Recent Progress on the Fabrication and Properties of Silver Nanowire-Based Transparent Electrodes
Abstract
:1. Introduction
2. Theoretical Approaches
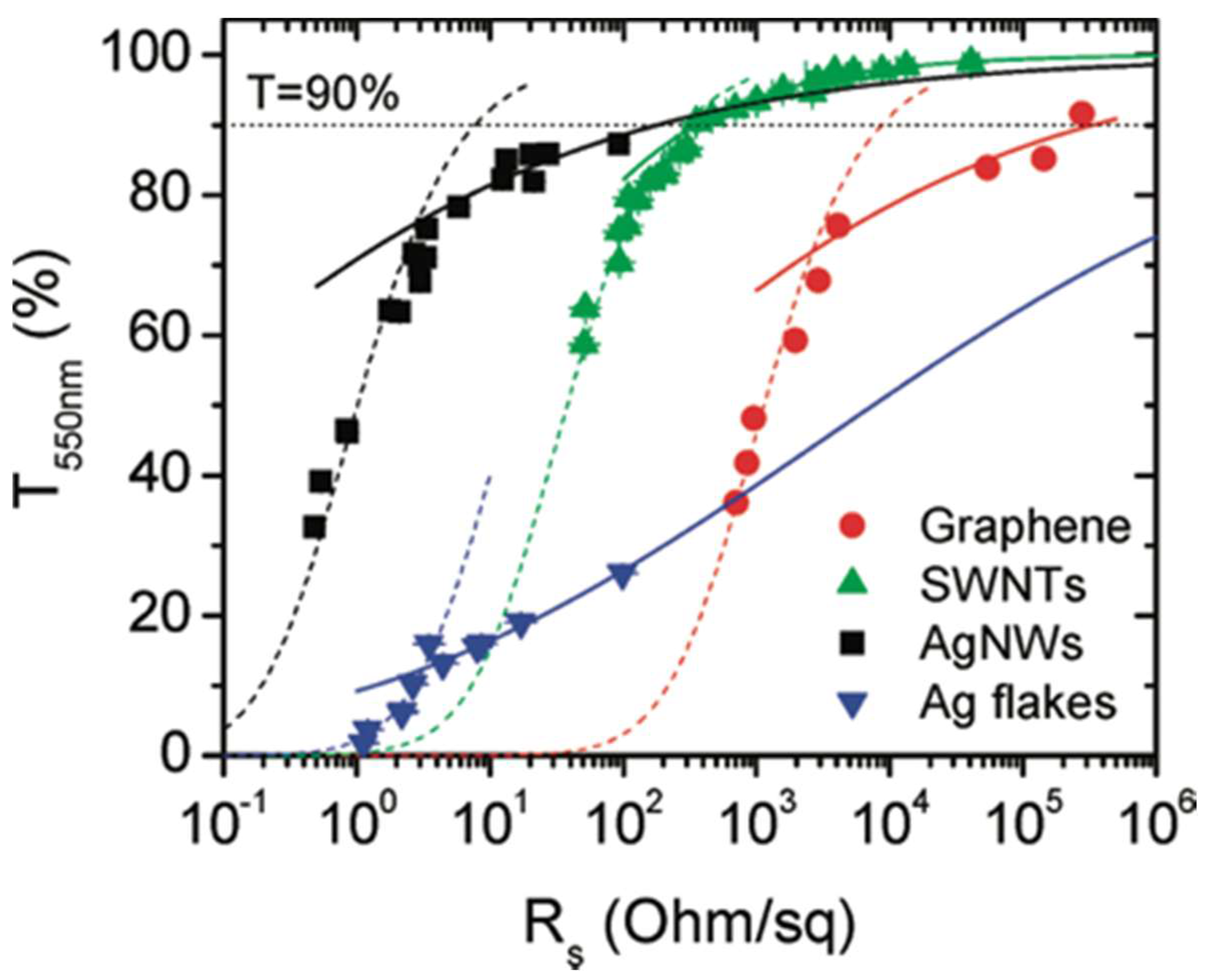
2.1. Transparency and Conductivity
2.2. Transmission and Film Parameters
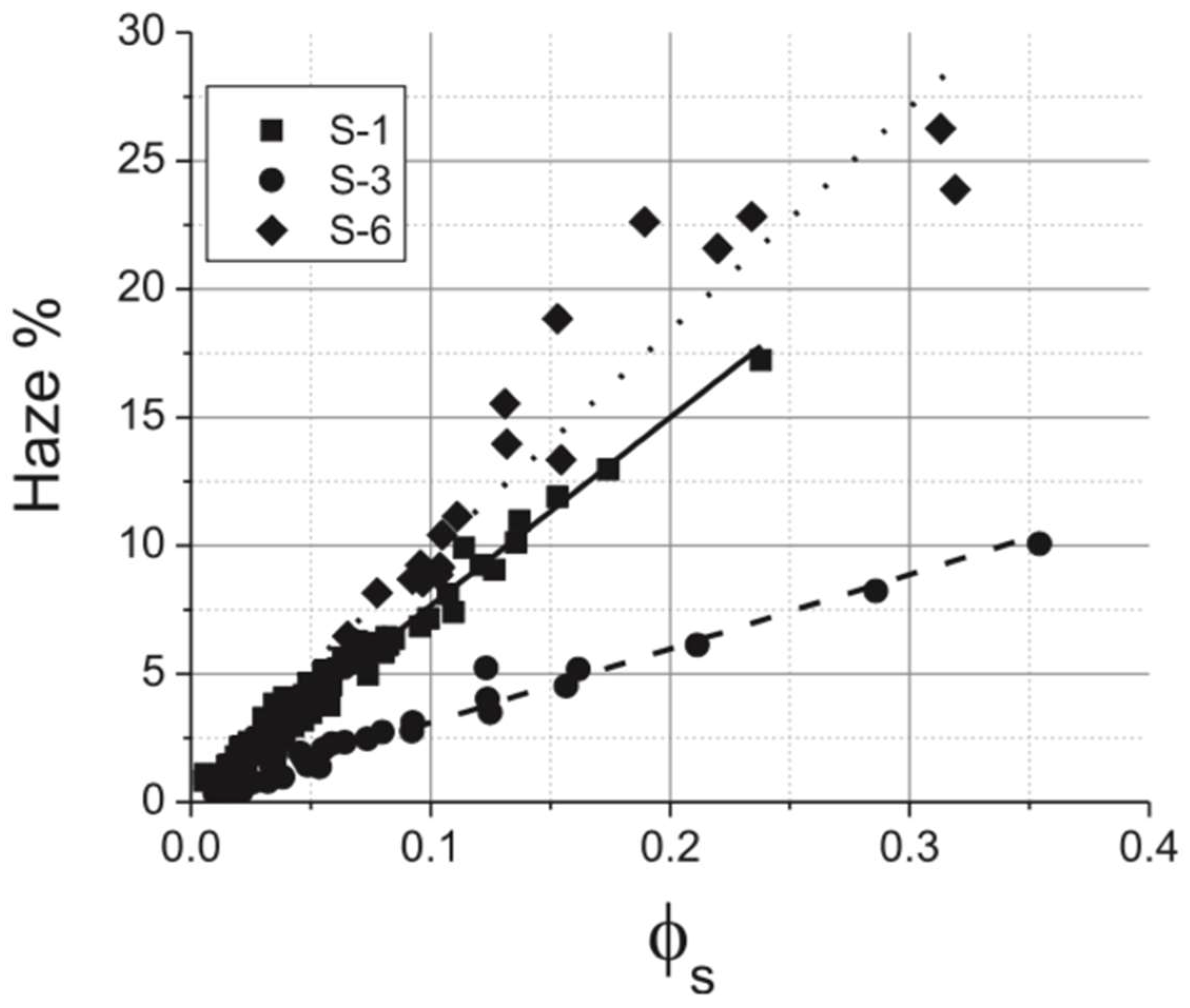
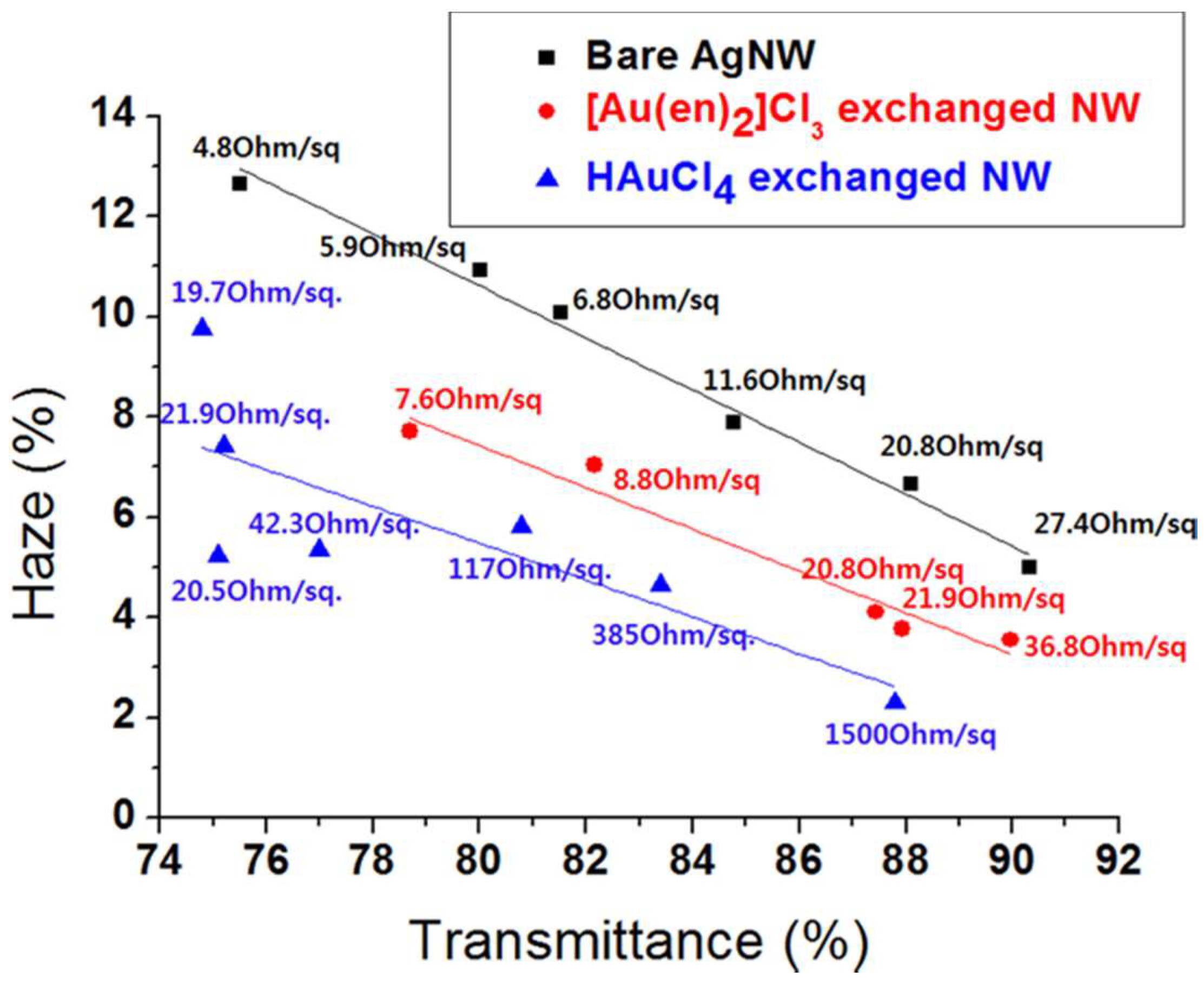
2.3. Haze
3. Experimental Approaches
3.1. Electrical Stability
3.2. Mechanical Stability
3.2.1. Flexibility
3.2.2. Adhesion
3.2.3. Stretchability
3.3. Chemical Stability
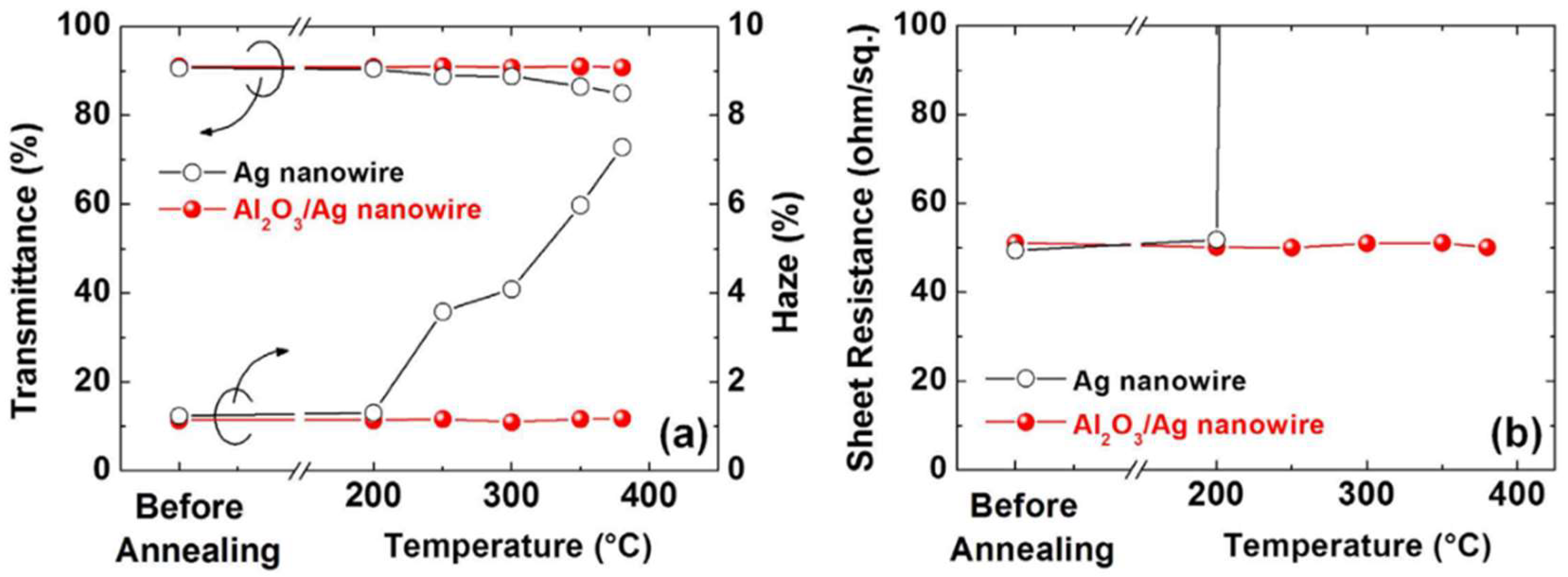
3.4. Thermal Stability
3.5. Tuning of the Optical Properties
4. Conclusion and Future Perspectives
- (1)
- The flexibility of the TEs is becoming increasingly important due to the fast growth of flexible electronics. It is also of great importance for applications of TEs in bio-electronics such as electronic skin. A further requirement of the TEs for biological or biomedical application is the stretchability. Efforts have been made to address this issue, while more studies are needed.
- (2)
- The chemical stability of silver is better than other metals such as copper or nickel that have been used for making TEs. However, TEs made by silver are still sensitive when exposed to air. The current protocol is to cover the silver nanowires with a layer of other materials that can stabilize the TEs. However, deposition of an extra layer such as ZnO usually requires a vacuum process that will limit the production and increase the cost. Improved solutions are expected to solve this problem.
- (3)
- The thermal stability of silver nanowire based TEs has been studied intensively and the results show that the TEs can survive at temperatures higher than 300 °C. Such stability is quite good and can be used in most of the applications.
