosheet for high-efficient dyes scavenger. ACS Sustain. Chem. Eng. 2017, 5, 4948–4956. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, K.; Jiao, T.; Xing, R.; Ma, X.; Hu, J.; Huang, H.; Zhang, L.; Yan, X. Fabrication of hierarchical layer-by-layer assembled diamond based core-shell nanocomposites as highly efficient dye absorbents for wastewater treatment. Sci. Rep. 2017, 7, 44076. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jiao, T.; Zhang, Q.; Guo, W.; Peng, Q.; Yan, X. Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res. Lett. 2015, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiao, T.; Xing, R.; Zou, G.; Zhou, J.; Zhang, L.; Peng, Q. Fabrication of tunable hierarchical MXene@AuNPs nanocomposites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Sun, S.; Zhang, L.; Yin, J.; Jiao, T.; Zhang, L.; Xu, Y.; Zhou, J.; Peng, Q. Facile preparation and catalytic performance characterization of AuNPs-loaded hierarchical electrospun composite fibers by solvent vapor annealing treatment. Colloid Surf. A Physicochem. Eng. Asp. 2019, 561, 283–291. [Google Scholar] [CrossRef]
- Chen, K.; Li, J.; Zhang, L.; Xing, R.; Jiao, T.; Gao, F.; Peng, Q. Facile synthesis of self-assembled carbon nanotubes/dye composite films for sensitive electrochemical determination of Cd(II) ions. Nanotechnology 2018, 29, 445603. [Google Scholar] [CrossRef]
- Huang, X.; Jiao, T.; Liu, Q.; Zhang, L.; Zhou, J.; Li, B.; Peng, Q. Hierarchical electrospun nanofibers treated by solvent vapor annealing as air filtration mat for high-efficiency PM2.5 capture. Sci. China Mater. 2018. [Google Scholar] [CrossRef]
- Li, N.; Tang, S.; Rao, Y.; Qi, J.; Wang, P.; Jiang, Y.; Huang, H.; Gu, J.; Yuan, D. Improved dye removal and simultaneous electricity production in a photocatalytic fuel cell coupling with persulfate. Electrochim. Acta 2018, 270, 330–338. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Zou, J.; Zhu, L.H.; Huang, L.; Jiang, H.P.; Zhang, Y.X. Degradation of Methylene Blue with H2O2 Activated by Peroxidase-Like Fe3O4 Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2011, 11, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gao, F.; Jiao, T.; Xing, R.; Zhang, L.; Zhang, Q.; Peng, Q. Selective Cu(II) ion removal from wastewater via surface charged self-assembled polystyrene-Schiff base nanocomposites. Colloid Surf. A Physicochem. Eng. Asp. 2018, 545, 60–67. [Google Scholar] [CrossRef]
- Tang, S.; Li, X.; Zhang, C.; Liu, Y.; Zhang, W.; Yuan, D. Strengthening decomposition of oxytetracycline in DBD plasma coupling with Fe-Mn oxides loaded granular activated carbon. Plasma Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Luo, X.; Ma, K.; Jiao, T.; Xing, R.; Zhang, L.; Zhou, J.; Li, B. Graphene oxide-polymer composite Langmuir films constructed by interfacial thiol-ene photopolymerization. Nanoscale Res. Lett. 2017, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Duan, P.; Jiao, T.; Peng, Q.; Liu, M. Self-assembled luminescent quantum dots to generate full-color and white circularly polarized light. Angew. Chem. Int. Ed. 2017, 56, 12174–12178. [Google Scholar] [CrossRef]
- Song, J.; Xing, R.; Jiao, T.; Peng, Q.; Yuan, C.; Möhwald, H.; Yan, X. Crystalline dipeptide nanobelts based on solid-solid phase transformation self-assembly and their polarization imaging of cells. ACS Appl. Mater. Interfaces 2018, 10, 2368–2376. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Xing, R.R.; Jiao, T.F.; Ma, K.; Chen, C.J.; Ma, G.H.; Yan, X.H. Synergistic in vivo photodynamic and photothermal antitumor therapy based on collagen-gold hybrid hydrogels with inclusion of photosensitive drugs Colloids and Surfaces A: Physicochemical and Engineering Aspects. ACS Appl. Mater. Interfaces 2016, 8, 13262–13269. [Google Scholar] [CrossRef]
- Xing, R.; Jiao, T.; Liu, Y.; Ma, K.; Zou, Q.; Ma, G.; Yan, X. Co-assembly of graphene oxide and albumin/photosensitizer nanohybrids towards enhanced photodynamic therapy. Polymers 2016, 8, 181. [Google Scholar] [CrossRef]
