Fabrication and Characterization of a Highly-Sensitive Surface-Enhanced Raman Scattering Nanosensor for Detecting Glucose in Urine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
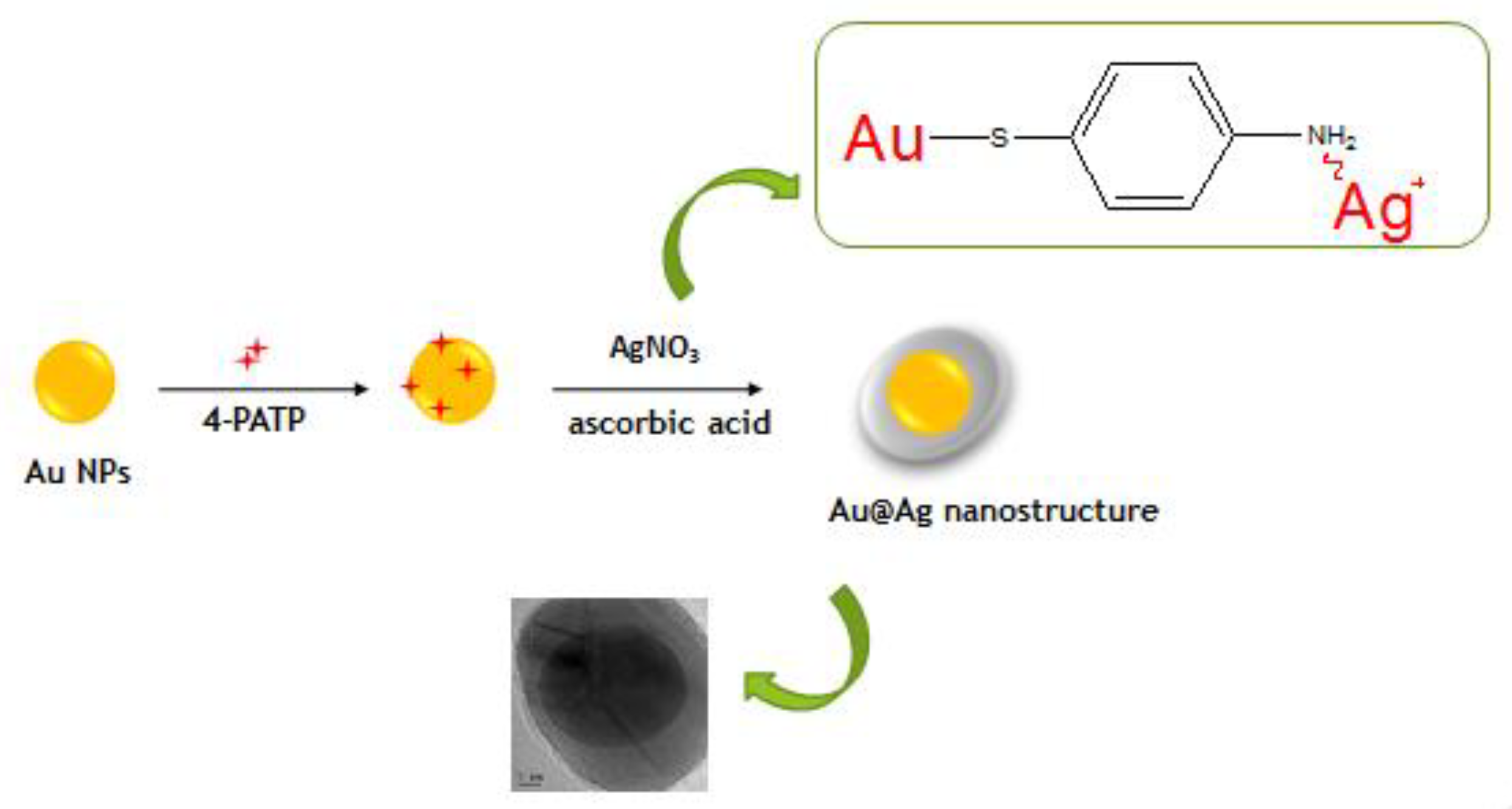
2.2. Synthesis of Au NPs
2.3. Synthesis of Core-Shell NPs
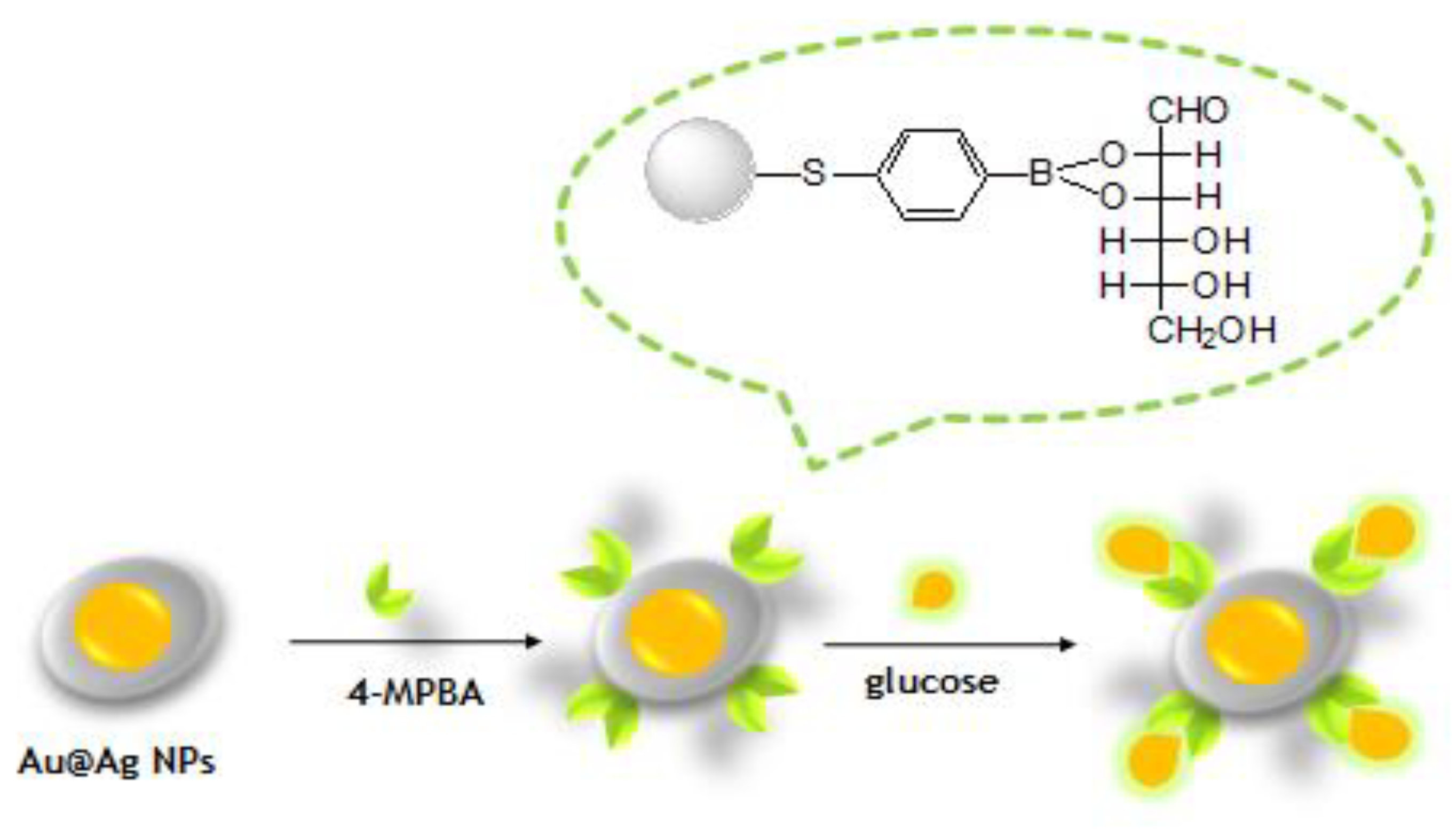
2.4. Functionalization of NPs by 4-MPBA
2.5. NPs Characterization
2.5.1. Transmission Electron Microscopy (TEM)
2.5.2. UV-Vis Spectrometer
2.5.3. Raman Measurement
2.5.4. EDX Analysis
3. Results and Discussion
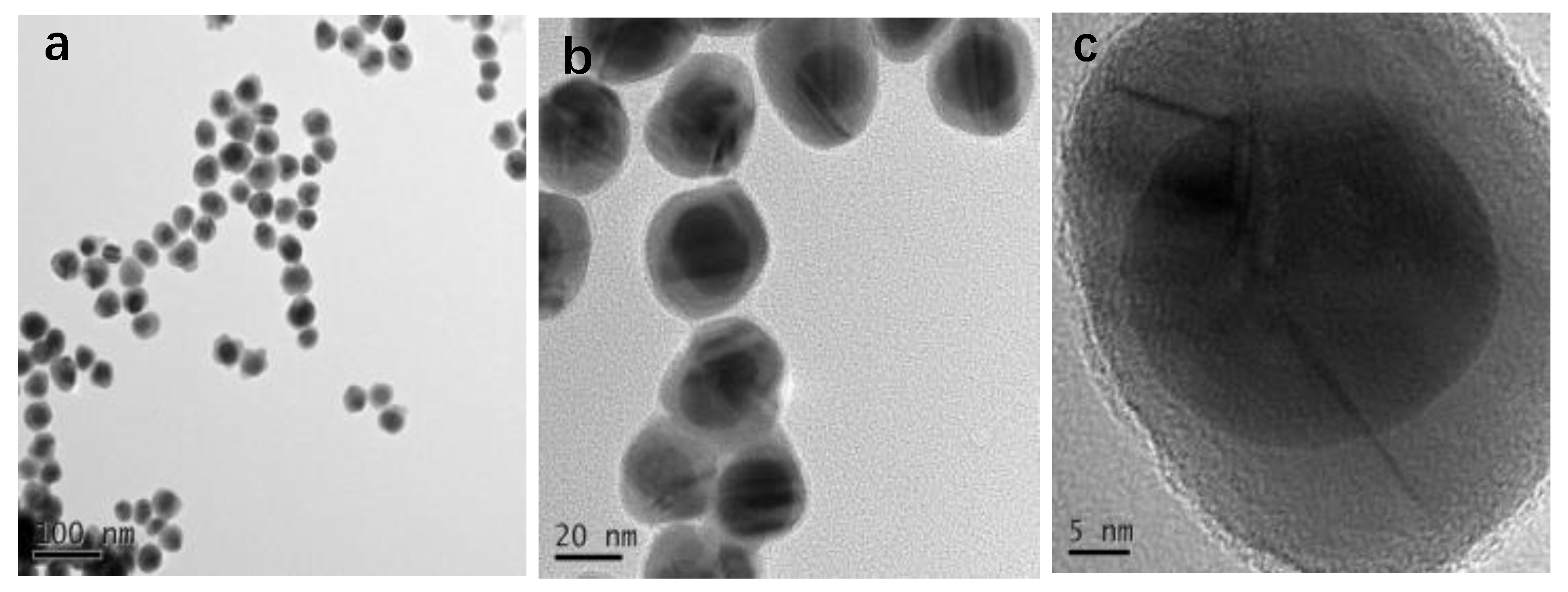
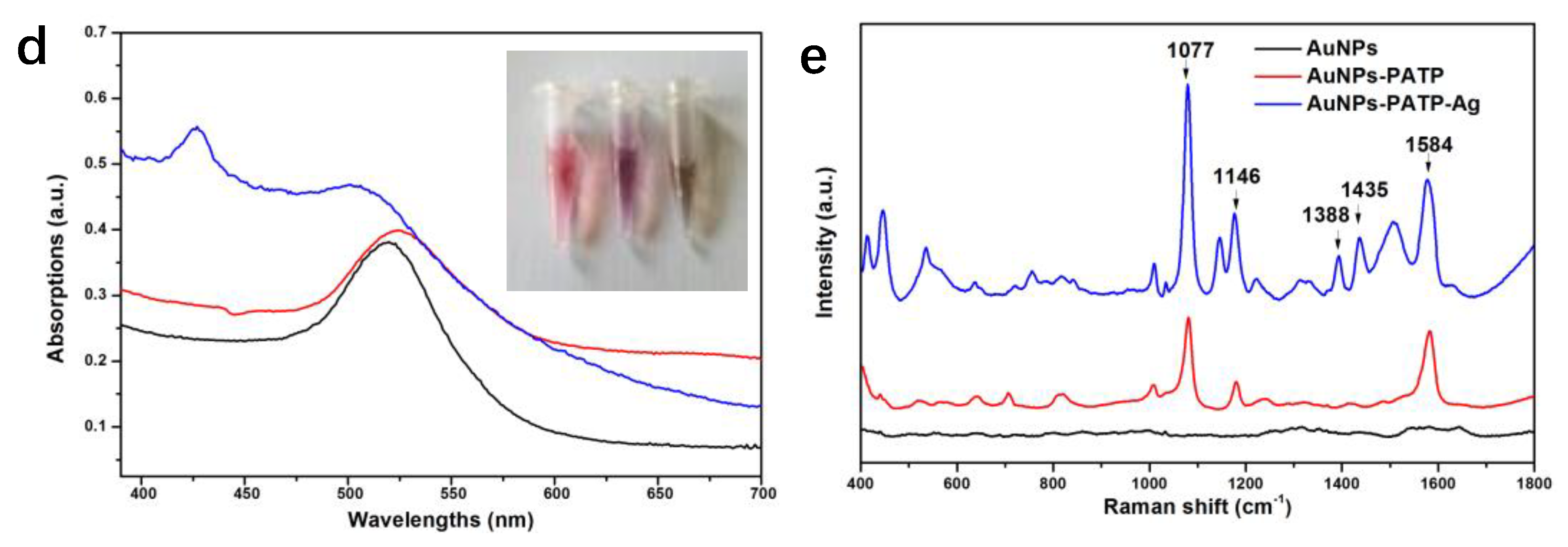
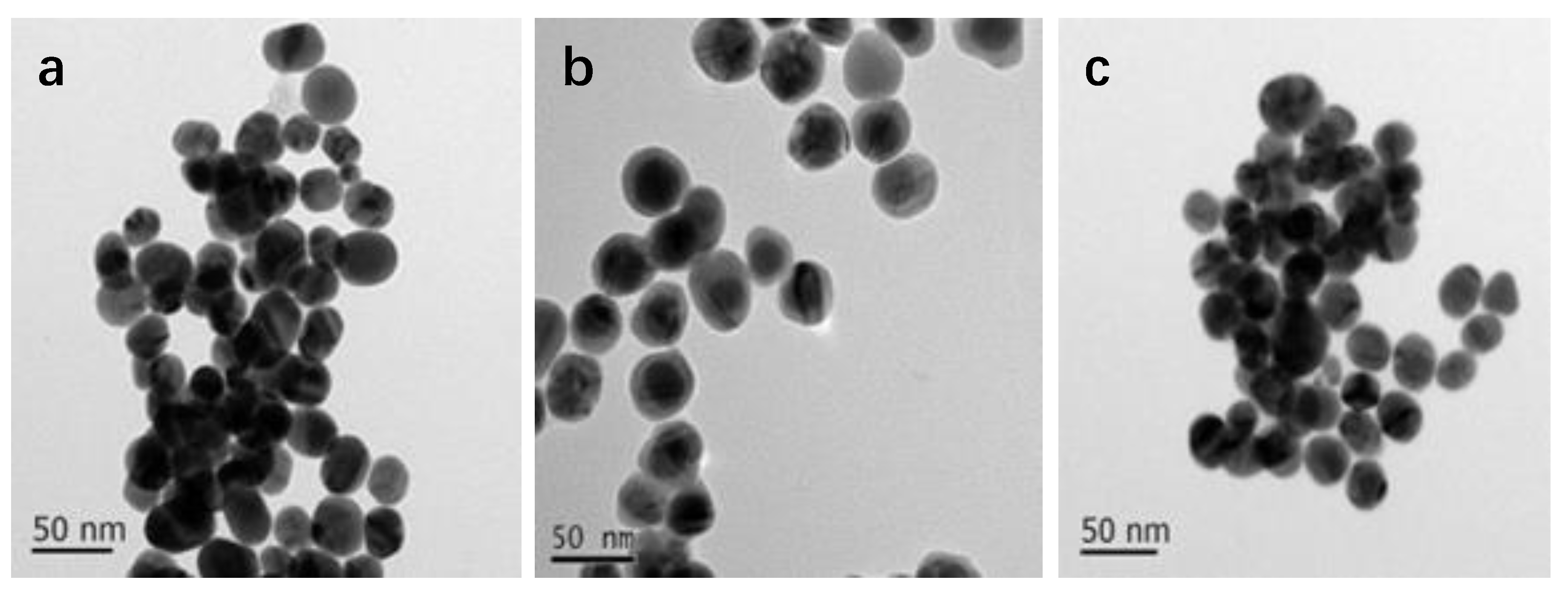
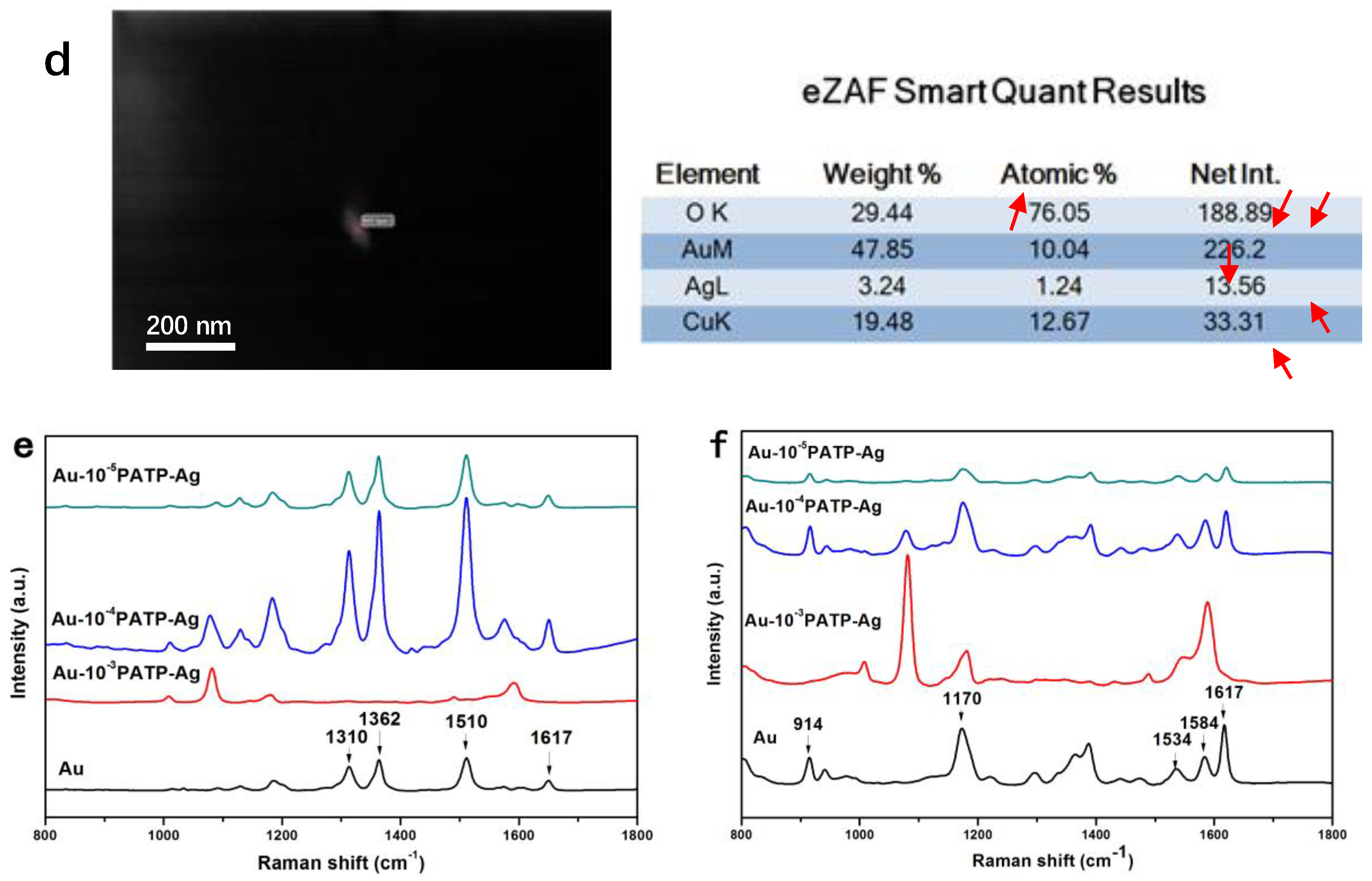
3.1. NPs Characterization
3.2. SERS Analysis of NPs Modified with Different Concentrations of 4-PATP
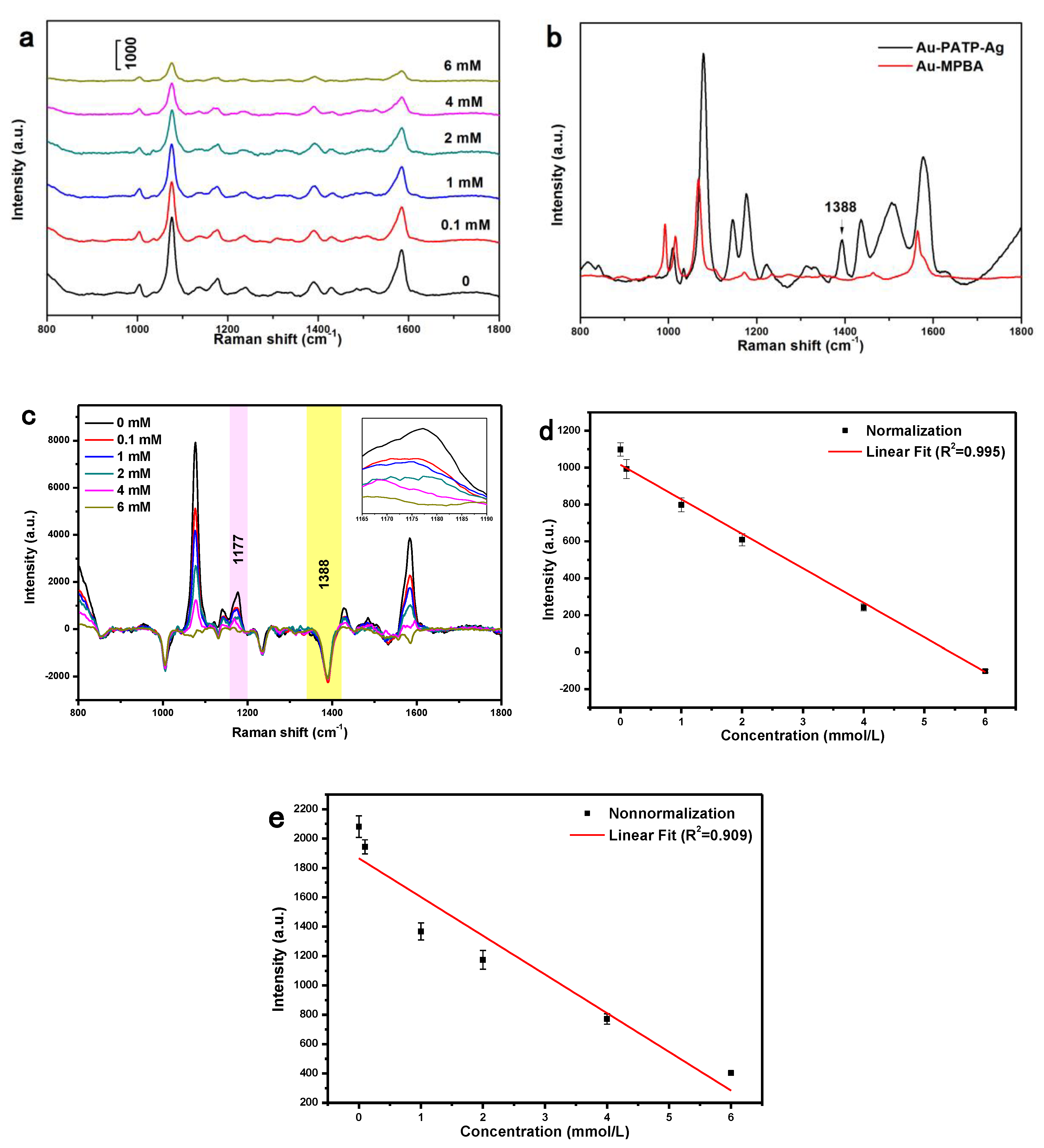
3.3. Detection of Glucose by SERS
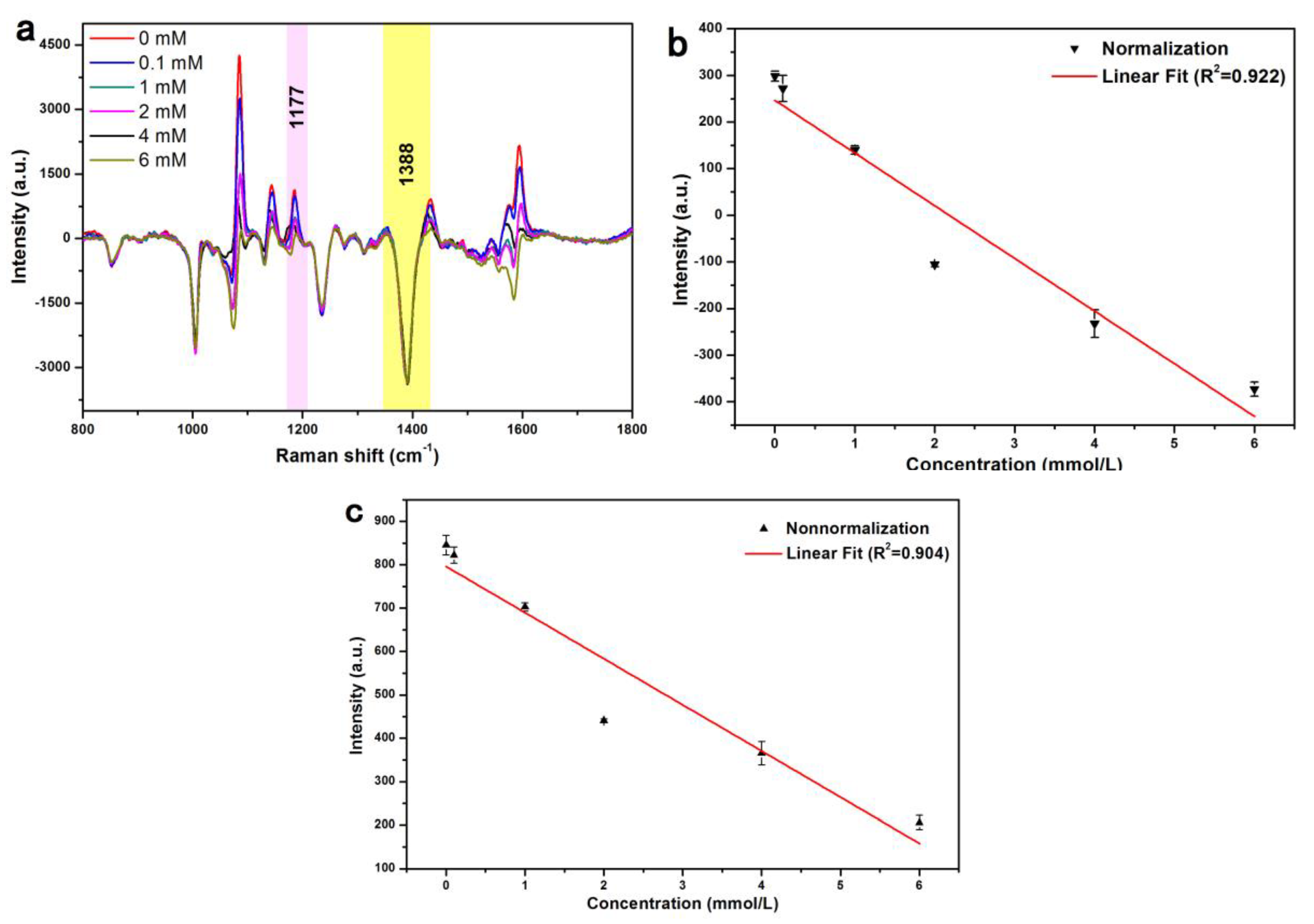
3.4. Detection of Glucose in Urine
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Yo.; Stevens, G.A.; et al. National, Regional, and Global Trends in Fasting Plasma Glucose and Diabetes Prevalence since 1980: Systematic Analysis of Health Examination Surveys and Epidemiological Studies with 370 Country-Years and 2·7 Million Participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Liakat, S.; Bors, K.A.; Xu, L.; Woods, C.M.; Doyle, J.; Gmachl, C.F. Noninvasive in vivo Glucose Sensing on Human Subjects Using Mid-Infrared Light. Biomed. Opt. Express 2014, 5, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Radhakumary, C.; Sreenivasan, K. Naked Eye Detection of Glucose in Urine Using Glucose Oxidase Immobilized Gold Nanoparticles. Anal. Chem. 2011, 83, 2829–2833. