Markers of Oxidative Stress in the Exhaled Breath Condensate of Workers Handling Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Facility and Operations Description
2.2. Subjects
2.3. Workplace Aerosol Measurements
2.4. Collection and Analysis of Oxidative Stress Markers in EBC
2.5. Environmental Contamination
2.6. Statistical Analysis
3. Results
3.1. Subjects
3.2. Workplace Aerosol Results
3.2.1. Number Size Distributions
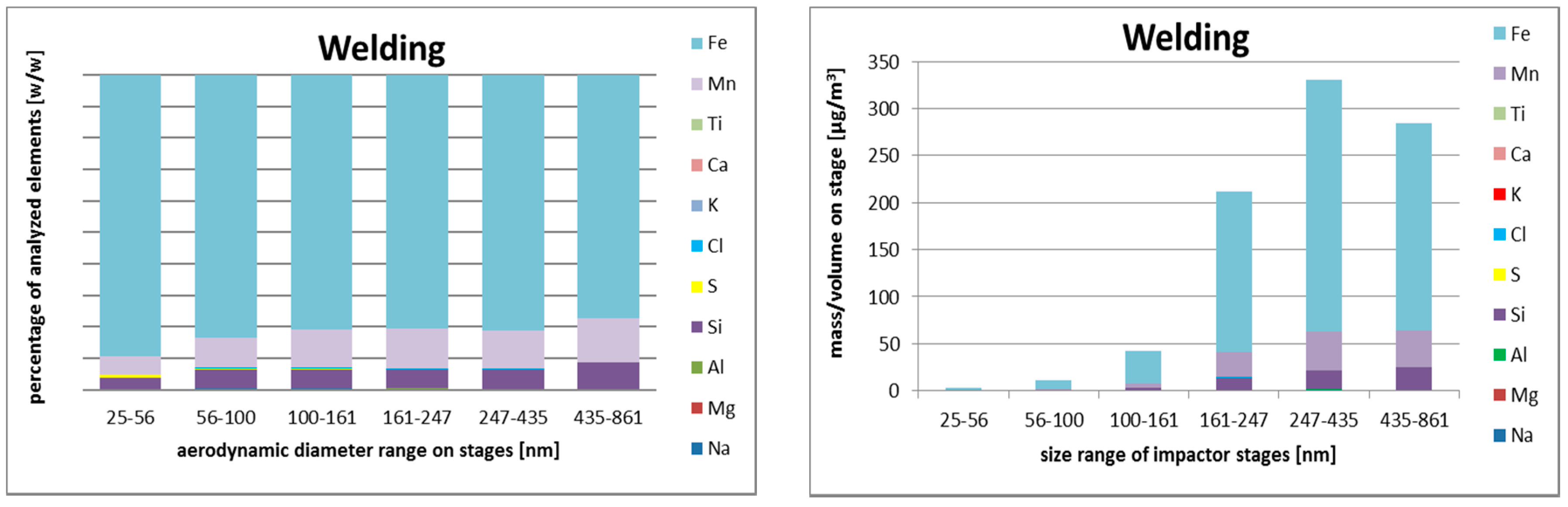
Metal Active Gas (MAG) Welding
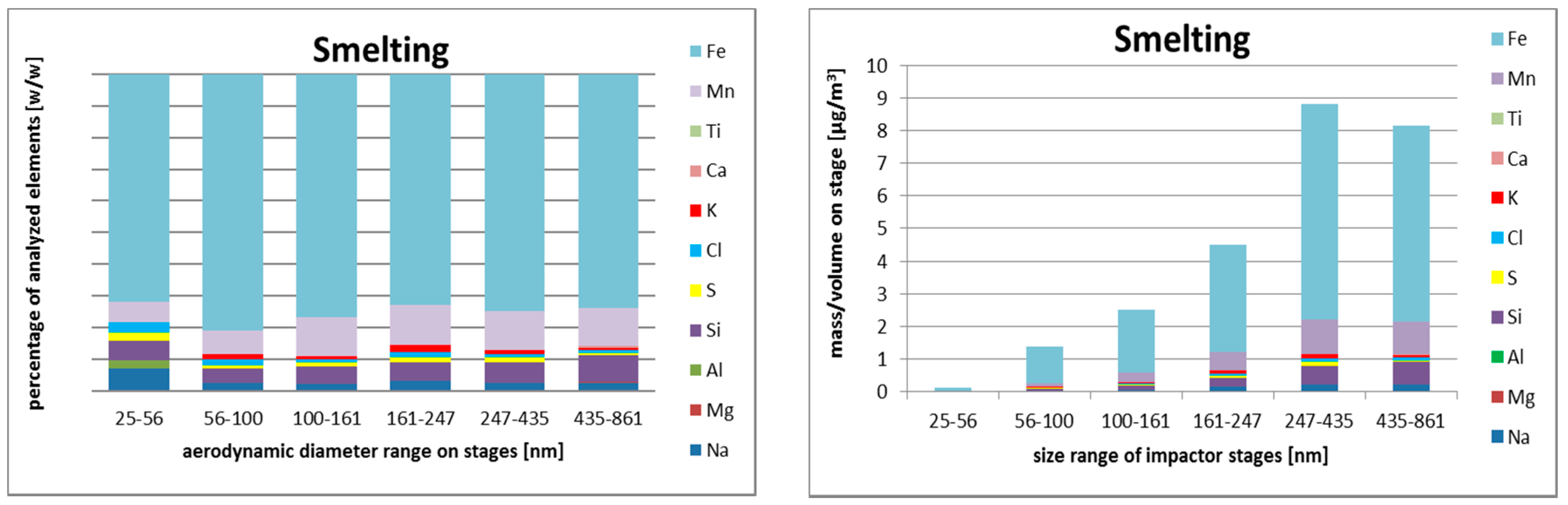
Smelting
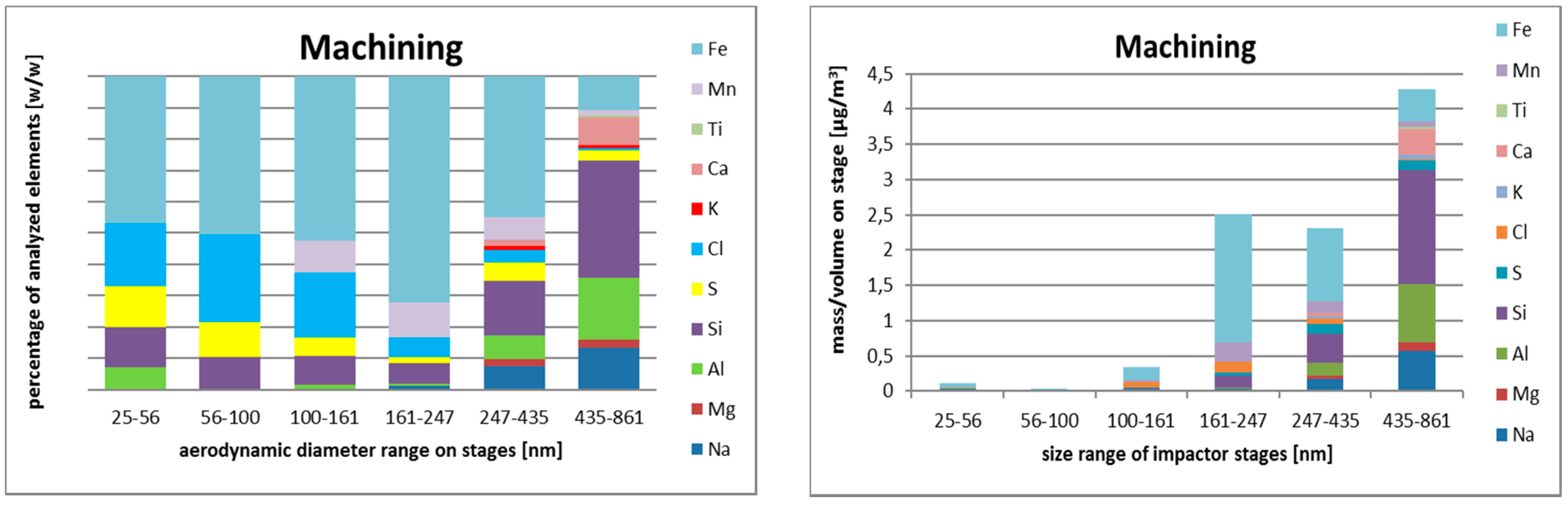
Nanocomposite Machining
Background
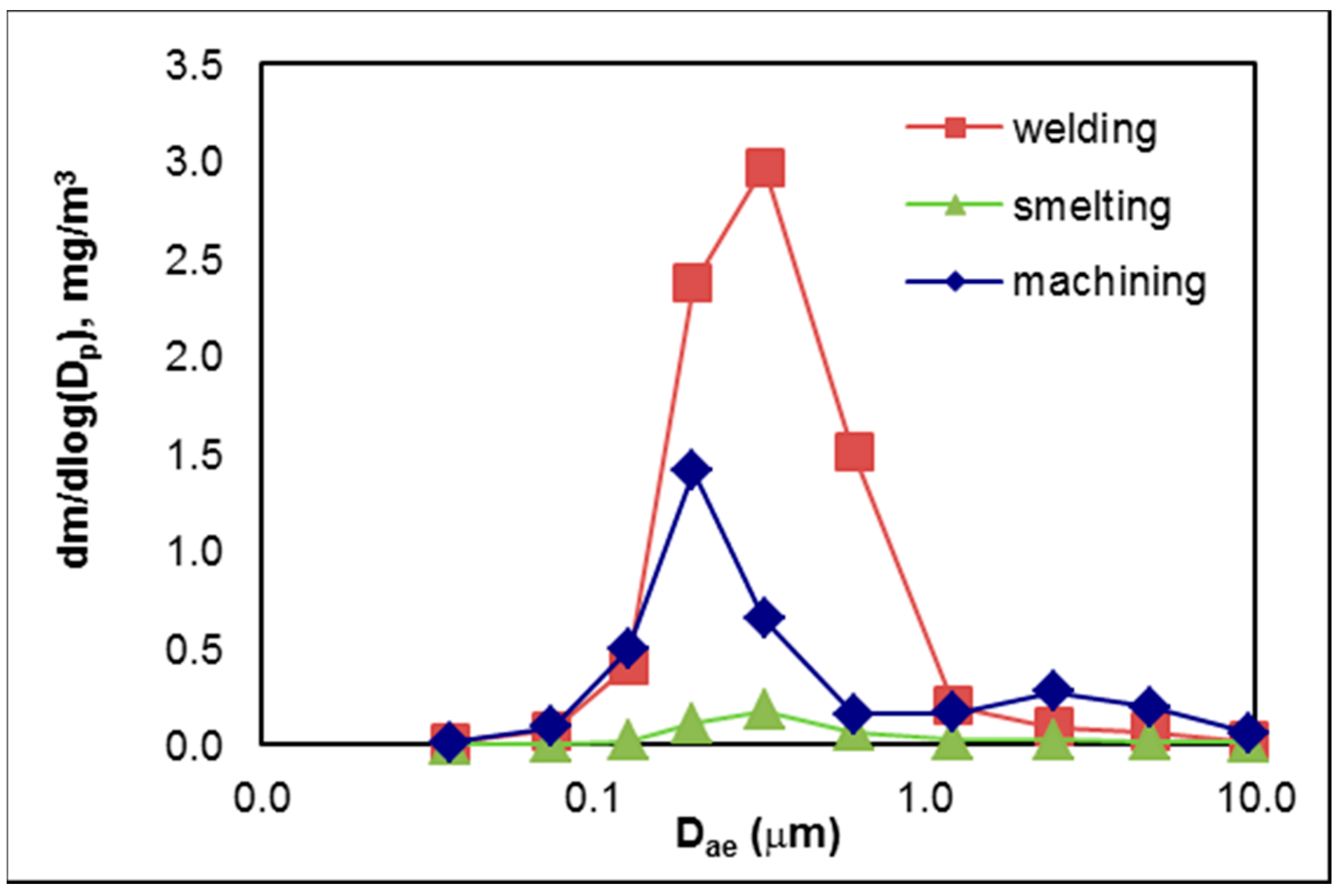
3.2.2. Mass Size Distributions
3.2.3. Size-Resolved Elemental Composition
3.3. Oxidative Stress Markers in EBC
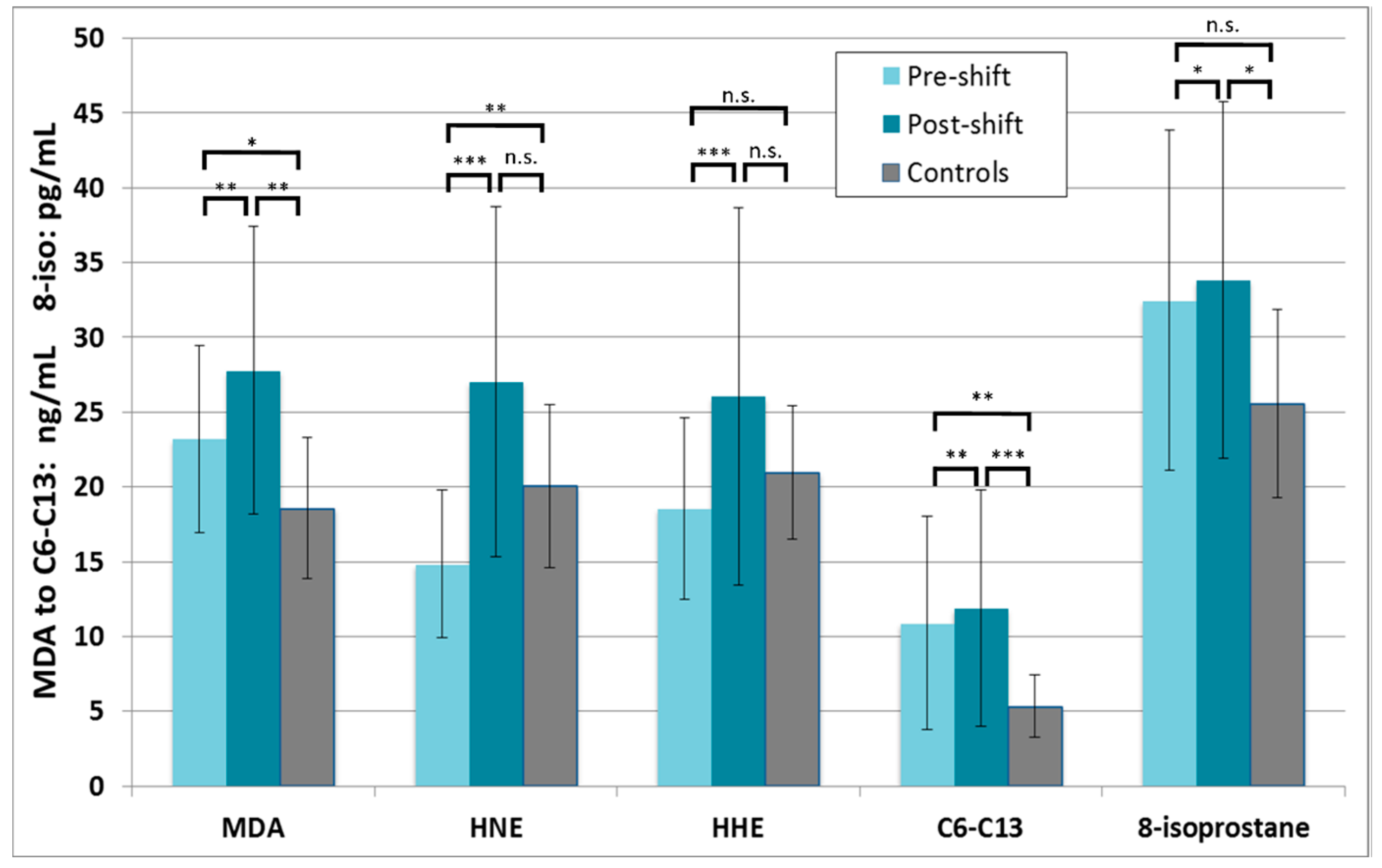
3.3.1. Markers of Oxidation of Lipids
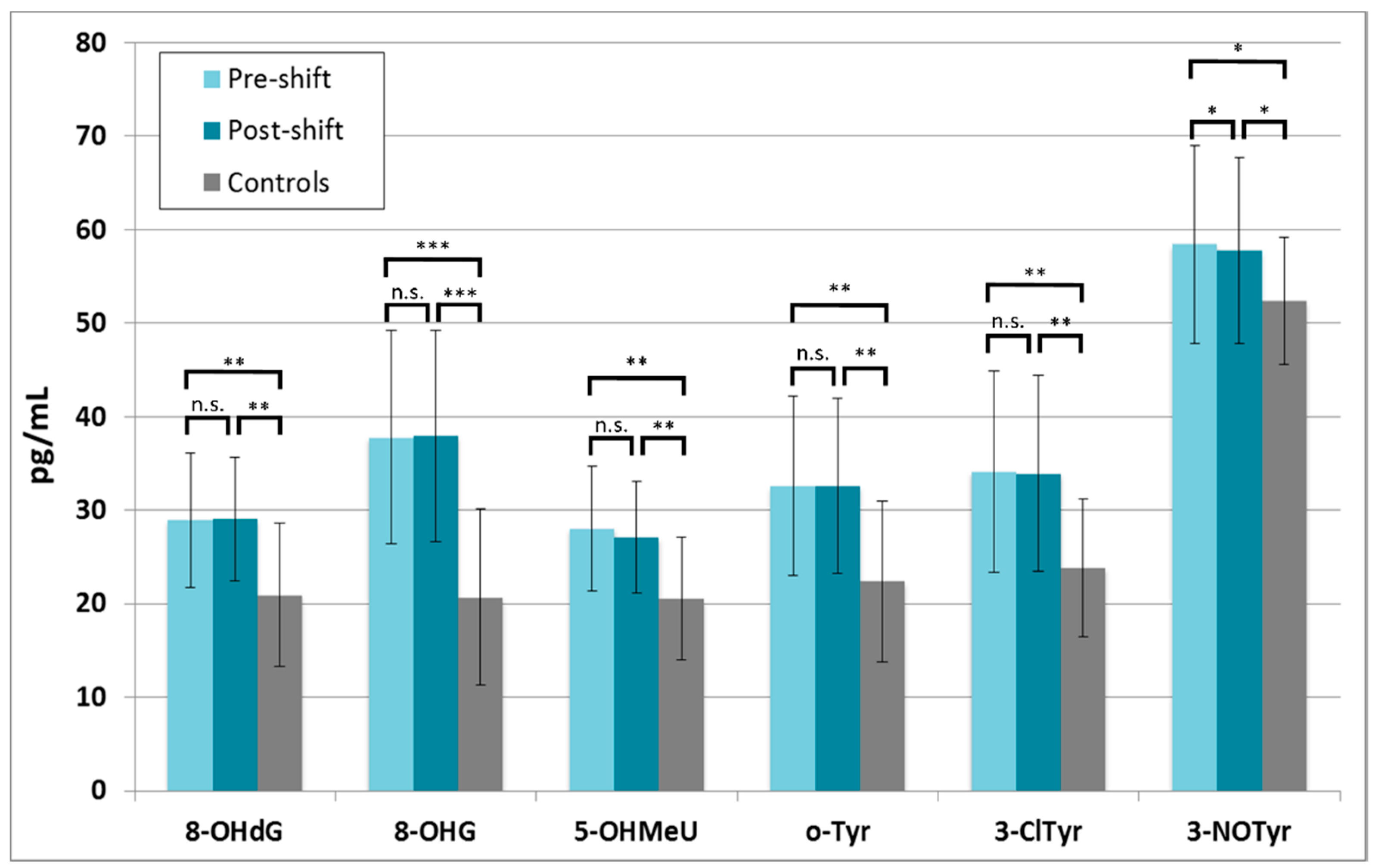
3.3.2. Markers of Oxidation of Nucleic Acids and Proteins
3.3.3. Correlations of Markers with Exposure and Symptoms
3.3.4. Correlations of the Levels of Markers in the Pre-Shift and Post-Shift Samples
3.3.5. Association of EBC Markers with Occupational Exposure
3.4. Environmental Contamination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bello, D.; Wardle, B.L.; Ahn, K.; Yamamoto, N.; Garcia, E.; deVilloria, R.G.; Hart, A.J.; Ellenbecker, M.J.; Hallock, M. Exposure to nanoscale particles and fibers during machining of hybrid advanced composites containing carbon nanotubes. J. Nanopart. Res. 2009, 11, 231–249. [Google Scholar] [CrossRef]
- Bello, D.; Wardle, B.L.; Zhang, J.; Yamamoto, N.; Santeufemio, C.; Hallock, M.; Virji, M.A. Characterization of exposures to nanoscale particles and fibers during drilling of hybrid advanced composites containing carbon nanotubes. Int. J. Occup. Environ. Health 2010, 16, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Boonruksa, P.; Bello, D.; Zhang, J.; Isaacs, J.A.; Mead, J.; Woskie, S. Characterization of potential exposures to nanoparticles and fibers during manufacturing and recycling of carbon nanotube reinforced polypropylene composites. Ann. Occup. Hyg. 2016, 60, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Boonruksa, P.; Bello, D.; Zhang, J.; Isaacs, J.A.; Mead, J.L.; Woskie, S.R. Exposures to nanoparticles and fibers during injection molding and recycling of carbon nanotube reinforced polycarbonate composites. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.H.; Tsai, C.S.; Pelclova, D.; Schubauer-Berigan, M.K.; Schulte, P.A. Assessing the first wave of epidemiological studies of nanomaterial workers. J. Nanopart. Res. 2015, 17, 413. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.A.; Iavicoli, I.; Rantanen, J.H.; Dahmann, D.; Iavicoli, S.; Pipke, R.; Guseva Canu, I.; Boccuni, F.; Ricci, M.; Polci, M.L.; et al. Assessing the protection of the nanomaterial workforce. Nanotoxicology 2016, 10, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Leso, V.; Schulte, P.A. Biomarkers of susceptibility. State of the art and implications for occupational exposure to engineered nanomaterials. Toxicol. Appl. Pharmacol. 2016, 299, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Guseva Canu, I.; Schulte, P.A.; Riediker, M.; Fatkhutdinova, L.; Bergamaschi, E. Methodological, political and legal issues in the assessment of the effects of nanotechnology on human health. J. Epidemiol. Community Health 2018, 72, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Chalbot, M.-C.; Pirela, S.V.; Schifman, L.; Kasaraneni, V.; Oyanedel-Craver, V.; Bello, D.; Castranova, V.; Qian, Y.; Thomas, T.; Kavouras, I.G.; et al. Synergistic effects of engineered nanoparticles and organics released from laser printers using nano-enabled toners: Potential health implications from exposures to the emitted organic aerosol. Environ. Sci. Nano 2017, 4, 2144–2156. [Google Scholar] [CrossRef]
- Keane, M.; Stone, S.; Chen, B. Welding fumes from stainless steel gas metal arc processes contain multiple manganese chemical species. J. Environ. Monit. 2010, 12, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Valuntaitė, V.; Girgždienė, R. Outdoor and indoor ozone level—A potential impact on human health. Vojnosanit. Pregl. 2015, 72, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Cena, L.G.; Chisholm, W.P.; Keane, M.J.; Chen, B.T. A field study on the respiratory deposition of the nano-sized fraction of mild and stainless steel welding fume metals. J. Occup. Environ. Hyg. 2015, 12, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Barnes, P.J.; Loukides, S.; Sterk, P.J.; Högman, M.; Olin, A.C.; Amann, A.; Antus, B.; Baraldi, E.; Bikov, A.; et al. A European respiratory society technical standard: Exhaled biomarkers in lung disease. Eur. Respir. J. 2017, 49, 1600965. [Google Scholar] [CrossRef] [PubMed]
- Almstrand, A.C.; Bake, B.; Ljungström, E.; Larsson, P.; Bredberg, A.; Mirgorodskaya, E.; Olin, A.C. Effect of airway opening on production of exhaled particles. J. Appl. Physiol. 2010, 108, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.F.; Bello, D.; Schmidt, D.F.; Pal, A.K.; Stella, A.; Isaacs, J.A.; Rogers, E.J. Mapping the biological oxidative damage of engineered nanomaterials. Small 2013, 9, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed. Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.H.; Bae, G.H. Nanotoxicity: An interplay of oxidative stress, inflammation and cell death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Bello, D.; Warheit, D.B. Biokinetics of engineered nano-TiO2 in rats administered by different exposure routes: Implications for human health. Nanotoxicology 2017, 11, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Molecular mechanisms of oxidative stress-induced carcinogenesis: From epidemiology to oxygenomics. IUBMB Life 2008, 60, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.F.; Tung, S.P.; Wang, D.; Yeh, D.Y.; Fong, Y.; Young, Y.C.; Leu, F.J. Lipoxygenase pathway mediates increases of airway resistance and lung inflation induced by exposure to nanotitanium dioxide in rats. Oxid. Med. Cell. Longev. 2014, 2014, 485604. [Google Scholar] [CrossRef] [PubMed]
- Kuka, S.; Hurbankova, M.; Drlickova, M.; Baska, T.; Hudeckova, H.; Tatarkova, Z. Nanomaterials—A new and former public health issue. The case of Slovakia Cent. Eur. J. Public Health 2016, 24, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Poland, C.A. Nanotoxicity: Challenging the myth of nano-specific toxicity. Curr. Opin. Biotechnol. 2013, 24, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Antus, B. Oxidative stress markers in sputum. Oxid. Med. Cell. Longev. 2016, 2016, 2930434. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Komarc, M.; Schwarz, J.; Kostejn, M.; Dvorackova, S.; Ondracek, J.; Kacer, P.; Vlckova, S.; Fenclova, Z.; et al. Respiratory symptoms and markers of inflammation in nanocomposite production workers. Occup. Environ. Med. submitted.
- Berner, A.; Lürzer, C. Mass size distributions of traffic aerosols at Vienna. J. Phys. Chem. 1980, 84, 2079–2083. [Google Scholar] [CrossRef]
- Stefancova, L.; Schwarz, J.; Mäkelä, T.; Hillamo, R.; Smolik, J. Comprehensive characterization of original 10-stage and 7-stage modified Berner Type Impactors. Aerosol Sci. Technol. 2011, 45, 88–100. [Google Scholar] [CrossRef]
- Talbot, N.; Kubelova, L.; Makes, O.; Ondracek, J.; Cusack, M.; Schwarz, J.; Vodicka, P.; Zikova, N.; Zdimal, V. Transformations of aerosol particles from an outdoor to indoor environment. Aerosol Air Qual. Res. 2017, 17, 653–665. [Google Scholar] [CrossRef]
- Syslova, K.; Kacer, P.; Kuzma, M.; Klusackova, P.; Fenclova, Z.; Lebedova, J.; Pelclova, D. Determination of 8-iso-prostaglandin F(2α) in exhaled breath condensate using combination of immunoseparation and LC-ESI-MS/MS. J. Chromatogr. B 2008, 867, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Syslova, K.; Kacer, P.; Kuzma, M.; Pankracova, A.; Fenclova, Z.; Vlckova, S.; Lebedova, J.; Pelclova, D. LC-ESI-MS/MS method for oxidative stress multimarker screening in the exhaled breath condensate of asbestosis/silicosis patients. J. Breath Res. 2010, 4, 017104. [Google Scholar] [CrossRef] [PubMed]
