Basic Medium Heterogeneous Solution Synthesis of α-MnO2 Nanoflakes as an Anode or Cathode in Half Cell Configuration (vs. Lithium) of Li-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of MnO2
2.2. Material Characterization
2.3. Electrochemistry
3. Results and Discussion
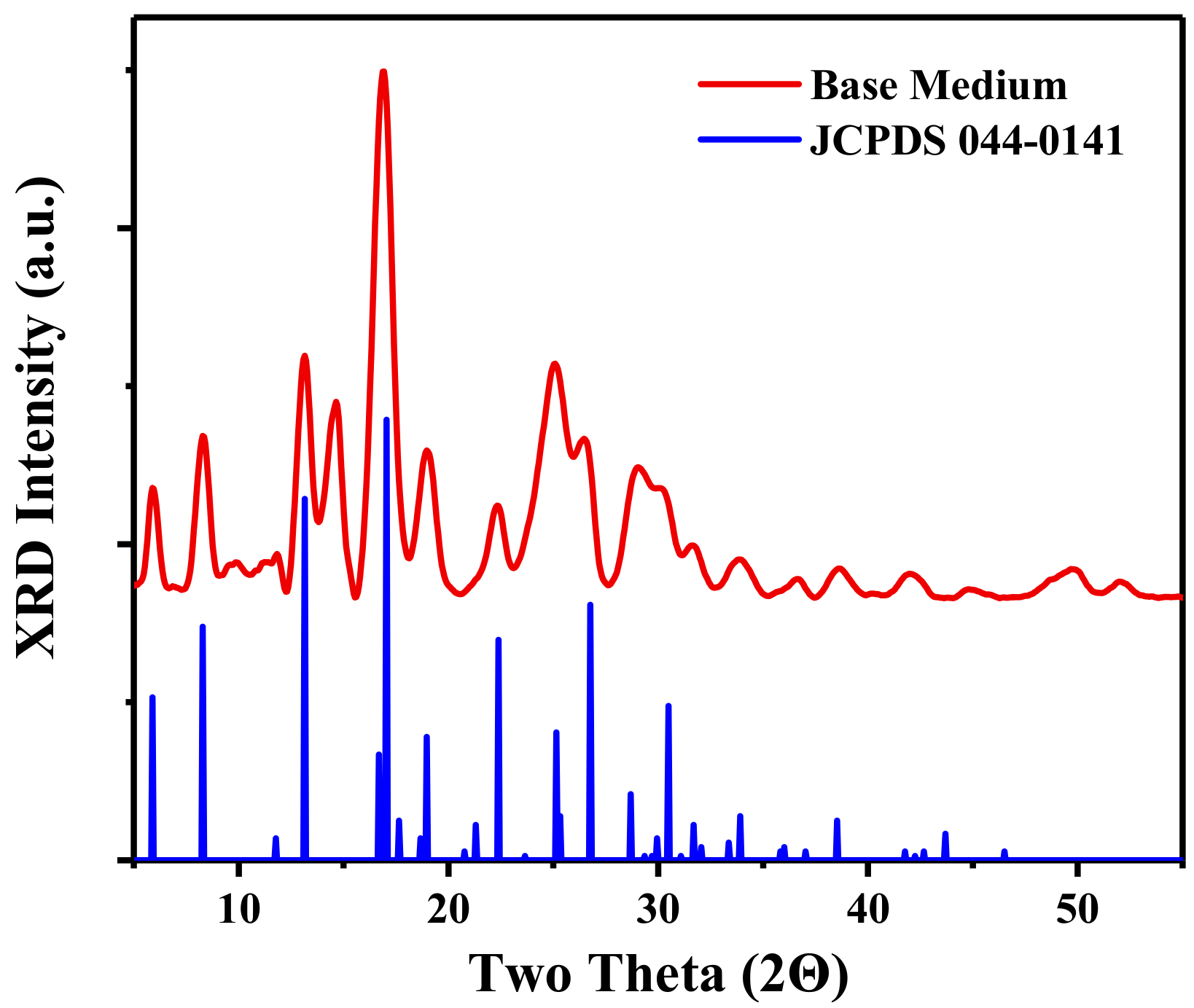
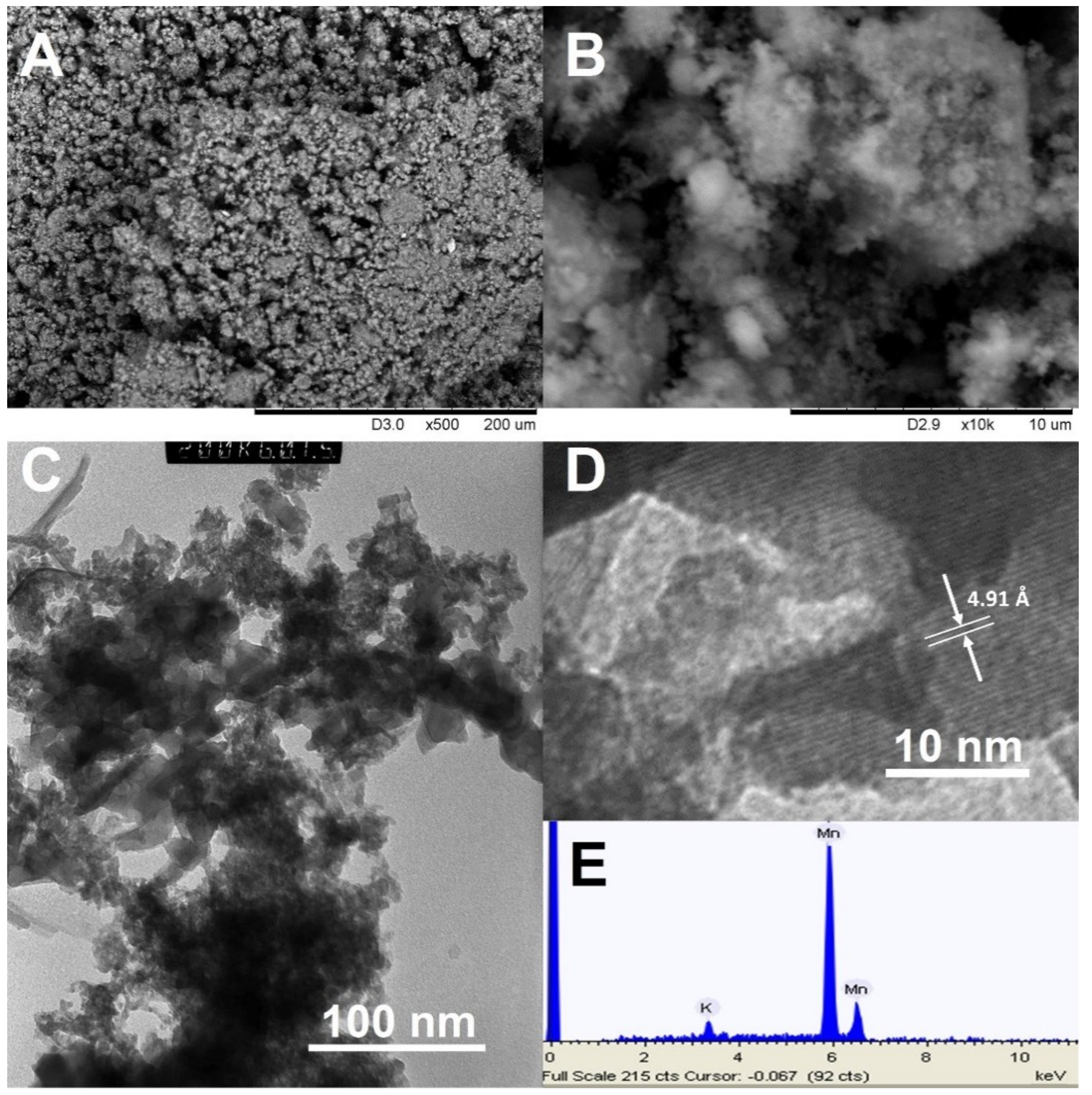
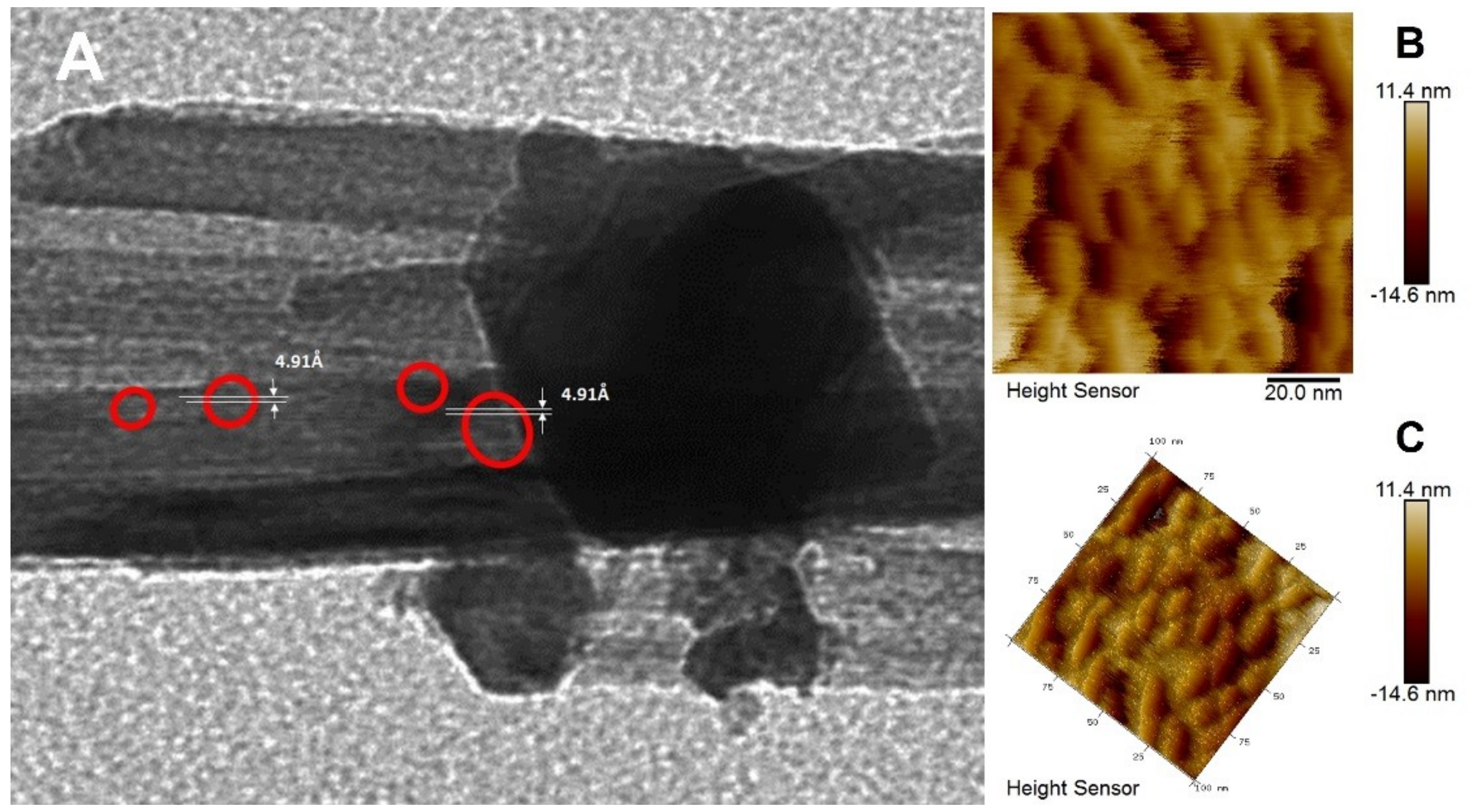
3.1. Synthesis and Structure of the Material
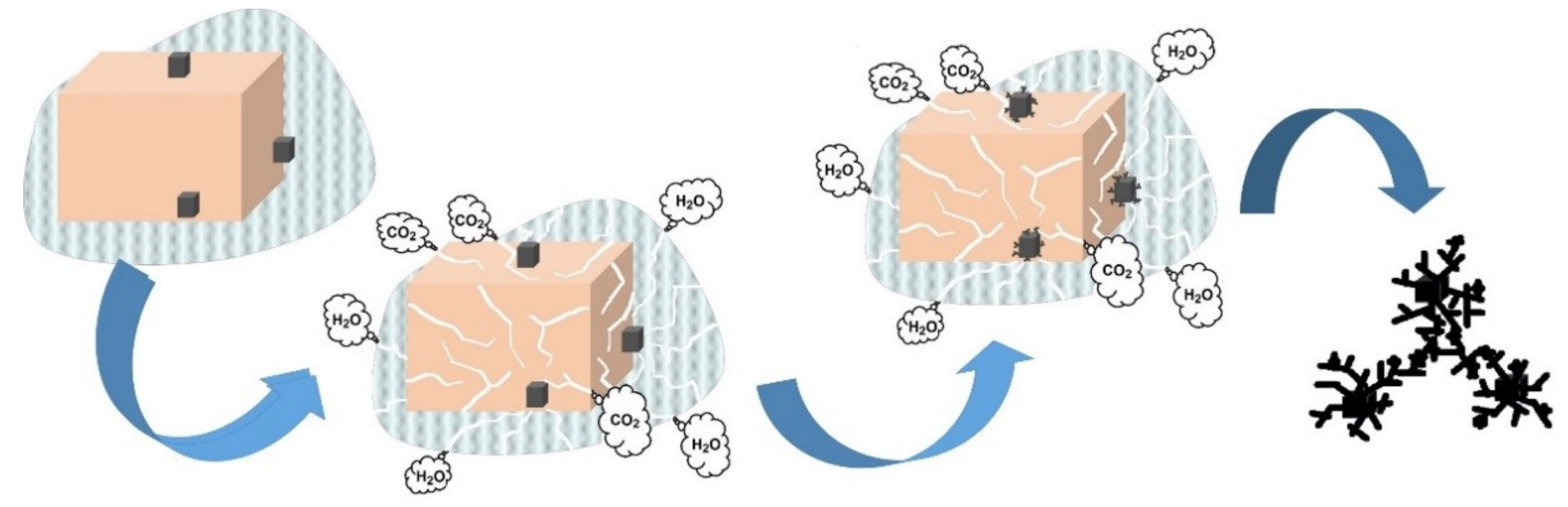
3.2. Stability Test of Produced α-MnO2
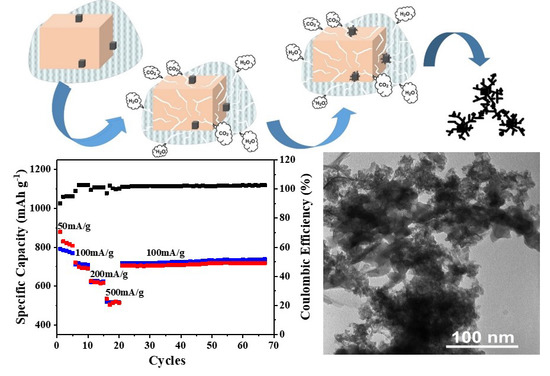
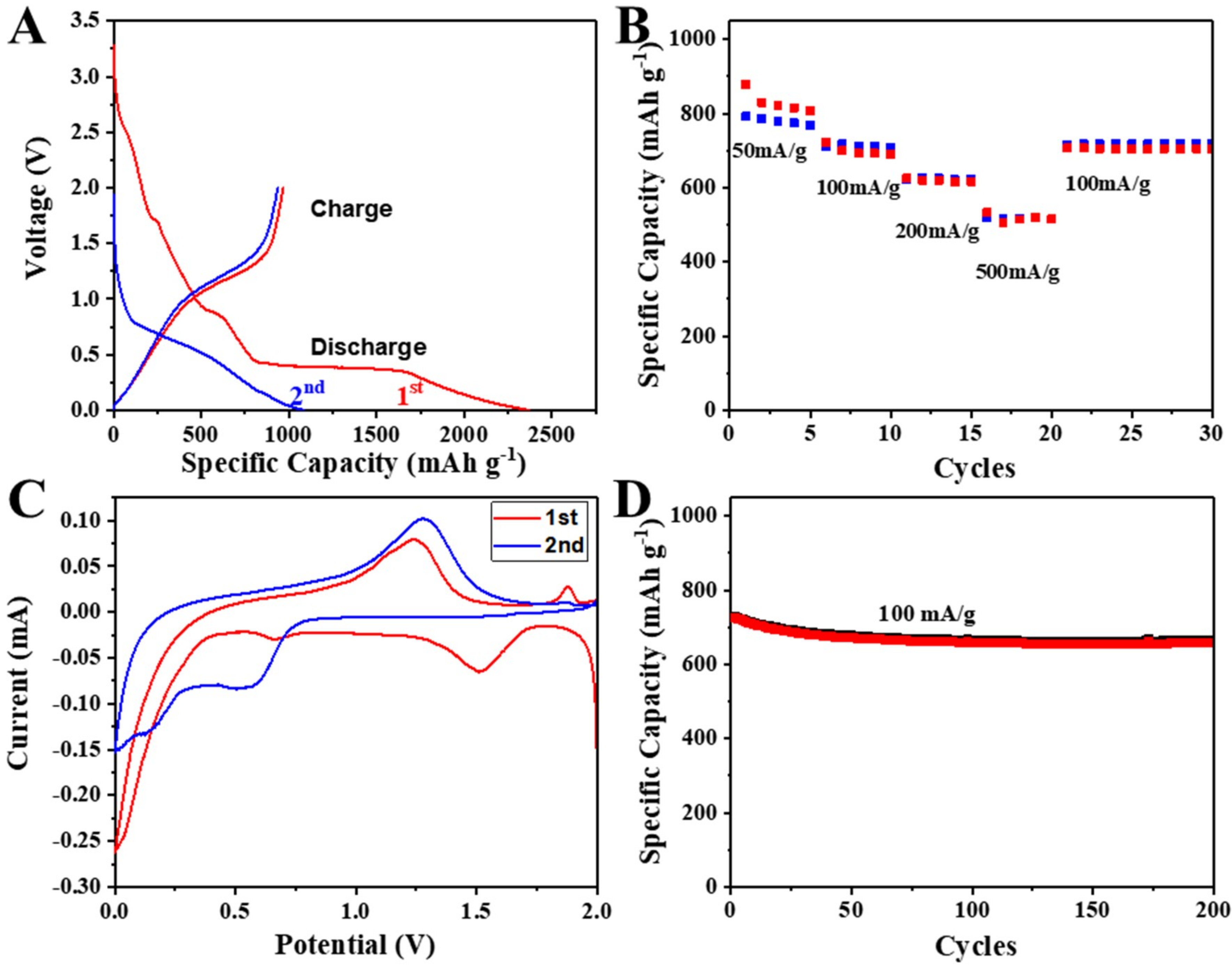
3.3. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Thirugunanam, L.; Kaveri, S.; Etacheri, V.; Ramaprabhu, S.; Dutta, M.; Pol, V.G. Electrospun nanoporous TiO2 nanofibers wrapped with reduced graphene oxide for enhanced and rapid lithium-ion storage. Mater. Charact. 2017, 131, 64–71. [Google Scholar] [CrossRef]
- Henzie, J.; Etacheri, V.; Jahan, M.; Rong, H.P.; Hong, C.N.; Pol, V.G. Biomineralization-inspired crystallization of monodisperse α-Mn2O3 octahedra and assembly of high-capacity lithium-ion battery anodes. J. Mater. Chem. A 2017, 5, 6079–6089. [Google Scholar] [CrossRef]