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Du, X.; Jiang, J.; Huang, X. Facile and Sensitive Glucose Sandwich Assay Using In Situ-Generated Raman Reporters. Anal. Chem. 2015, 87, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.Q.; Tabatabaei, M.; Zuin, M.S.; Workentin, M.S.; Lagugné-Labarthet, F. A Nanoaggregate-on-Mirror Platform for Molecular and Biomolecular Detection by Surface-Enhanced Raman Spectroscopy. Anal. Bioanal. Chem. 2016, 408, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent Advances in Electrochemical Glucose Biosensors: A Review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Yi, Y.; Deng, J.; Zhang, Y.; Li, H.; Yao, S. Label-Free Si Quantum Dots as Photoluminescence Probes for Glucose Detection. Chem. Commun. 2013, 49, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.L.; Liu, Y.-H.; Deng, H.-H.; Hong, G.-L.; Liu, A.-L.; Lin, X.-H.; Xia, X.-H.; Chen, W. Fluorescent Hydrogen Peroxide Sensor Based on Cupric Oxide Nanoparticles and Its Application for Glucose and L-Lactate Detection. Biosens. Bioelectron. 2014, 61, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Nacht, B.; Larndorfer, C.; Sax, S.; Borisov, S.M.; Hajnsek, M.; Sinner, F.; List-Kratochvil, E.J.W.; Klimant, I. Integrated catheter system for continuous glucose measurement and simultaneous insulin infusion. Biosens. Bioelectron. 2015, 64, 102–110. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xu, X.; Wu, H.; Jin, Y. Enzymatic Plasmonic Engineering of Ag/Au Bimetallic Nanoshells and Their Use for Sensitive Optical Glucose Sensing. Adv. Mater. 2012, 24, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ringstrand, B.S.; Stone, D.A.; Firestone, M.A. Electrochemical Activity of Glucose Oxidase on a Poly(ionic liquid)–Au Nanoparticle Composite. ACS Appl. Mater. Interfaces 2012, 4, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Boonyasit, Y.; Laiwattanapaisal, W.; Chailapakul, O.; Emnéus, J.; Heiskanen, A.R. Boronate-Modified Interdigitated Electrode Array for Selective Impedance-Based Sensing of Glycated Hemoglobin. Anal. Chem. 2016, 88, 9582–9589. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.-Y.; Choi, Y.-B.; Kim, H.-H. Disposable Non-Enzymatic Glucose Sensors Using Screen-Printed Nickel/Carbon Composites on Indium Tin Oxide Electrodes. Sensors 2015, 15, 31083–31091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghayeri, M.; Amiri, A.; Farhadi, S. Development of Non-Enzymatic Glucose Sensor Based on Efficient Loading Ag Nanoparticles on Functionalized Carbon Nanotubes. Sens. Actuators B 2016, 225, 354–362. [Google Scholar] [CrossRef]
- Karim, M.N.; Anderson, S.R.; Singh, S.; Ramanathan, R.; Bansal, V. Nanostructured silver fabric as a free-standing NanoZyme for colorimetric detection of glucose in urine. Biosens. Bioelectron. 2018, 110, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Qi, G.; Xu, S.; Xu, W. Construction of highly sensitive surface-enhanced Raman scattering (SERS) nanosensor aimed for the testing of glucose in urine. RSC Adv. 2016, 6, 53800–53803. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, Y.; Zhang, W.; Ye, S.; Zhang, H.; Xie, F.; Gong, L.; Wei, Z.; Jin, H.; Chen, J. Highly sensitive detection of glucose: A quantitative approach employing nanorods assembled plasmonic substrate. Talanta 2017, 165, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Panneerselvam, R.; Tian, Z.-Q. Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Haynes, C.L.; McFarland, A.D.; van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2005, 77, 338A–346A. [Google Scholar] [CrossRef]