- (4)
- High transmission of silver nanowire based TEs is aimed for in most of the studies, while few studies focus on the haze property. In most of the applications, high transmission is needed such as display. However, haze is of great importance in applications such as solar cells. There is, on the other hand a lack of methods to tune the transmission and the haze easily to fulfill different requirements.
- (5)
- The conductivity of the silver nanowire based TEs is not a problem as shown by the studies. However, the electrical stability of the TEs has attracted more and more attention because the conductivity of the TEs could reduce over time. Electromigration and Joule heating are the two main reasons. Deposition of an extra layer such as a thin ZnO film could delay the failure although better solutions are expected. Joule heating could be reduced by welding the nanowires to decrease junctions, while simpler methods need to be developed to increase the procedure and reduce the costs.
Author Contributions
Funding
Conflicts of Interest
References
- Kou, P.; Yang, L.; Chang, C.; He, S. Improved Flexible Transparent Conductive Electrodes based on Silver Nanowire Networks by a Simple Sunlight Illumination Approach. Sci. Rep. 2017, 7, 42052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.C.; Chang, Y.C.; Lin, Y.; Chang, S.H.; Chang, W.C.; Li, G.A.; Tuan, H.Y. Spray-Deposited Large-Area Copper Nanowire Transparent Conductive Electrodes and Their Uses for Touch Screen Applications. ACS Appl. Mater. Interfaces 2016, 8, 13009–13017. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Canlier, A.; Kim, G.H.; Choi, J.; Park, M.; Han, S.M. Electrostatic spray deposition of highly transparent silver nanowire electrode on flexible substrate. ACS Appl. Mater. Interfaces 2013, 5, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; Shin, H.A.S.; Kim, T.; Joo, Y.C.; Han, S.M. Highly reliable Ag nanowire flexible transparent electrode with mechanically welded junctions. Small 2014, 10, 3397–3404. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; An, Y.; Lee, H.; Lee, E.; Becker, S.; Kim, Y.-H.; Kim, H. Highly Flexible and Transparent Ag Nanowire Electrode Encapsulated with Ultra-Thin Al2O3: Thermal, Ambient, and Mechanical Stabilities. Sci. Rep. 2017, 7, 41336. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.C.; Kong, D.; Wang, S.; Wang, H.; Welch, A.J.; Wu, H.; Cui, Y. Electrolessly deposited electrospun metal nanowire transparent electrodes. J. Am. Chem. Soc. 2014, 136, 10593–10596. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, R.; Shi, L.; Sun, J. Synthesis of Metal/Bimetal Nanowires and Their Applications as Flexible Transparent Electrodes. Small 2015, 11, 4737–4744. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Jun, L.; Kun, L.; Hu, W.; Ping, C.; Ya-Hui, D.; Zheng, C.; Yun-Fei, L.; Hao-Ran, W.; Yu, D. Inkjet-printed Ag grid combined with Ag nanowires to form a transparent hybrid electrode for organic electronics. Org. Electron. 2017, 41, 179–185. [Google Scholar] [CrossRef]
- Han, J.; Yuan, S.; Liu, L.; Qiu, X.; Gong, H.; Yang, X.; Li, C.; Hao, Y.; Cao, B. Fully indium-free flexible Ag nanowires/ZnO:F composite transparent conductive electrodes with high haze. J. Mater. Chem. A 2015, 3, 5375–5384. [Google Scholar] [CrossRef]
- Sannicolo, T.; Lagrange, M.; Cabos, A.; Celle, C.; Simonato, J.P.; Bellet, D. Metallic Nanowire-Based Transparent Electrodes for Next Generation Flexible Devices: a Review. Small 2016, 12, 6052–6075. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Song, J.; Dong, Y.; Xu, L.; Li, J.; Zeng, H. Nanowire-based transparent conductors for flexible electronics and optoelectronics. Sci. Bull. 2017, 62, 143–156. [Google Scholar] [CrossRef]