- Syslova, K.; Böhmova, A.; Mikoska, M.; Kuzma, M.; Pelclova, D.; Kacer, P. Multimarker screening of oxidative stress in aging. Oxid. Med. Cell. Longev. 2014, 2014, 562860. [Google Scholar] [CrossRef] [PubMed]
- Klusackova, P.; Lebedova, J.; Kacer, P.; Kuzma, M.; Brabec, M.; Pelclova, D.; Fenclova, Z.; Navratil, T. Leukotrienes and 8-isoprostane in exhaled breath condensate in bronchoprovocation tests with occupational allergens. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.M.; Biller, J.; Foss, B.; Hoagland, K.; Dunning, M.B.; Castillo, D.; Bosbous, M.; Sun, F.; Shaker, R. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am. J. Respir. Crit. Care Med. 2003, 168, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; Tan, S.L.; Stockley, R.A. Chronic obstructive pulmonary disease: Towards pharmacogenetics. Genome Med. 2009, 1, 112. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [PubMed]
- Runa, S.; Hussey, M.; Payne, C.K. Nanoparticle-Cell interactions: Relevance for public health. J. Phys. Chem. B 2018, 122, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Theriaulta, M.; Yoeutha, S.; Matara, J.; Martin, J.; Bello, D.; Barry, C. Investigation of nanoparticles emitted when injection molding neat and additive-filled polypropylene and polycarbonate. In Proceedings of the 32nd International Conference of the Polymer Processing Society, Lyon, France, 25–29 July 2016. [Google Scholar]
- Malvindi, M.A.; De Matteis, V.; Galeone, A.; Brunetti, V.; Anyfantis, G.C.; Athanassiou, A.; Cingolani, R.; Pompa, P.P. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS ONE 2014, 9, e85835. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Fenclova, Z.; Vlckova, S.; Turci, F.; Corazzari, I.; Kacer, P.; Schwarz, J.; Zikova, N.; Makes, O.; et al. Markers of oxidative damage of nucleic acids and proteins among workers exposed to TiO2 (nano) particles. Occup. Environ. Med. 2016, 73, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Zikova, N.; Komarc, M.; Fenclova, Z.; Vlckova, S.; Schwarz, J.; Makes, O.; Syslova, K.; et al. Markers of lipid oxidative damage in the exhaled breath condensate of nanoTiO2 production workers. Nanotoxicology 2017, 11, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Fenclova, Z.; Vlckova, S.; Syslova, K.; Navratil, T.; Schwarz, J.; Zikova, N.; Barosova, H.; et al. Oxidative stress markers are elevated in exhaled breath condensate of workers exposed to nanoparticles during iron oxide pigment production. J. Breath Res. 2016, 10, 016004. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Komarc, M.; Fenclova, Z.; Vlckova, S.; Zikova, N.; Schwarz, J.; Makes, O.; Navratil, T.; et al. Markers of lipid oxidative damage among office workers exposed intermittently to air pollutants including nanoTiO2 particles. Rev. Environ. Health 2017, 32, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Zdimal, V.; Kacer, P.; Vlckova, S.; Fenclova, Z.; Navratil, T.; Komarc, M.; Schwarz, J.; Zikova, N.; Makes, O.; et al. Markers of nucleic acids and proteins oxidation among office workers exposed to air pollutants including (nano)TiO2 particles. Neuro Endocrinol. Lett. 2016, 37, 3–16. [Google Scholar] [PubMed]
- Pelclova, D.; Barosova, H.; Kukutschova, J.; Zdimal, V.; Navratil, T.; Fenclova, Z.; Vlckova, S.; Schwarz, J.; Zikova, N.; Kacer, P.; et al. Raman microspectroscopy of exhaled breath condensate and urine in workers exposed to fine and nanoTiO2 particles: A cross-sectional study. J. Breath Res. 2015, 9, 036008. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Yang, Y.S.; Yang, H.S.; Lee, J.; Kang, M.S.; Lee, B.S.; Lee, K.; Song, C.W. Nasal and pulmonary toxicity of titanium dioxide nanoparticles in rats. Toxicol. Res. 2012, 28, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Bello, D.; Bunker, K.; Shafer, M.; Christiani, D.; Woskie, S.; Demokritou, P. Occupational exposure to nanoparticles at commercial photocopy centers. J. Hazard. Mater. 2015, 298, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Bello, D.; Gaines, P.; Martin, J.; Pal, A.K.; Gore, R.; Woskie, S. Nanoparticles from photocopiers induce oxidative stress and upper respiratory tract inflammation in healthy volunteers. Nanotoxicology 2013, 7, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Huang, H.B.; Chang, Y.C.; Su, T.Y.; Wang, Y.C.; Wang, G.C.; Chen, J.E.; Tang, C.S.; Wu, T.N.; Liou, S.H. Exposure to fine particulate matter causes oxidative and methylated DNA damage in young adults: A longitudinal study. Sci. Total Environ. 2017, 598, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Khatri, M.; Bello, D.; Martin, J.; Bello, A.; Gore, R.; Demokritou, P.; Gaines, P. Chronic upper airway inflammation and oxidative stress in photocopier operators: Mechanistic insights. NanoImpact 2017, 5, 133–145. [Google Scholar] [CrossRef]