- Shafer-Peltier, K.E.; Haynes, C.L.; Glucksberg, M.R.; van Duyne, R.P. Toward a Glucose Biosensor Based on Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2003, 125, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Yonzon, C.R.; Haynes, C.L.; Zhang, X.; Walsh, J.T.; van Duyne, R.P. A Glucose Biosensor Based on Surface-Enhanced Raman Scattering: Improved Partition Layer, Temporal Stability, Reversibility, and Resistance to Serum Protein Interference. Anal. Chem. 2004, 76, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kaminský, J.; Kapitán, J.; Baumruk, V.; Bednárová, L.; Bour, P. Interpretation of Raman and Raman Optical Activity Spectra of a Flexible Sugar Derivative, the Gluconic Acid Anion. J. Phys. Chem. A 2009, 113, 3594–3601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.C.; Jin, R.; Mirkin, C.A. Nanoparticles with Raman Spectroscopic Fingerprints for DNA and RNA Detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Küstner, B.; Gellner, M.; Schütz, M.; Schöppler, F.; Marx, A.; Ströbel, P.; Adam, P.; Schmuck, C.; Schlücker, S. SERS Labels for Red Laser Excitation: Silica-Encapsulated SAMs on Tunable Gold/Silver Nanoshells. Angew. Chem. Int. Ed. 2009, 48, 1950–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zong, S.; Li, W.; Wang, C.; Xu, S.; Chen, H.; Cui, Y. SERS-Fluorescence Joint Spectral Encoding Using Organic–Metal–QD Hybrid Nanoparticles with a Huge Encoding Capacity for High-Throughput Biodetection: Putting Theory into Practice. J. Am. Chem. Soc. 2012, 134, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Eggert, H.; Frederiksen, J.; Morin, C.; Norrild, J.C. A New Glucose-Selective Fluorescent Bisboronic Acid. First Report of Strong α-Furanose Complexation in Aqueous Solution at Physiological pH. J. Org. Chem. 1999, 64, 3846–3852. [Google Scholar] [CrossRef]
- Yang, W.; He, H.; Drueckhammer, D.G. Computer-Guided Design in Molecular Recognition: Design and Synthesis of a Glucopyranose Receptor. Angew. Chem. Int. Ed. 2001, 40, 1714–1718. [Google Scholar] [CrossRef]