- De, S.; King, P.J.; Lyons, P.E.; Khan, U.; Coleman, J.N. Size Effects and the Problem with Percolation in Nanostructured Transparent Conductors. ACS Nano 2010, 4, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Coleman, J.N. The effects of percolation in nanostructured transparent conductors. MRS Bull. 2011, 36, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Lagrange, M.; Langley, D.P.; Giusti, G.; Jiménez, C.; Bréchet, Y.; Bellet, D. Optimization of silver nanowire-based transparent electrodes: effects of density, size and thermal annealing. Nanoscale 2015, 7, 17410–17423. [Google Scholar] [CrossRef] [PubMed]
- Aharony, A.; Stauffer, D. Introduction to percolation theory; Taylor & Francis: London, UK, 1991. [Google Scholar]
- Hu, L.; Hecht, D.S.; Grüner, G. Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hecht, D.S.; Grüner, G. Percolation in Transparent and Conducting Carbon Nanotube Networks. Nano Lett. 2004, 4, 2513–2517. [Google Scholar] [CrossRef] [Green Version]
- Mutiso, R.M.; Sherrott, M.C.; Rathmell, A.R.; Wiley, B.J.; Winey, K.I. Integrating Simulations and Experiments To Predict Sheet Resistance and Optical Transmittance in Nanowire Films for Transparent Conductors. ACS Nano 2013, 7, 7654–7663. [Google Scholar] [CrossRef] [PubMed]
- Khanarian, G.; Joo, J.; Liu, X.-Q.; Eastman, P.; Werner, D.; O’Connell, K.; Trefonas, P. The optical and electrical properties of silver nanowire mesh films. J. Appl. Phys. 2013, 114, 024302. [Google Scholar] [CrossRef]
- Van de Hulst, H.C. Light Scattering by Small Particles; Dover Publications, Inc.: New York, NY, USA, 1957; ISBN 0-486-64228-3. [Google Scholar]
- Meeten, G.H. Transparency, translucency and gloss. In Optical Properties Of Polymers; Elsevier: London, UK, 1986. [Google Scholar]
- Preston, C.; Xu, Y.; Han, X.; Munday, J.N.; Hu, L. Optical haze of transparent and conductive silver nanowire films. Nano Res. 2013, 6, 461–468. [Google Scholar] [CrossRef]
- Deignan, G.; Goldthorpe, I.A. The dependence of silver nanowire stability on network composition and processing parameters. RSC Adv. 2017, 7, 35590–35597. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Sun, H.; Dai, S.; Wang, Y.; Zhu, J. Electrical Breakdown of Nanowires. Nano Lett. 2011, 11, 4647–4651. [Google Scholar] [CrossRef] [PubMed]
- Khaligh, H.H.; Goldthorpe, I.A. Failure of silver nanowire transparent electrodes under current flow. Nanoscale Res. Lett. 2013, 8, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Nguyen, V.H.; Muñoz-Rojas, D.; Aghazadehchors, S.; Jiménez, C.; Nguyen, N.D.; Bellet, D. Stability Enhancement of Silver Nanowire Networks with Conformal ZnO Coatings Deposited by Atmospheric Pressure Spatial Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 19208–19217. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, F.; Tong, K.; Saldanha, G.; Liu, C.; Pei, Q. Mitigation of Electrical Failure of Silver Nanowires under Current Flow and the Application for Long Lifetime Organic Light-Emitting Diodes. Adv. Electron. Mater. 2016, 2, 1600167. [Google Scholar] [CrossRef]
- Sannicolo, T.; Muñoz-Rojas, D.; Nguyen, N.D.; Moreau, S.; Celle, C.; Simonato, J.-P.; Bréchet, Y.; Bellet, D. Direct Imaging of the Onset of Electrical Conduction in Silver Nanowire Networks by Infrared Thermography: Evidence of Geometrical Quantized Percolation. Nano Lett. 2016, 16, 7046–7053. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L. On The Instability Of Jets. Proc. London Math. Soc. 1878, 1, 4–13. [Google Scholar] [CrossRef]
- Hummelgård, M.; Zhang, R.Y.; Nilsson, H.-E.; Olin, H. Electrical sintering of silver nanoparticle ink studied by in-situ TEM probing. PLoS ONE 2011, 6, e17209. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, D.; Cheng, J.; Liu, J.; Mao, J.; Choy, W.C.H. Locally Welded Silver Nano-Network Transparent Electrodes with High Operational Stability by a Simple Alcohol-Based Chemical Approach. Adv. Funct. Mater. 2015, 25, 4211–4218. [Google Scholar] [CrossRef]
- Song, T.-B.; Chen, Y.; Chung, C.-H.; Yang, Y.M.; Bob, B.; Duan, H.-S.; Li, G.; Tu, K.-N.; Huang, Y.; Yang, Y. Nanoscale Joule Heating and Electromigration Enhanced Ripening of Silver Nanowire Contacts. ACS Nano 2014, 8, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Spechler, J.A.; Nagamatsu, K.A.; Sturm, J.C.; Arnold, C.B. Improved Efficiency of Hybrid Organic Photovoltaics by Pulsed Laser Sintering of Silver Nanowire Network Transparent Electrode. ACS Appl. Mater. Interfaces 2015, 7, 10556–10562. [Google Scholar] [CrossRef] [PubMed]