- Pirela, S.V.; Martin, J.; Bello, D.; Demokritou, P. Nanoparticle exposures from nano-enabled toner-based printing equipment and human health: State of science and future research needs. Crit. Rev. Toxicol. 2017, 47, 683–709. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.H.; Chen, Y.C.; Liao, H.Y.; Wang, C.J.; Chen, J.S.; Lee, H.L. Increased levels of oxidative stress biomarkers in metal oxides nanomaterial-handling workers. Biomarkers 2016, 21, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.H.; Wu, W.T.; Liao, H.Y.; Chen, C.Y.; Tsai, C.Y.; Jung, W.T.; Lee, H.L. Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J. Hazard. Mater. 2017, 331, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Chen, Z.; Xu, H.; Zhou, J.; Tang, S.; Xu, Z.; Kong, F.; Li, X.; Zhang, Y.; et al. Cardiopulmonary effects induced by occupational exposure to titanium dioxide nanoparticles. Nanotoxicology 2018, 12, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Demokritou, P.; Woskie, S.; Bello, D. Indoor air quality in photocopy centers, nanoparticle exposures at photocopy workstations, and the need for exposure controls. Ann. Work Expo. Health 2017, 61, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Fenclova, Z.; Kacer, P.; Kuzma, M.; Navratil, T.; Lebedova, J. 8-isoprostane and leukotrienes in exhaled breath condensate in Czech subjects with silicosis. Ind. Health 2007, 45, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Fenclova, Z.; Kacer, P.; Kuzma, M.; Navratil, T.; Lebedova, J. Increased 8-isoprostane, a marker of oxidative stress in exhaled breath condensate in subjects with asbestos exposure. Ind. Health 2008, 46, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Pelclova, D.; Fenclova, Z.; Syslova, K.; Vlckova, S.; Lebedova, J.; Pecha, O.; Belacek, J.; Navratil, T.; Kuzma, M.; Kacer, P. Oxidative stress markers in exhaled breath condensate in lung fibroses are not significantly affected by systemic diseases. Ind. Health 2011, 49, 746–754. [Google Scholar] [CrossRef] [PubMed]
| Subjects | Exposed | Controls |
|---|---|---|
| Number of Subjects | 19 | 19 |
| Age (years) | 42.4 ± 11.4 | 45.4 ± 11.7 |
| Body Mass Index (kg/m2) | 27.69 ± 6.30 | 24.39 ± 4.04 |
| Alcohol occasionally (n, %) | 17 (90%) | 16 (84%) |
| Employment in nanocomposite production (years) | Mean 18.0 ± 10.3 | - |
| Operation | N (Number of SMPS/APS Samples) | TNC (particles/cm3), Median | TNC, Maximum | Size Distribution (Main Mode) | % of Particles <100 nm | Ratio Operation/Background | Mass Concentration (mg/m3) |
|---|---|---|---|---|---|---|---|
| Smelting | 32 | 4.8 × 104 | 2.0 × 105 | <25 nm | 95 | 2.3 | 0.120 |
| Machining | 39 | 5.4 × 105 | 8.2 × 105 | 100 nm | 61 | 1.9 | 0.804 |
| Welding | 28 | 1.3 × 105 | 2.5 × 105 | 200 nm | 40 | 6.2 | 1.840 |
| Background smelting/welding | 5 | 2.1 × 104 | 2.5 × 104 | <10 nm | 97 | 1 | not measured |
| Background machining | 5 | 2.8 × 105 | 3.4 × 105 | 130 nm | 41 | 1 | not measured |