- Kong, K.V.; Lam, Z.; Lau, W.K.O.; Leong, W.K.; Olivo, M. A Transition Metal Carbonyl Probe for Use in a Highly Specific and Sensitive SERS-Based Assay for Glucose. J. Am. Chem. Soc. 2013, 135, 18028–18031. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.V.; Ho, C.J.H.; Gong, T.; Lau, W.K.O.; Olivo, M. Sensitive SERS Glucose Sensing in Biological Media Using Alkyne Functionalized Boronic Acid on Planar Substrates. Biosens. Bioelectron. 2014, 56, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Wang, M. A Novel SERS-Active Tag Based on Bimetallic Flowerlike Au-Ag Nanoparticles. Curr. Nanosci. 2011, 7, 969–978. [Google Scholar] [CrossRef]
- Kruss, S.; Srot, V.; van Aken, P.A.; Spatz, J.P. Au–Ag Hybrid Nanoparticle Patterns of Tunable Size and Density on Glass and Polymeric Supports. Langmuir 2012, 28, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Wang, L.; Shen, A.; Hu, J. Simultaneous enzymatic and SERS properties of bifunctional chitosanmodified popcorn-like Au-Ag nanoparticles for high sensitive detection of melamine in milk powder. Talanta 2015, 140, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, P.; Muhammed, M.A.H.; Alsaiari, S.K.; Moosa, B.; Almalik, A.; Kumar, A.; Ringe, E.; Khashab, N.M. Tunable and Linker Free Nanogaps in Core–Shell Plasmonic Nanorods for Selective and Quantitative Detection of Circulating Tumor Cells by SERS. ACS Appl. Mater. Interfaces 2017, 9, 37597–37605. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, T.; Wang, L.; Dong, S. Surface-enhanced Raman scattering of 4-aminothiophenol self-assembled monolayers in sandwich structure with nanoparticle shape dependence: Off-surface plasmon resonance condition. J. Phys. Chem. C 2007, 111, 6962–6969. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Khanadeev, V.; Khlebtsov, N. Surface-enhanced Raman scattering inside Au@Ag core/shell nanorods. Nano Research 2016, 9, 2303–2318. [Google Scholar] [CrossRef]