- Mayousse, C.; Celle, C.; Fraczkiewicz, A.; Simonato, J.-P. Stability of silver nanowire based electrodes under environmental and electrical stresses. Nanoscale 2015, 7, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Z.; Hu, A.; Zhang, T.; Oakes, K.D. Direct Writing on Paper of Foldable Capacitive Touch Pads with Silver Nanowire Inks. ACS Appl. Mater. Interfaces 2014, 6, 21721–21729. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hwang, G.-T.; Kim, S.; Seo, J.; Park, H.-J.; Yu, K.; Kim, T.-S.; Lee, K.J. Flash-Induced Self-Limited Plasmonic Welding of Silver Nanowire Network for Transparent Flexible Energy Harvester. Adv. Mater. 2017, 29, 1603473. [Google Scholar] [CrossRef] [PubMed]
- Garnett, E.C.; Cai, W.; Cha, J.J.; Mahmood, F.; Connor, S.T.; Greyson Christoforo, M.; Cui, Y.; McGehee, M.D.; Brongersma, M.L. Self-limited plasmonic welding of silver nanowire junctions. Nat. Mater. 2012, 11, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gao, Y.; Hu, B.; Li, J.; Su, J.; Fan, Z.; Zhou, J. Transferable self-welding silver nanowire network as high performance transparent flexible electrode. Nanotechnology 2013, 24, 335202. [Google Scholar] [CrossRef] [PubMed]
- Cheong, H.-G.; Triambulo, R.E.; Lee, G.-H.; Yi, I.-S.; Park, J.-W. Silver Nanowire Network Transparent Electrodes with Highly Enhanced Flexibility by Welding for Application in Flexible Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2014, 6, 7846–7855. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Gao, H.; Wang, Y.; Liu, Q.; Huang, S.; Guo, C.F.; Ren, Z. Capillary-Force-Induced Cold Welding in Silver-Nanowire-Based Flexible Transparent Electrodes. Nano Lett. 2017, 17, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Liu, H.; Chen, Y.; Zheng, M.; Zhao, Y.; Kong, X.; Wang, Y.; Zhang, X.; Kong, X.; Wang, P.; Jiang, L. Highly Conductive, Air-Stable Silver Nanowire@Iongel Composite Films toward Flexible Transparent Electrodes. Adv. Mater. 2016, 28, 7167–7172. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Lee, D.; Yoo, J.S.; Lee, S.; Jung, H.S. Effective passivation of Ag nanowire-based flexible transparent conducting electrode by TiO2 nanoshell. Nano Converg. 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jun, S.; Ju, B.-K.; Kim, J.-W. Heterogeneous Configuration of a Ag Nanowire/Polymer Composite Structure for Selectively Stretchable Transparent Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 7505–7514. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Yu, K.C.; Kim, Y.; Kim, J.W. Highly Stretchable and Mechanically Stable Transparent Electrode Based on Composite of Silver Nanowires and Polyurethane-Urea. ACS Appl. Mater. Interfaces 2015, 7, 15214–15222. [Google Scholar] [CrossRef] [PubMed]
- Madaria, A.R.; Kumar, A.; Ishikawa, F.N.; Zhou, C. Uniform, Highly Conductive, and Patterned Transparent Films of a Percolating Silver Nanowire Network on Rigid and Flexible Substrates Using a Dry Transfer Technique. Nano Res 2010, 3, 564–573. [Google Scholar] [CrossRef]
- Kim, A.; Won, Y.; Woo, K.; Kim, C.H.; Moon, J. Highly transparent low resistance ZnO/Ag nanowire/ZnO composite electrode for thin film solar cells. ACS Nano 2013, 7, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Göbelt, M.; Keding, R.; Schmitt, S.W.; Hoffmann, B.; Jäckle, S.; Latzel, M.; Radmilović, V.V.; Radmilović, V.R.; Spiecker, E.; Christiansen, S. Encapsulation of silver nanowire networks by atomic layer deposition for indium-free transparent electrodes. Nano Energy 2015, 16, 196–206. [Google Scholar] [CrossRef]
- Song, T.B.; Rim, Y.S.; Liu, F.; Bob, B.; Ye, S.; Hsieh, Y.T.; Yang, Y. Highly Robust Silver Nanowire Network for Transparent Electrode. ACS Appl. Mater. Interfaces 2015, 7, 24601–24607. [Google Scholar] [CrossRef] [PubMed]