| Percentage from Total <10 µm | <25–100 nm | 100 nm–10 µm | ||||||
|---|---|---|---|---|---|---|---|---|
| <25 nm | 25–100 nm | 100 nm–1 µm | 1–2.5 µm | 2.5–10 µm | Total <1 µm | 1–10 µm | ||
| Basement | Metal Active Gas (MAG) Welding | 3.35 | 36.78 | 59.85 | 0.02 | 0.00 | 99.97 | 0.03 |
| Smelting | 69.64 | 25.00 | 5.36 | 0.01 | 0.00 | 99.99 | 0.01 | |
| Background—15 min before welding | 74.37 | 22.39 | 3.23 | 0.00 | 0.00 | 99.99 | 0.01 | |
| Ground floor | Machining (Milling & Grinding) | 2.61 | 58.62 | 38.76 | 0.00 | 0.00 | 99.99 | 0.01 |
| Background—15 min before machining | 0.27 | 40.62 | 59.10 | 0.01 | 0.00 | 99.99 | 0.01 | |
| Background-night—15 h | 4.24 | 66.40 | 29.32 | 0.04 | 0.00 | 99.96 | 0.04 | |
| Operation | Mass Concentration (µg/m3) | Diagnostic Ratios | ||||||
|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Ti | Ca | Si | Mg | Mn/Fe | Si/Fe | |
| Welding | 703 | 114 | 0 | 0 | 59 | 0 | 0.16 | 0.08 |
| Smelting | 19 | 3.00 | 0 | 0.04 | 1.70 | 0.01 | 0.16 | 0.09 |
| Machining | 3.60 | 0.55 | 0.03 | 0.42 | 2.20 | 0.17 | 0.16 | 0.63 |
| Pre-Shift Marker, Correlation Coefficient (p Value) | Post-Shift Marker, Correlation Coefficient (p Value) | |
|---|---|---|
| Employment in nanocomposite production (years) | 5-OHMeU, 0.477 (0.039) o-Tyr, 0.488 (0.034) | - o-Tyr, 0.511 (0.025) |
| Chronic bronchitis | - | 3-NOTyr, 0.496 (0.031) |
| Pre-Shift Markers | MDA | C6–C13 | 8-isoprostane | 8-OHdG | 8-OHG | 5-OHMeU | o-Tyr | 3-ClTyr | 3-NOTyr |
|---|---|---|---|---|---|---|---|---|---|
| Nanocomposites production (Yes/No) | 4.71 * (1.19, 8.23) | 7.06 *** (3.58, 10.54) | 8.02 * (1.36, 14.69) | 8.61 ** (3.26, 13.97) | 17.66 *** (9.97, 23.35) | 7.46 ** (2.82, 12.09) | 10.12 ** (3.64, 16.59) | 11.31 *** (5.00, 17.61) | 6.74 * (0.36, 13.12) |
| Age (years) | −0.23 ** (−0.40, −0.70) | 0.53 (−0.11, 0.22) | 0.03 (−2.29, 0.34) | 0.10 (−0.15, 0.35) | 0.11 (−0.25, 0.47) | 0.18 (−0.04, 0.39) | 0.17 (−0.13, 0.47) | 0.34 * (0.44, 0.63) | −0.02 (−0.31, 0.28) |
| Gender (Male/Female) | 0.73 (−3.56, 5.01) | −0.84 (−5.07, 3.40) | 0.10 (−8.01, 8.21) | 4.00 (−2.51, 10.52) | 1.96 (−7.40, 11.31) | 2.38 (−3.27, 8.02) | 5.61 (−2.26, 13.49) | 3.92 (−3.76, 11.60) | 4.34 (−3.42, 12.10) |
| Alcohol (Yes/No) | −1.53 (−7.18, 4.11) | 2.61 (−2.97, 8.18) | 3.79 (−6.89, 14.47) | 3.73 (−4.85, 12.31) | 3.50 (−8.83, 15.82) | 4.59 (−2.84, 12.03) | 4.63 (−5.74, 15.00) | 2.72 (−7.39, 12.83) | 2.73 (−7.49, 12.94) |
| BMI (kg/m2) | −0.83 (−0.45, 0.28) | −0.38 * (−0.74, −0.20) | −0.28 (−0.97, 0.40) | −0.25 (−0.80, 0.31) | −0.18 (−0.97, 0.62) | 0.26 (−0.45, 0.51) | −0.07 (−0.74, 0.60) | −0.37 (−1.02, 0.28) | −0.30 (−0.96, 0.36) |