- Tang, J.L.; Lugo, C.E.Z.; Guzman, S.F.A.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A.; Pol, V.G. Pushing the theoretical capacity limits of iron oxide anodes: Capacity rise of γ-Fe2O3 nanoparticles in lithium-ion batteries. J. Mater. Chem. A 2016, 4, 18107–18115. [Google Scholar] [CrossRef]
- Tang, J.L.; Dysart, A.D.; Kim, D.H.; Saraswat, R.; Shaver, G.M.; Pol, V.G. Fabrication of Carbon/Silicon Composite as Lithium-ion Anode with Enhanced Cycling Stability. Electrochim. Acta 2017, 247, 626–633. [Google Scholar] [CrossRef]
- Abuzeid, H.M.; Hashem, A.M.; Kaus, M.; Knapp, M.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Electrochemical performance of nanosized MnO2 synthesized by redox route using biological reducing agents. J. Alloy. Compd. 2018, 746, 227–237. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J.; Gou, X.; Shen, P. High-Power Alkaline Zn–MnO2 Batteries Using γ-MnO2 Nanowires/Nanotubes and Electrolytic Zinc Powder. Adv. Mater. 2005, 17, 2753–2756. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Yu, J.; Zeng, J. Anisotropic expansion and high rate discharge performance of V-doped MnO2 for Li/MnO2 primary battery. Int. J. Electrochim. Sci. 2012, 7, 1242–1250. [Google Scholar]
- Chen, J.B.; Wang, Y.W.; He, X.M.; Xu, S.M.; Fang, M.; Zhao, X.; Shang, Y.M. Electrochemical properties of MnO2 nanorods as anode materials for lithium ion batteries. Electrochim. Acta 2014, 142, 152–156. [Google Scholar] [CrossRef]
- Barbato, S.; Restovic, A.; Gautier, J.L. Hollandite cathodes for lithium ion batteries. 1. Synthesis and characterization of the MM′Mn7O16 compounds (M′ = K., Ba; M′ = Mg, Mn, Fe, Ni). Bol. Soc. Chil. Quim. 2001, 46, 165–173. [Google Scholar]
- Li, L.; Raji, A.R.O.; Tour, J.M. Graphene-Wrapped MnO2–Graphene Nanoribbons as Anode Materials for High-Performance Lithium Ion Batteries. Adv. Mater. 2013, 25, 6298–6302. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2012, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Lai, M.; Lu, L. Nanoflaky MnO2/carbon nanotube nanocomposites as anode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 6896–6902. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, J.; Liu, K.; Huang, Z.; Ren, X.; Wang, C.A. Simple synthesis of a double-shell hollow structured MnO2@TiO2 composite as an anode material for lithium ion batteries. RSC Adv. 2017, 7, 46263–46270. [Google Scholar] [CrossRef]
- Zhang, N.; Ding, Y.; Zhang, J.; Fu, B.; Zhang, X.; Zheng, X.; Fang, Y. Construction of MnO2 nanowires@ Ni1−xCoxOy nanoflake core-shell heterostructure for high performance supercapacitor. J. Alloy. Compd. 2017, 694, 1302–1308. [Google Scholar] [CrossRef]
- Liu, C.; Peng, T.; Wang, C.; Lu, Y.; Yan, H.; Luo, Y. Three-dimensional ZnFe2O4@MnO2 hierarchical core/shell nanosheet arrays as high-performance battery-type electrode materials. J. Alloy. Compd. 2017, 720, 86–94. [Google Scholar] [CrossRef]
- Feng, L.L.; Xuan, Z.W.; Zhao, H.B.; Bai, Y.; Guo, J.M.; Su, C.W.; Chen, X.K. MnO2 prepared by hydrothermal method and electrochemical performance as anode for lithium-ion battery. Nanoscale Res. Lett. 2014, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Li, B.X.; Rong, G.X.; Xie, Y.; Huang, L.F.; Feng, C.Q. Low-temperature