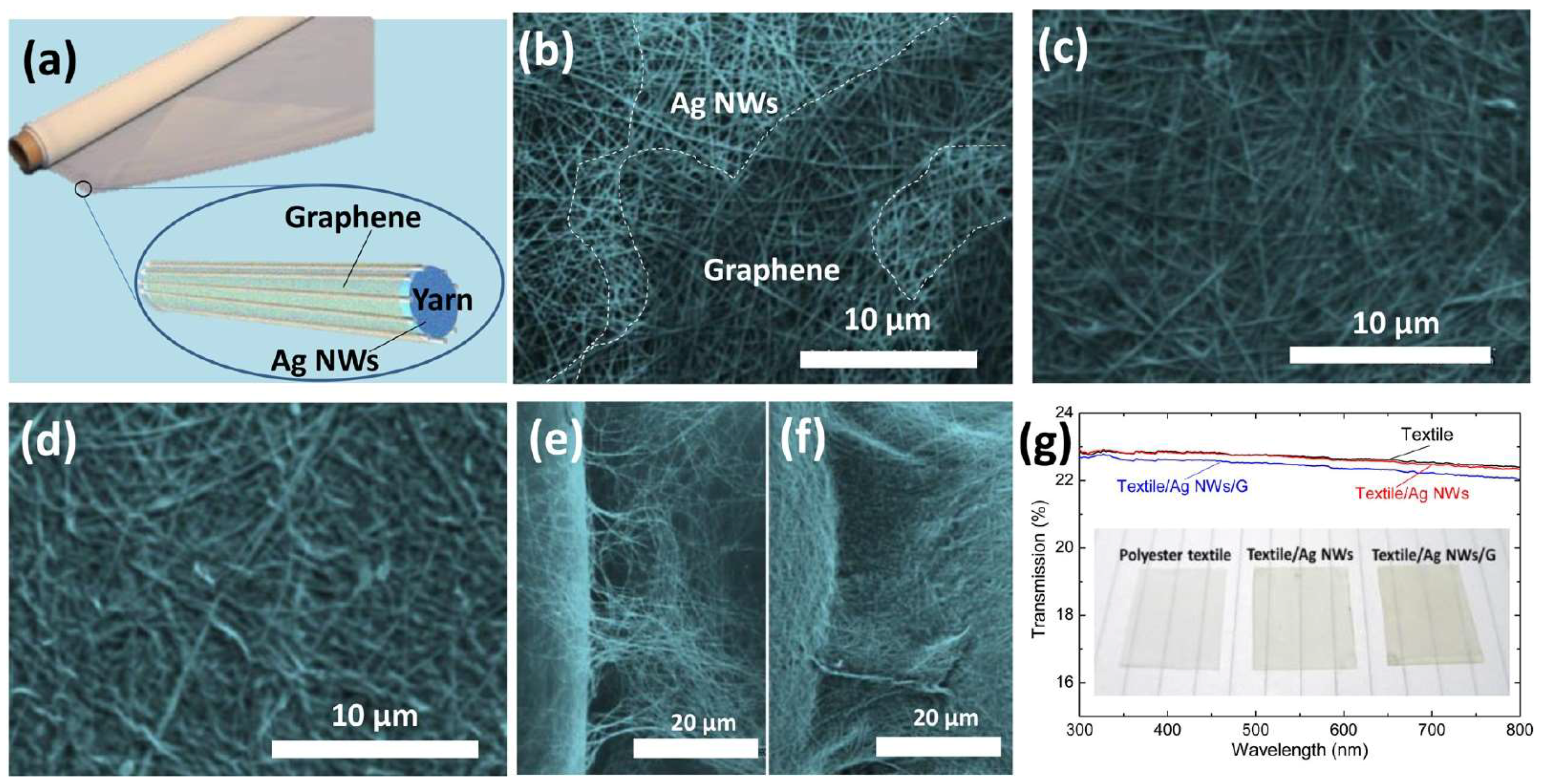
- Wu, C.; Kim, T.W.; Li, F.; Guo, T. Wearable Electricity Generators Fabricated Utilizing Transparent Electronic Textiles Based on Polyester/Ag Nanowires/Graphene Core-Shell Nanocomposites. ACS Nano 2016, 10, 6449–6457. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Song, M.; Kim, D.-H.; Cho, B.; Lee, H.M.; Kwon, J.-D.; Park, S.-G.; Nam, K.-S.; Jeong, Y.; Kwon, S.-H.; et al. Ultrasmooth, extremely deformable and shape recoverable Ag nanowire embedded transparent electrode. Sci. Rep. 2014, 4, 4788. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, K.; Cheng, Y.; Pei, Q.; Xu, Y.; Xiao, F. Removable Large-Area Ultrasmooth Silver Nanowire Transparent Composite Electrode. ACS Appl. Mater. Interfaces 2017, 9, 4733–4741. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Dong, D.; Yang, S.; Wei, B.; He, G. Highly Conductive and Uniform Alginate/Silver Nanowire Composite Transparent Electrode by Room Temperature Solution Processing for Organic Light Emitting Diode. ACS Appl. Mater. Interfaces 2017, 9, 11811–11818. [Google Scholar] [CrossRef] [PubMed]
- He, X.; He, R.; Liu, A.; Chen, X.; Zhao, Z.; Feng, S.; Chen, N.; Zhang, M. A highly conductive, flexible, transparent composite electrode based on the lamination of silver nanowires and polyvinyl alcohol. J. Mater. Chem. C 2014, 2, 9737–9745. [Google Scholar] [CrossRef]
- Kim, B.S.; Pyo, J.B.; Son, J.G.; Zi, G.; Lee, S.-S.; Park, J.H.; Lee, J. Biaxial Stretchability and Transparency of Ag Nanowire 2D Mass-Spring Networks Prepared by Floating Compression. ACS Appl. Mater. Interfaces 2017, 9, 10865–10873. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Hong, S.; Cho, H.M.; Lee, J.; Suh, Y.D.; Ham, J.; Ko, S.H. Highly Sensitive and Stretchable Multidimensional Strain Sensor with Prestrained Anisotropic Metal Nanowire Percolation Networks. Nano Lett. 2015, 15, 5240–5247. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Park, K.H.; Han, C.J.; Oh, M.S.; You, B.; Kim, Y.-S.; Kim, J.-W. Crack-induced Ag nanowire networks for transparent, stretchable, and highly sensitive strain sensors. Sci. Rep. 2017, 7, 7959. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Kim, Y.; Ju, B.-K.; Kim, J.-W. Highly Stretchable and Waterproof Electroluminescence Device Based on Superstable Stretchable Transparent Electrode. ACS Appl. Mater. Interfaces 2017, 9, 5486–5494. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, L.; Niu, X.; Yu, Z.; Pei, Q. Elastomeric polymer light-emitting devices and displays. Nat. Photonics 2013, 7, 817–824. [Google Scholar] [CrossRef]
- Chen, D.; Liang, J.; Liu, C.; Saldanha, G.; Zhao, F.; Tong, K.; Liu, J.; Pei, Q. Thermally Stable Silver Nanowire-Polyimide Transparent Electrode Based on Atomic Layer Deposition of Zinc Oxide on Silver Nanowires. Adv. Funct. Mater. 2015, 25, 7512–7520. [Google Scholar] [CrossRef]
- Bellet, D.; Lagrange, M.; Sannicolo, T.; Aghazadehchors, S.; Nguyen, V.H.; Langley, D.P.; Muñoz-Rojas, D.; Jiménez, C.; Bréchet, Y.; Nguyen, N.D. Transparent Electrodes Based on Silver Nanowire Networks: From Physical Considerations towards Device Integration. Materials 2017, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Im, H.-G.; Jin, J.; Ko, J.-H.; Lee, J.; Lee, J.-Y.; Bae, B.-S. Flexible transparent conducting composite films using a monolithically embedded AgNW electrode with robust performance stability. Nanoscale 2014, 6, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, Q.; Li, L.; Chen, Q.; Niu, X.; Liu, J.; Pei, Q. Highly Flexible Silver Nanowire Electrodes for Shape-Memory Polymer Light-Emitting Diodes. Adv. Mater. 2011, 23, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, S.; Araki, T.; Jiu, J.; Sugahara, T.; Wang, J.; Vanfleteren, J.; Sekitani, T.; Suganuma, K. Facile fabrication of stretchable Ag nanowire/polyurethane electrodes using high intensity pulsed light. Nano Res. 2016, 9, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Jiu, J.; Wang, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; Suganuma, K.; Hara, M.; Nakazawa, E.; Uchida, H. The effect of light and humidity on the stability of silver nanowire transparent electrodes. RSC Adv. 2015, 5, 27657–27664. [Google Scholar] [CrossRef]