| Post-Shift Markers | MDA | HNE | C6–C13 | 8-isoprostane | 8-OHdG | 8-OHG | 5-OHMeU | o-Tyr | 3-ClTyr |
|---|---|---|---|---|---|---|---|---|---|
| Nanocomposites production (Yes/No) | 8.71 ** (4.33, 14.00) | 8.48 ** (2.20, 14.75) | 8.27 *** (4.47, 12.08) | 9.37 ** (2.44, 16.31) | 8.23 ** (3.00, 13.47) | 17.78 *** (10.12, 25.43) | 5.74 ** (1.21, 10.27) | 10.27 ** (3.88, 16.65) | 11.09 *** (4.88, 17.31) |
| Age (years) | −0.20 (−0.45, 0.49) | 0.18 (−0.28, 0.31) | 0.05 (−0.13, 0.23) | 0.05 (−0.28, 0.37) | −0.05 (−0.29, 0.20) | 0.11 (−0.25, 0.47) | −0.03 (−0.24, 0.19) | 0.17 (−0.13, 0.47) | 0.33 * (0.04, 0.62) |
| Gender (Male/Female) | 2.33 (−4.10, 8.76) | 6.06 (−1.58, 13.70) | −0.66 (−5.29, 4.00) | 0.18 (−8.26, 8.62) | 2.09 (−4.28, 8.45) | 1.70 (−7.62, 11.01) | 1.55 (−3.96, 7.06) | 5.52 (−2.25, 13.29) | 3.73 (−3.84, 11.29) |
| Alcohol (Yes/No) | −0.88 (−9.34, 7.58) | 2.15 (−7.91, 12,21) | 2.81 (−3.28, 8.90) | 3.10 (−8.01, 14.21) | 1.34 (−7.05, 9.72) | 3.32 (−8.94, 15.59) | 1.79 (−5.47, 9.05) | 4.75 (−5.48, 14.98) | 2.63 (−7.34, 12.59) |
| BMI (kg/m2) | 0.06 (−0.49, 0.60) | −0.61 (−1.25, 0.42) | −0.45 * (−0.84, −0.05) | −0.29 (−1.01, 0.43) | −0.09 (−0.63, 0.45) | −0.16 (−0.95, 0.63) | 0.23 (−0.24, 0.70) | −0.93 (−0.75, 0.57) | 0.36 (−1.01, 0.28) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelclova, D.; Zdimal, V.; Schwarz, J.; Dvorackova, S.; Komarc, M.; Ondracek, J.; Kostejn, M.; Kacer, P.; Vlckova, S.; Fenclova, Z.; et al. Markers of Oxidative Stress in the Exhaled Breath Condensate of Workers Handling Nanocomposites. Nanomaterials 2018, 8, 611. https://doi.org/10.3390/nano8080611
Pelclova D, Zdimal V, Schwarz J, Dvorackova S, Komarc M, Ondracek J, Kostejn M, Kacer P, Vlckova S, Fenclova Z, et al. Markers of Oxidative Stress in the Exhaled Breath Condensate of Workers Handling Nanocomposites. Nanomaterials. 2018; 8(8):611. https://doi.org/10.3390/nano8080611
Chicago/Turabian StylePelclova, Daniela, Vladimir Zdimal, Jaroslav Schwarz, Stepanka Dvorackova, Martin Komarc, Jakub Ondracek, Martin Kostejn, Petr Kacer, Stepanka Vlckova, Zdenka Fenclova, and et al. 2018. "Markers of Oxidative Stress in the Exhaled Breath Condensate of Workers Handling Nanocomposites" Nanomaterials 8, no. 8: 611. https://doi.org/10.3390/nano8080611
APA StylePelclova, D., Zdimal, V., Schwarz, J., Dvorackova, S., Komarc, M., Ondracek, J., Kostejn, M., Kacer, P., Vlckova, S., Fenclova, Z., Popov, A., Lischkova, L., Zakharov, S., & Bello, D. (2018). Markers of Oxidative Stress in the Exhaled Breath Condensate of Workers Handling Nanocomposites. Nanomaterials, 8(8), 611. https://doi.org/10.3390/nano8080611