- Xu, J.-F.; Luo, S.-Y.; Liu, G.-K. Different Behaviors in the Transformation of PATP Adsorbed on Ag or Au Nanoparticles Investigated by Surface-Enhanced Raman spectroscopy – a Study of the Effects from Laser Energy and Annealing. Spectrochim. Acta, Part A 2005, 143, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wiley, B.; Li, Z.-Y.; Xia, Y. Synthesis and Optical Properties of Nanorattles and Multiple-Walled Nanoshells/Nanotubes Made of Metal Alloys. J. Am. Chem. Soc. 2004, 126, 9399–9406. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Wang, Y.; Zhang, X.; Wang, X.; Cheng, W.; Su, H.; Wang, X.; Sun, X.; Han, X.X.; Zhao, B.; Ozaki, Y. Ultrasensitive Detection of Thyrotropin-Releasing Hormone Based on Azo Coupling and Surface-Enhanced Resonance Raman Spectroscopy. Analyst 2016, 141, 5181–5188. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lin, L.; Li, Z.; Zhang, Z.; Feng, J. Novel Ag-Cu substrates for surface-enhanced Raman scattering. Materials Letters 2009, 63, 2306–2308. [Google Scholar] [CrossRef]
- Chen, X.; Nguyen, T.H.D.; Gu, L.; Lin, M. Use of Standing Gold Nanorods for Detection of Malachite Green and Crystal Violet in Fish by SERS. J. Food Science. 2017, 82, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Cardini, G.; Muniz-Miranda, M.; Pagliai, M.; Schettino, V. A Density Functional Study of the SERS Spectra of Pyridine Adsorbed on Silver Clusters. Theor. Chem. Acc. 2007, 117, 451–458. [Google Scholar] [CrossRef]
- Richter, A.P.; Lombardi, J.R.; Zhao, B. Size and Wavelength Dependence of the Charge-Transfer Contributions to Surface-Enhanced Raman Spectroscopy in Ag/PATP/ZnO Junctions. J. Phys. Chem. C 2010, 114, 1610–1614. [Google Scholar] [CrossRef]