- Wang, J.; Jiu, J.; Sugahara, T.; Nagao, S.; Nogi, M.; Koga, H.; He, P.; Suganuma, K.; Uchida, H. Highly Reliable Silver Nanowire Transparent Electrode Employing Selectively Patterned Barrier Shaped by Self-Masked Photolithography. ACS Appl. Mater. Interfaces 2015, 7, 23297–23304. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Rana, T.R.; Kim, S.; Kim, K.; Yun, J.H.; Kim, J. Silver Nanowires Binding with Sputtered ZnO to Fabricate Highly Conductive and Thermally Stable Transparent Electrode for Solar Cell Applications. ACS Appl. Mater. Interfaces 2016, 8, 12764–12771. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.T.; Kwon, S.-Y.; Park, H.S.; Kim, S.Y. Negative Thermal Expansion of Ultrathin Metal Nanowires: A Computational Study. Nano Lett. 2017, 17, 5113–5118. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.-S.; Dong, H.; Maric, R.; Pothukuchi, S.; Hunt, A.; Li, Y.; Wong, C.P. Thermal Behavior of Silver Nanoparticles for Low-Temperature Interconnect Applications. J. Electron. Mater. 2005, 34, 168–175. [Google Scholar] [CrossRef]
- Nanda, K.K.; Maisels, A.; Kruis, F.E.; Fissan, H.; Stappert, S. Higher Surface Energy of Free Nanoparticles. Phys. Rev. Lett. 2003, 91, 106102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Hummelgård, M.; Olin, H. Single layer porous gold films grown at different temperatures. Phys. B Condens. Matter 2010, 405, 4517–4522. [Google Scholar] [CrossRef]
- Yeh, M.-H.; Chen, P.-H.; Yang, Y.-C.; Chen, G.-H.; Chen, H.-S. Investigation of Ag-TiO2 Interfacial Reaction of Highly Stable Ag Nanowire Transparent Conductive Film with Conformal TiO2 Coating by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 10788–10797. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhu, H.; Yuan, Y.; Ha, D.; Zhu, S.; Preston, C.; Chen, Q.; Li, Y.; Han, X.; Lee, S.; et al. Novel Nanostructured Paper with Ultrahigh Transparency and Ultrahigh Haze for Solar Cells. Nano Lett. 2014, 14, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Rathmell, A.R.; Chen, Z.; Stewart, I.E.; Wiley, B.J. Metal Nanowire Networks: The Next Generation of Transparent Conductors. Adv. Mater. 2014, 26, 6670–6687. [Google Scholar] [CrossRef] [PubMed]
- Menamparambath, M.M.; Yang, K.; Kim, H.H.; Bae, O.S.; Jeong, M.S.; Choi, J.-Y.; Baik, S. Reduced haze of transparent conductive films by smaller diameter silver nanowires. Nanotechnology 2016, 27, 465706. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Jiu, J.; Nogi, M.; Koga, H.; Nagao, S.; Sugahara, T.; Suganuma, K. Low haze transparent electrodes and highly conducting air dried films with ultra-long silver nanowires synthesized by one-step polyol method. Nano Res. 2014, 7, 236–245. [Google Scholar] [CrossRef]
- Kim, T.; Canlier, A.; Cho, C.; Rozyyev, V.; Lee, J.Y.; Han, S.M. Highly transparent Au-coated ag nanowire transparent electrode with reduction in haze. ACS Appl. Mater. Interfaces 2014, 6, 13527–13534. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Engholm, M. Recent Progress on the Fabrication and Properties of Silver Nanowire-Based Transparent Electrodes. Nanomaterials 2018, 8, 628. https://doi.org/10.3390/nano8080628
Zhang R, Engholm M. Recent Progress on the Fabrication and Properties of Silver Nanowire-Based Transparent Electrodes. Nanomaterials. 2018; 8(8):628. https://doi.org/10.3390/nano8080628
Chicago/Turabian StyleZhang, Renyun, and Magnus Engholm. 2018. "Recent Progress on the Fabrication and Properties of Silver Nanowire-Based Transparent Electrodes" Nanomaterials 8, no. 8: 628. https://doi.org/10.3390/nano8080628
APA StyleZhang, R., & Engholm, M. (2018). Recent Progress on the Fabrication and Properties of Silver Nanowire-Based Transparent Electrodes. Nanomaterials, 8(8), 628. https://doi.org/10.3390/nano8080628