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An injectable self-assembling collagen-gold hybrid hydrogel for combinatorial antitumor photothermal/photodynamic therapy. Adv. Mater. 2016, 28, 3669–3676. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Y.; Jiao, T.; Xing, R.; Yang, Z.; Fan, J.; Liu, J.; Li, B.; Peng, Q. Preparation and enhanced structural integrity of electrospun poly(ε-caprolactone)-based fibers by freezing amorphous chains through thiol-ene click reaction. Colloid Surf. A Physicochem. Eng. Asp. 2018, 538, 7–13. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.Q.; Zou, Q.L.; Xie, Z.C.; Yan, X.H. Self-assembled Zinc/cystine-based chloroplast mimics capable of photoenzymatic reactions for sustainable fuel synthesis. Angew. Chem. Int. Ed. 2017, 56, 7876–7880. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.R.; Li, Y.X.; Zou, Q.L.; Möhwald, H.; Yan, X.H. Mimicking primitive photobacteria: Sustainable hydrogen evolution based on peptide-porphyrin co-assemblies with self-mineralized reaction center. Angew. Chem. Int. Ed. 2016, 55, 12503–12507. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xing, R.R.; Chen, C.J.; Shen, G.Z.; Yan, L.Y.; Zou, Q.L.; Ma, G.H.; Möhwald, H.; Yan, X.H. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem. Int. Ed. 2015, 54, 500–505. [Google Scholar] [CrossRef]
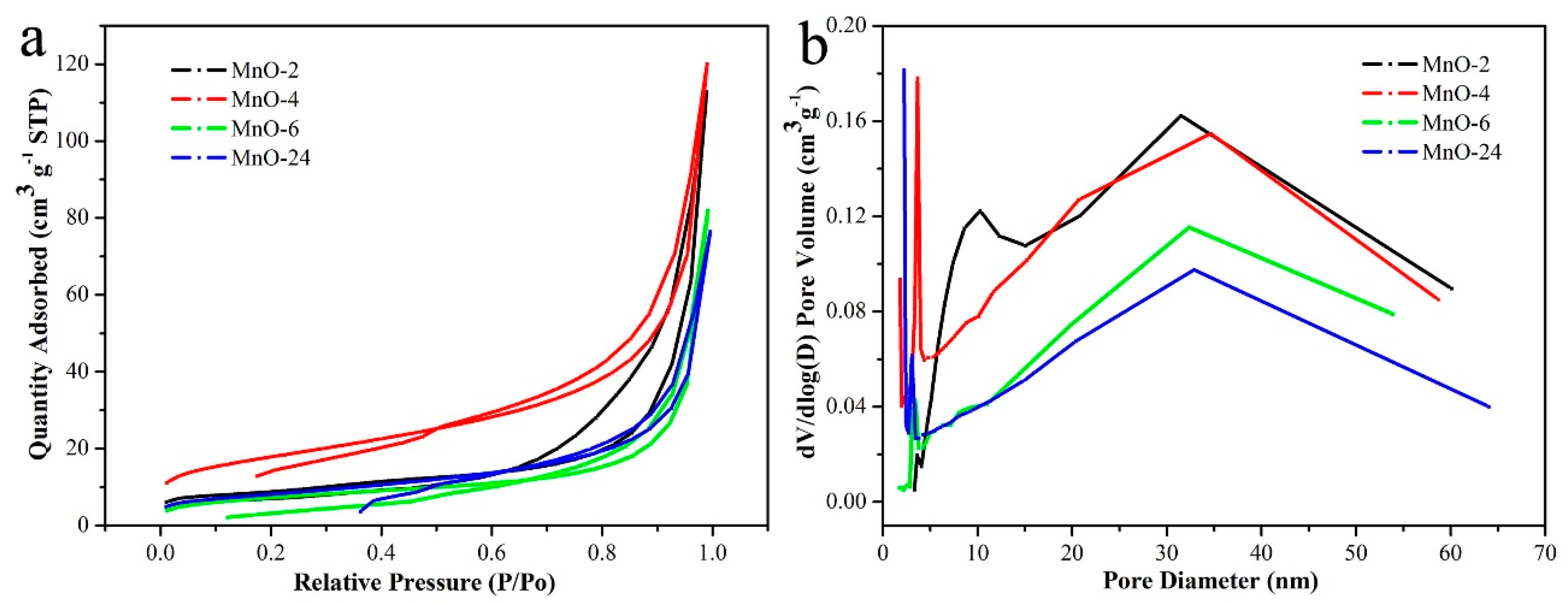
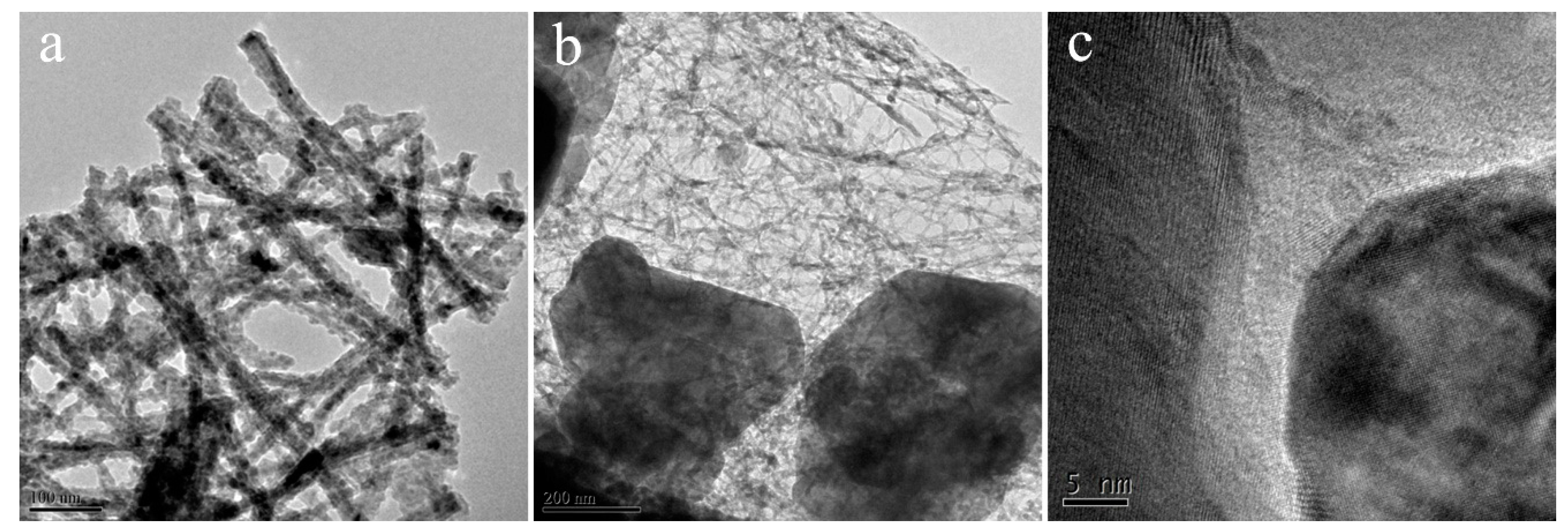
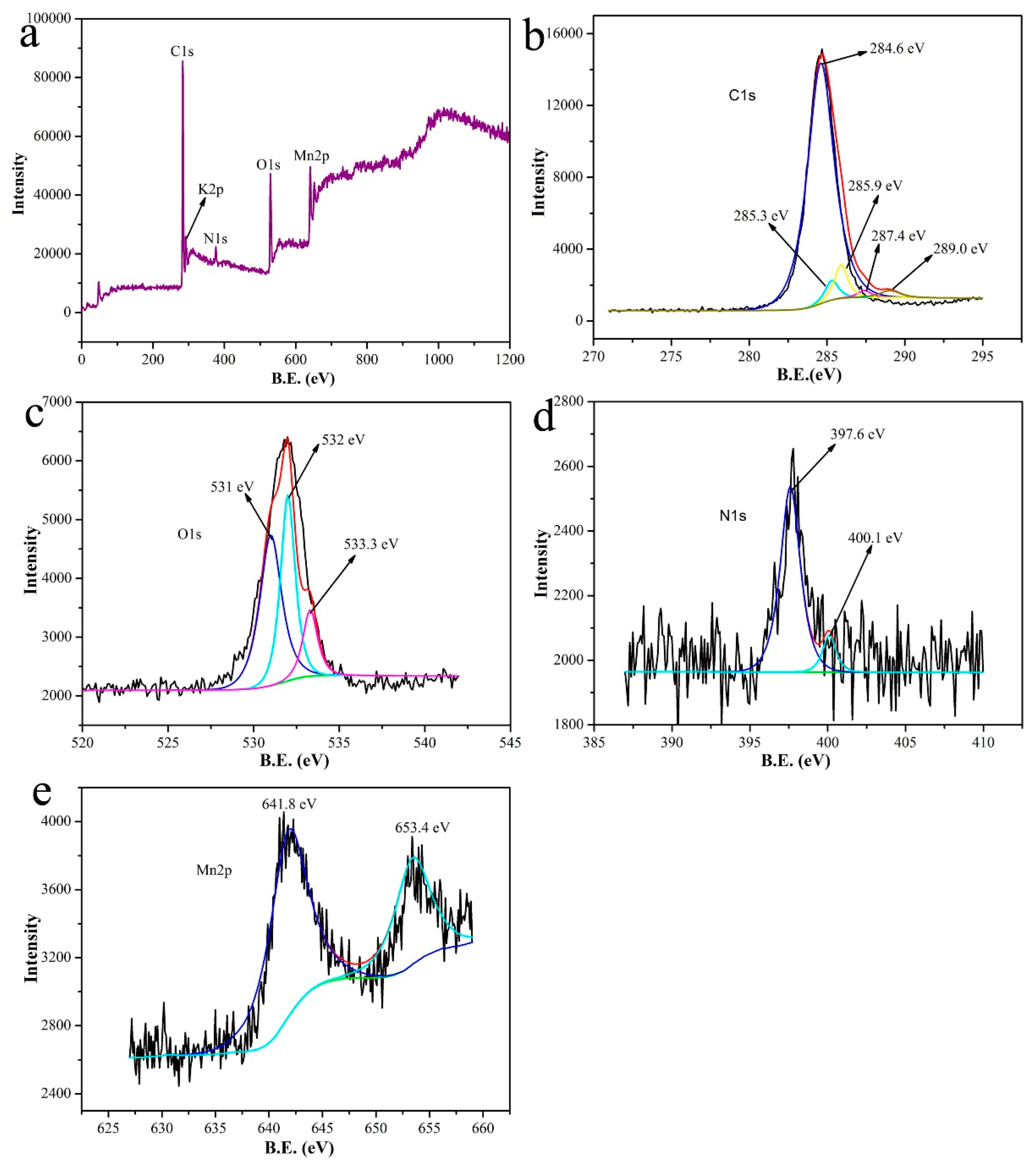
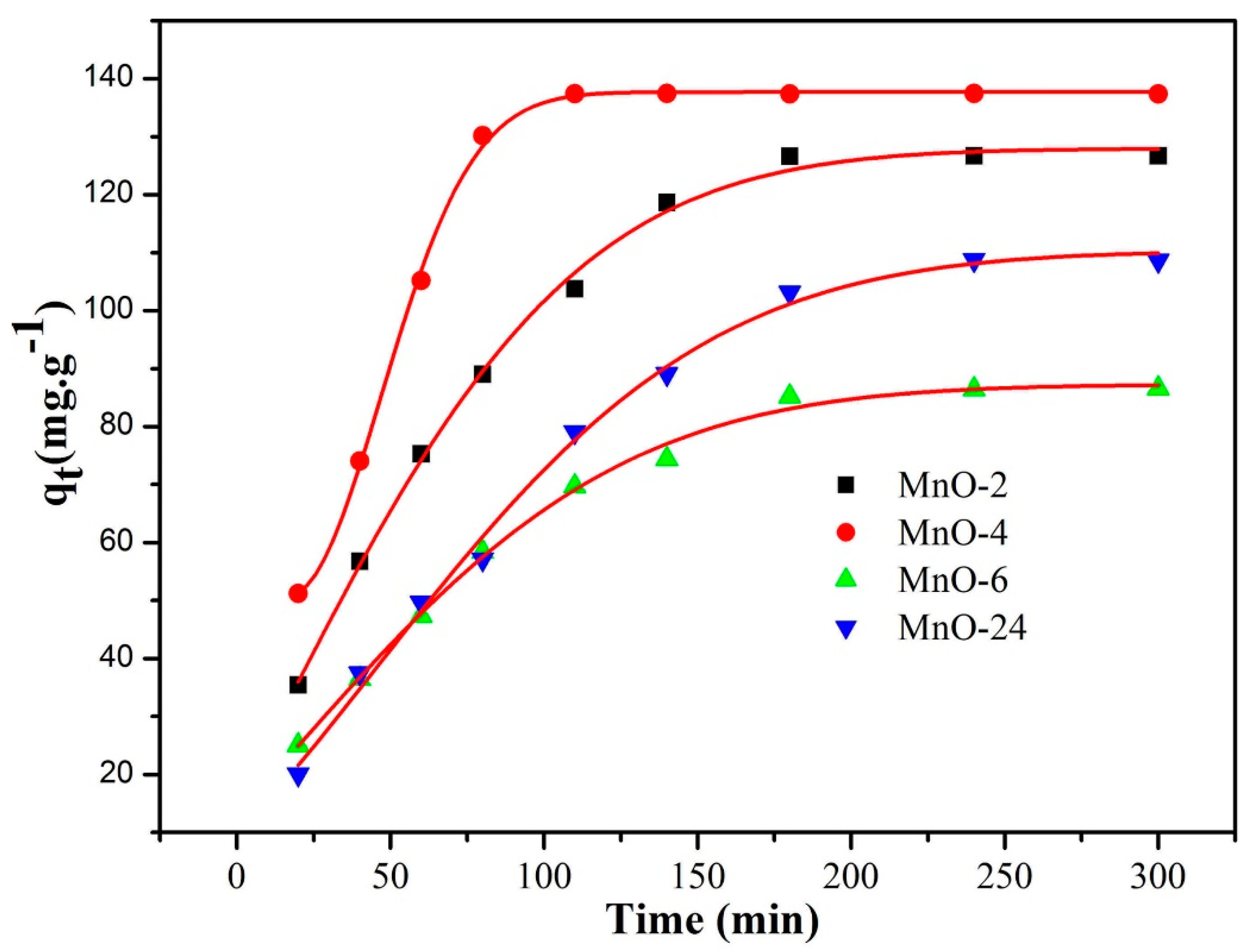
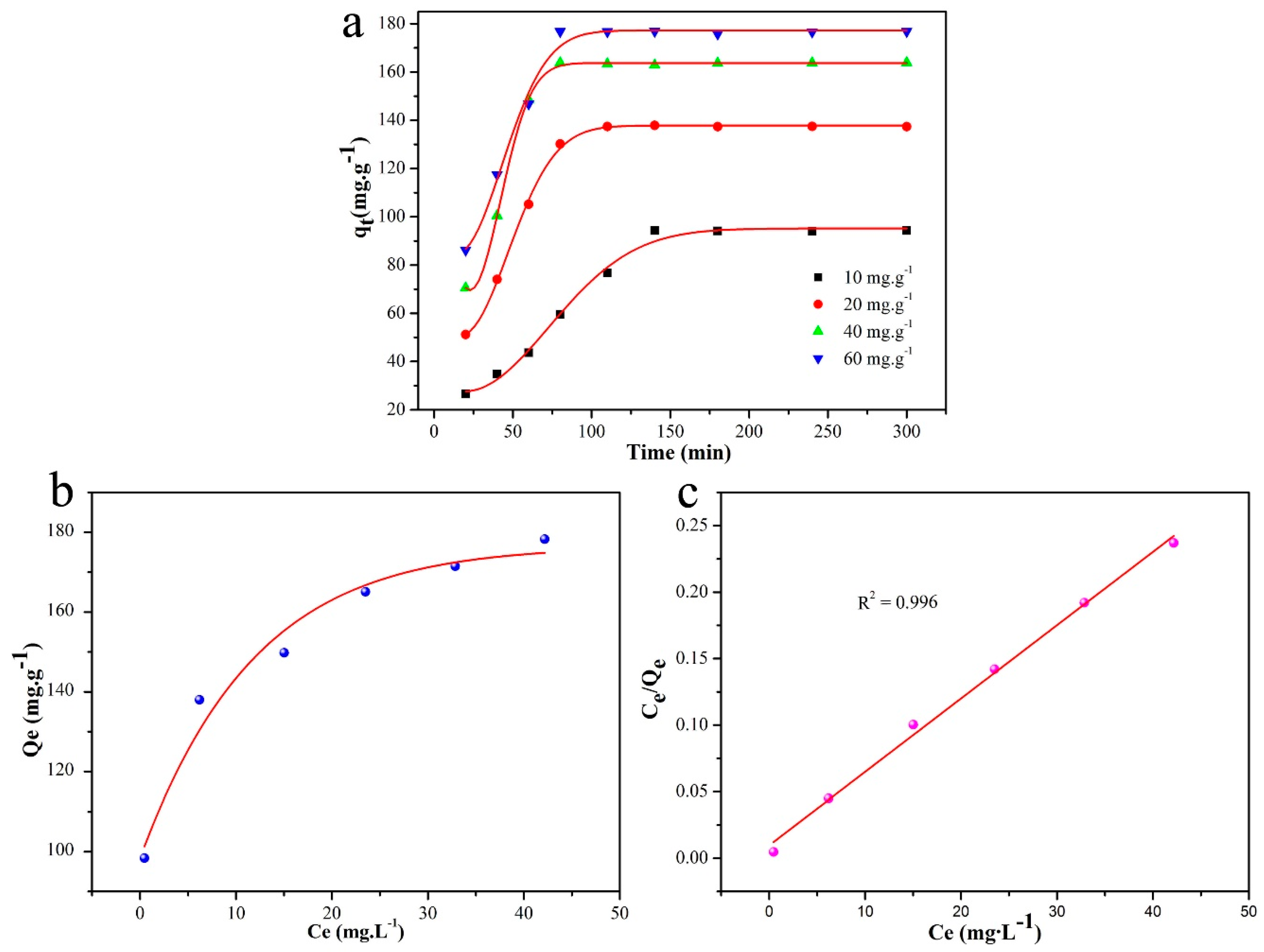
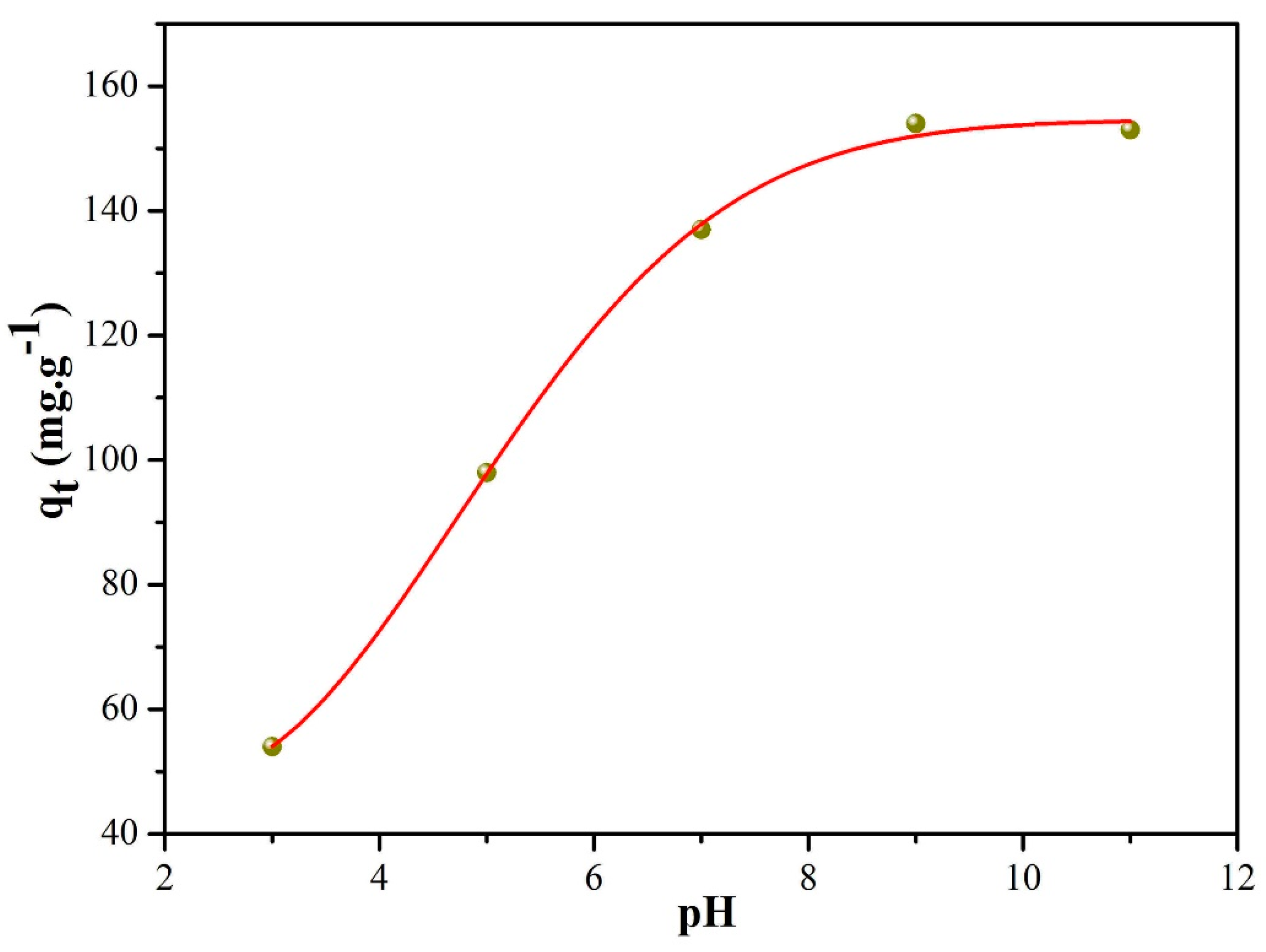
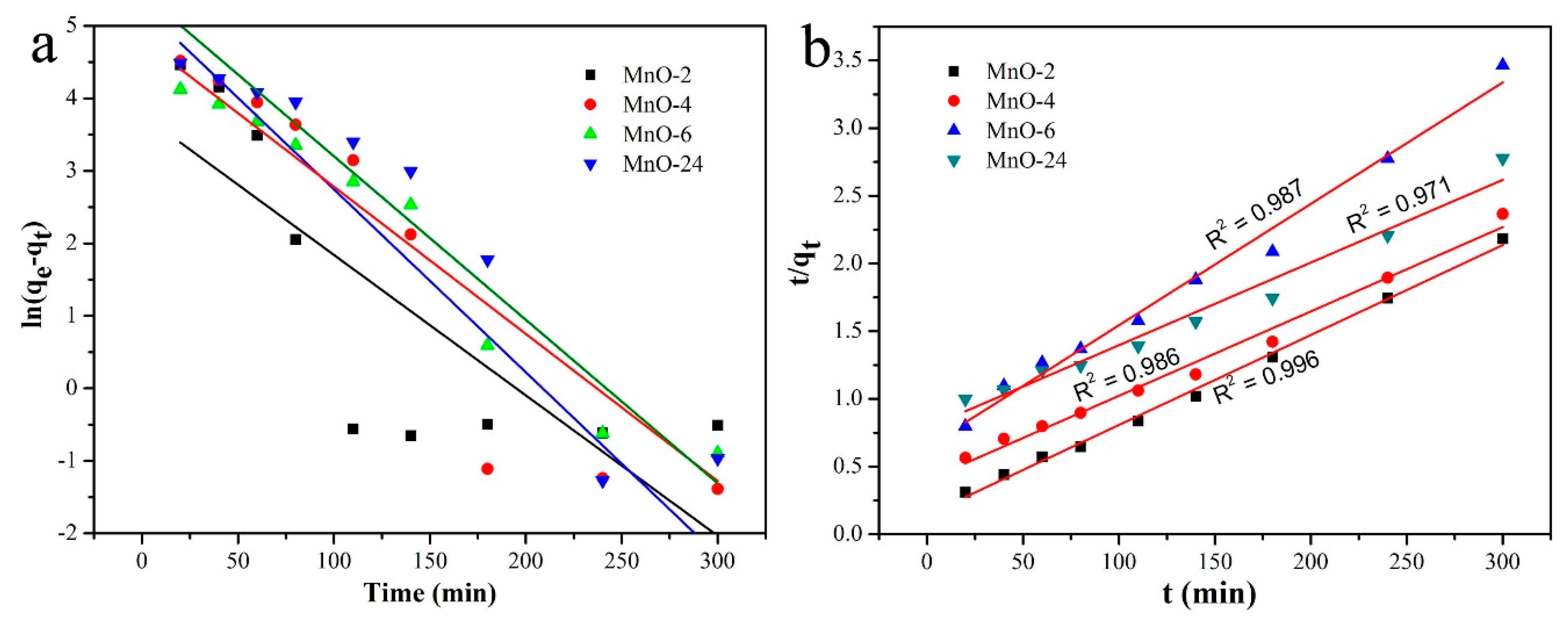
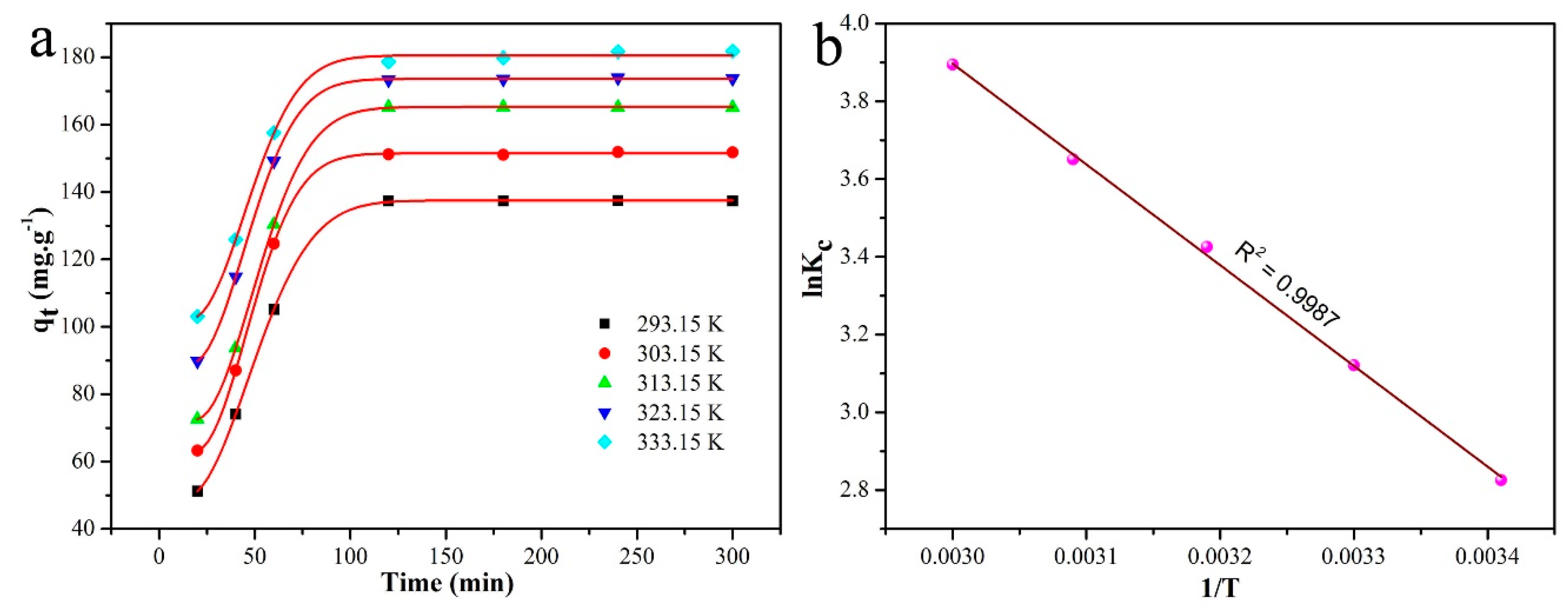

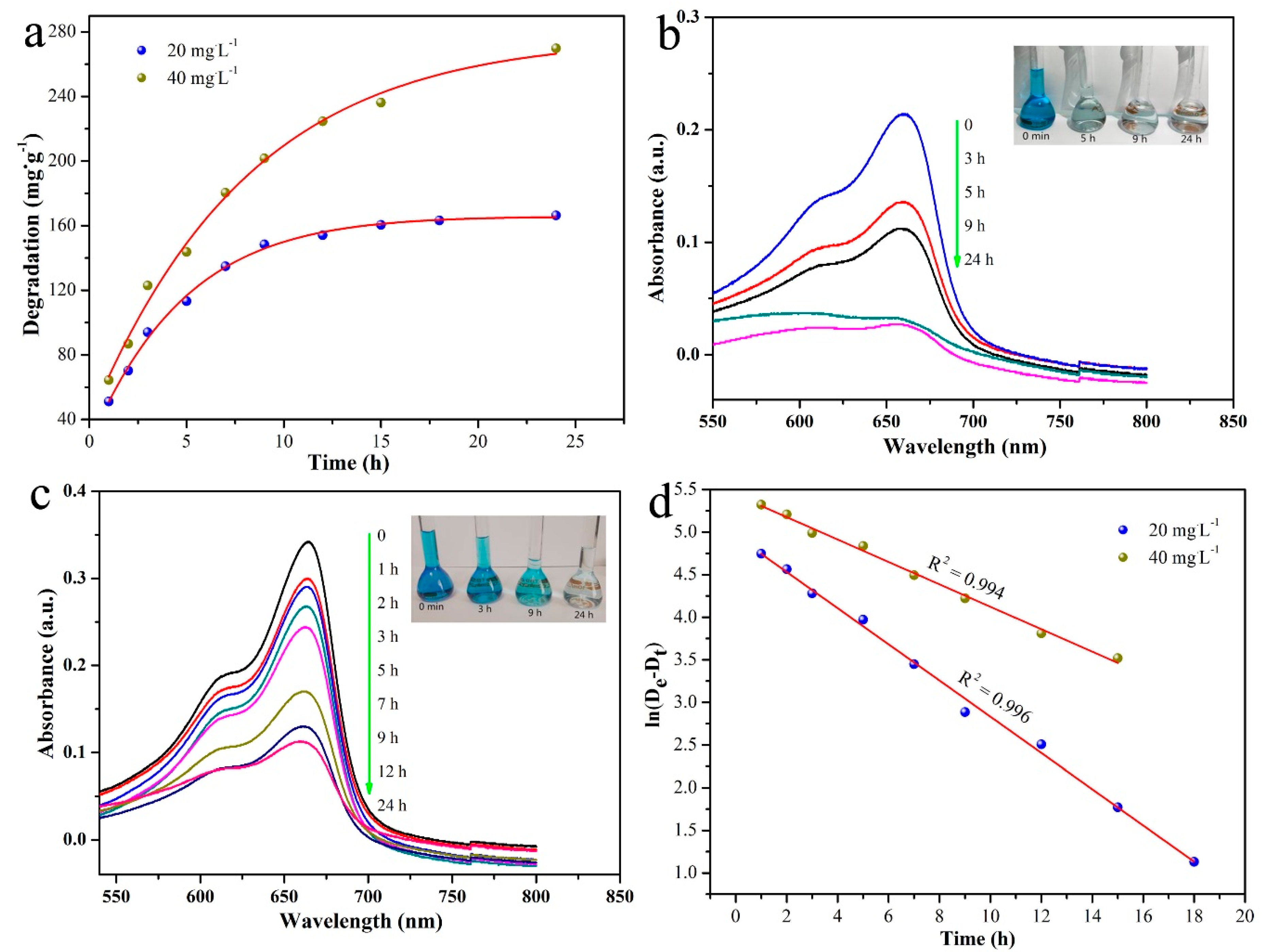
| Entry | SBET (m2/g) | Smicro (m2/g) | Daverage (nm) | Vtotal (cm3/g) |
|---|---|---|---|---|
| MnO-2 | 30.6 | 6.3 | 22.81 | 0.175 |