- Xu, J.F.; Liu, G.-K. Laser-Induced Chemical Transformation of PATP Adsorbed on Ag Nanoparticles by Surface-Enhanced Raman Spectroscopy – a Study of the Effects from Surface Morphology of Substrate and Surface Coverage of PATP. Spectrochim. Acta, Part A 2015, 138, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Torul, H.; Çiftçi, H.; Çetin, D.; Suludere, Z.; Boyacı, I.H.; Tamer, U. Paper Membrane-Based SERS Platform for the Determination of Glucose in Blood Samples. Anal. Bioanal. Chem. 2015, 407, 8243–8251. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, Y.; Yu, Z.; Liu, Y.; Zhang, X.; Wang, X.; Sui, H.; Sun, C.; Zhao, B. Surface-Enhanced Raman Spectroscopy Study on the Structure Changes of 4-Mercaptophenylboronic Acid under Different pH Conditions. Spectrochim. Acta, Part A 2017, 185, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Zhong, Y.; Cui, Y. A wide range optical pH sensor for living cells using Au@Ag nanoparticles functionalized carbon nanotubes based on SERS signals. Anal. Bioanal. Chem. 2014, 406, 6337–6346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Rong, Z.; Wang, J.; Jiang, N.; Pang, Y.; Xiao, R.; Wang, S. Seed-Mediated Synthesis of High-Performance Silver-Coated Magnetic Nanoparticles and Their Use as Effective SERS substrates. Colloids Surf. A 2016, 506, 393–401. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Hassan, M.M.; Chen, X.; Ouyang, Q.; Guo, Z.; Zhao, J. A Magnetite/PMAA Nanospheres-Targeting SERS Aptasensor for Tetracycline Sensing Using Mercapto Molecules Embedded Core/Shell Nanoparticles for Signal Amplification. Biosens. Bioelectron. 2017, 92, 192–199. [Google Scholar] [CrossRef] [PubMed]
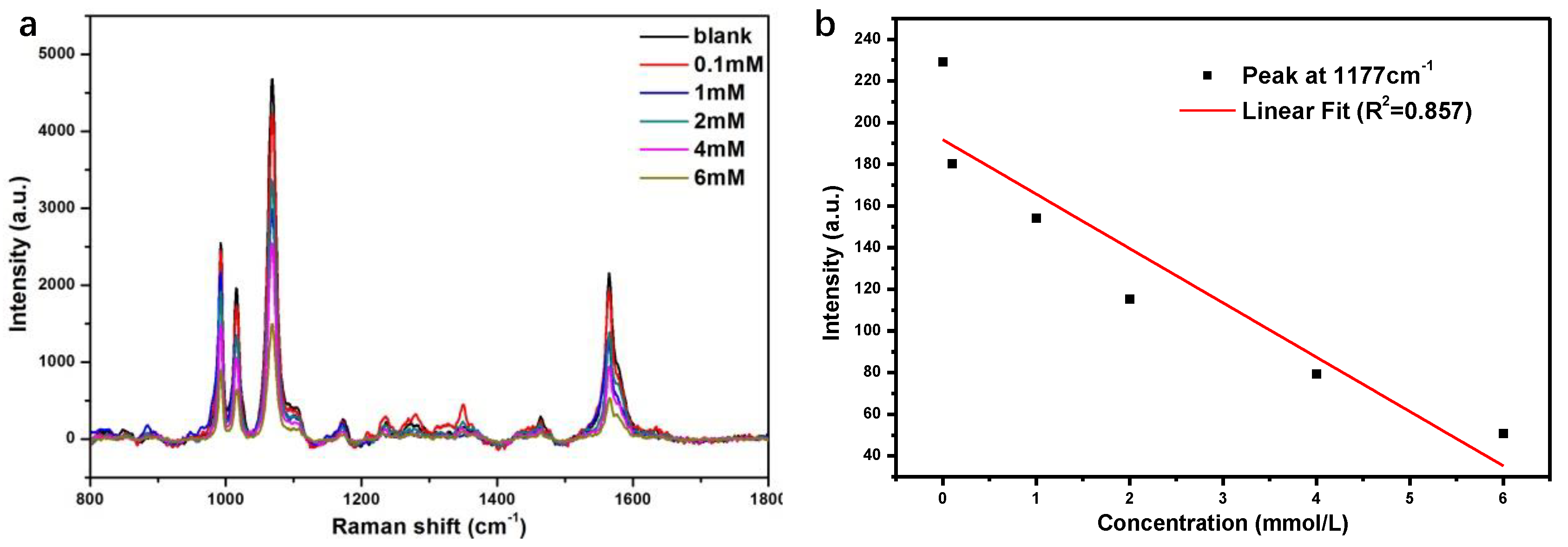
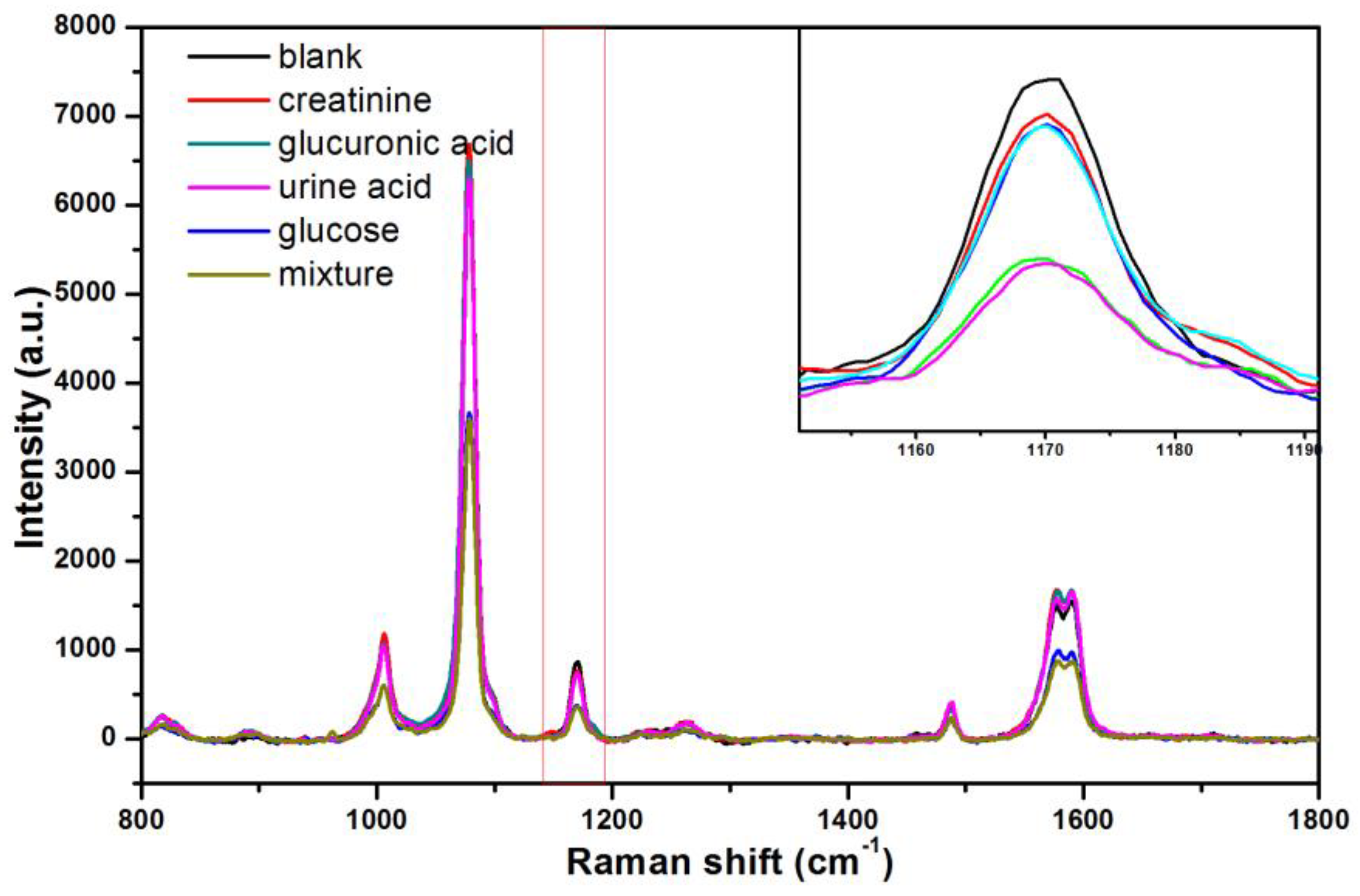