synthesis of α-MnO2 hollow urchins and their application in rechargeable Li+ batteries. Inorg. Chem. 2006, 45, 6404–6410. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.X.; Li, S.F.Y.; Siow, K.S.; Gao, Z.Q. Synthesis and characterization of the hollandite-type MnO2 as a cathode material in lithium batteries. Electrochim. Acta 2000, 45, 2211–2217. [Google Scholar] [CrossRef]
- Liu, H.D.; Hu, Z.L.; Su, Y.Y.; Hu, R.; Tian, L.L.; Zhang, L.; Ruan, H.B. Facile Preparation of 1D α-MnO2 as Anode Materials for Li-ion Batteries. Int. J. Electrochem. Sci. 2016, 11, 8964–8971. [Google Scholar] [CrossRef]
- Walanda, D.K.; Lawrance, G.A.; Donne, S.W. Hydrothermal MnO2: Synthesis, structure, morphology and discharge performance. J. Power Sources 2005, 139, 325–341. [Google Scholar] [CrossRef]
- Kijima, N.; Sakata, Y.; Takahashi, Y.; Akimoto, J.; Kumagai, T.; Igarashi, K.; Shimizu, T. Synthesis and lithium ion insertion/extraction properties of hollandite-type MnO2 prepared by acid digestion of Mn2O3. Solid State Ion. 2009, 180, 616–620. [Google Scholar] [CrossRef]