| MnO-4 | 38.7 | 8.5 | 23.09 | 0.193 |
| MnO-6 | 35.5 | 1.0 | 21.46 | 0.127 |
| MnO-24 | 33.9 | 1.6 | 15.97 | 0.168 |
| Entry | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||
|---|---|---|---|---|
| K1 | qe (mg g−1) | K2 | qe (mg g−1) | |
| MnO-2 | 0.019 | 43.70 | 0.00031 | 150.38 |
| MnO-4 | 0.025 | 194.03 | 0.000097 | 164.12 |
| MnO-6 | 0.021 | 122.85 | 0.00012 | 111.48 |
| MnO-24 | 0.022 | 234.40 | 0.000047 | 160.67 |
| Adsorbent | mg g−1 | Reference |
|---|---|---|
| Wheat shells | 21.5 | [39] |
| Chitosan-modified zeolite | 37 | [40] |
| Fe3O4@Ag/SiO2 nanospheres | 128.5 | [41] |
| α-Fe2O3@carboxyl-functionalized yeast composite | 49.5 | [42] |
| N, O-codoped porous carbon | 100.2 | [43] |
| Kaolin | 52.7 | [44] |
| C, N-doped MnO | 154 | Present work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ren, B.; Wang, R.; Zhang, L.; Jiao, T.; Liu, Z. Facile Preparation of Rod-like MnO Nanomixtures via Hydrothermal Approach and Highly Efficient Removal of Methylene Blue for Wastewater Treatment. Nanomaterials 2019, 9, 10. https://doi.org/10.3390/nano9010010
Xu Y, Ren B, Wang R, Zhang L, Jiao T, Liu Z. Facile Preparation of Rod-like MnO Nanomixtures via Hydrothermal Approach and Highly Efficient Removal of Methylene Blue for Wastewater Treatment. Nanomaterials. 2019; 9(1):10. https://doi.org/10.3390/nano9010010
Chicago/Turabian StyleXu, Yuelong, Bin Ren, Ran Wang, Lihui Zhang, Tifeng Jiao, and Zhenfa Liu. 2019. "Facile Preparation of Rod-like MnO Nanomixtures via Hydrothermal Approach and Highly Efficient Removal of Methylene Blue for Wastewater Treatment" Nanomaterials 9, no. 1: 10. https://doi.org/10.3390/nano9010010
APA StyleXu, Y., Ren, B., Wang, R., Zhang, L., Jiao, T., & Liu, Z. (2019). Facile Preparation of Rod-like MnO Nanomixtures via Hydrothermal Approach and Highly Efficient Removal of Methylene Blue for Wastewater Treatment. Nanomaterials, 9(1), 10. https://doi.org/10.3390/nano9010010