| Limit of Detection (LOD) | Detection Method | Detection Selectivity | References |
|---|---|---|---|
| 0.1 mM | SERS with Au@Ag NPs modified with internal standard | Urine, Creatinine, Uric acid, Glucuronic acid | This work |
| 0.56 mM | Naked eye detection using glucose oxidase | Creatinine, Cysteine | [4] |
| 0.11 mM | Near-infrared (NIR) | Blood | [10] |
| 1 mM | electrochemical method | Uric acid, Ascorbic acid | [14] |
| 0.1 mM | SERS with Ag@Fabric NanoZymes | Urine | [18] |
| 1 mM | SERS with Au NPs substrate | Urine | [19] |
| 10 nM | SERS with nanorods assembled substrate | Galactose, Fructose | [20] |
| 5.1 mM | SERS substrate with metal carbonyl probe | Urine | [32] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhou, T.; You, R.; Wu, Y.; Shen, H.; Feng, S.; Su, J. Fabrication and Characterization of a Highly-Sensitive Surface-Enhanced Raman Scattering Nanosensor for Detecting Glucose in Urine. Nanomaterials 2018, 8, 629. https://doi.org/10.3390/nano8080629
Lu Y, Zhou T, You R, Wu Y, Shen H, Feng S, Su J. Fabrication and Characterization of a Highly-Sensitive Surface-Enhanced Raman Scattering Nanosensor for Detecting Glucose in Urine. Nanomaterials. 2018; 8(8):629. https://doi.org/10.3390/nano8080629
Chicago/Turabian StyleLu, Yudong, Ting Zhou, Ruiyun You, Yang Wu, Huiying Shen, Shangyuan Feng, and Jingqian Su. 2018. "Fabrication and Characterization of a Highly-Sensitive Surface-Enhanced Raman Scattering Nanosensor for Detecting Glucose in Urine" Nanomaterials 8, no. 8: 629. https://doi.org/10.3390/nano8080629
APA StyleLu, Y., Zhou, T., You, R., Wu, Y., Shen, H., Feng, S., & Su, J. (2018). Fabrication and Characterization of a Highly-Sensitive Surface-Enhanced Raman Scattering Nanosensor for Detecting Glucose in Urine. Nanomaterials, 8(8), 629. https://doi.org/10.3390/nano8080629