- Vernardou, D.; Kazas, A.; Apostolopoulou, M.; Katsarakis, N.; Koudoumas, E. Hydrothermal Growth of MnO2 at 95 °C as an anode material. Int. J. Thin Films Sci. Technol. 2016, 5, 121–127. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, D.L.; Lou, X.W. Shape-Controlled Synthesis of MnO2 Nanostructures with Enhanced Electrocatalytic Activity for Oxygen Reduction. J. Phys. Chem. C 2010, 114, 1694–1700. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhao, T.B. Reduced graphene oxide anchored with MnO2 nanorods as anode for high rate and long cycle Lithium ion batteries. Electrochim. Acta 2016, 201, 165–171. [Google Scholar] [CrossRef]
- Luo, Y.L. Preparation of MnO2 nanoparticles by directly mixing potassium permanganate and polyelectrolyte aqueous solutions. Mater. Lett. 2007, 61, 1893–1895. [Google Scholar] [CrossRef]
- Raj, B.G.S.; Asiri, A.M.; Qusti, A.H.; Wu, J.J.; Anandan, S. Sonochemically synthesized MnO2 nanoparticles as electrode material for supercapacitors. Ultrason Sonochem. 2014, 21, 1933–1938. [Google Scholar]
- Zhang, X.; Yu, P.; Wang, D.L.; Ma, Y.W. Controllable Synthesis of alpha-MnO2 Nanostructures and Phase Transformation to β-MnO2 Microcrystals by Hydrothermal Crystallization. J. Nanosci. Nanotechnol. 2010, 10, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, A.; Bystrom, A.M. The Crystal Structure of Hollandite, the Related Manganese Oxide Minerals, and α-MnO2. Acta Crystallogr. 1950, 3, 146–154. [Google Scholar] [CrossRef]
- Hicks, A.L.; Dysart, A.D.; Pol, V.G. Environmental impact, life cycle analysis and battery performance of upcycled carbon anodes. Environ. Sci. Nano 2018, 5, 1237–1250. [Google Scholar] [CrossRef]
- Liang, G.; Qin, X.; Zou, J.; Luo, L.; Wang, Y.; Wu, M.; Zhu, H.; Chen, G.; Kang, F.; Li, B. Electrosprayed silicon-embedded porous carbon microspheres as lithium-ion battery anodes with exceptional rate capacities. Carbon 2018, 127, 424–431. [Google Scholar] [CrossRef]
- Peng, B.; Xu, Y.; Wang, X.; Shi, X.; Mulder, F.M. The electrochemical performance of super P carbon black in reversible Li/Na ion uptake. Sci. China Phys. Mech. Astron. 2017, 60, 064611. [Google Scholar] [CrossRef]
- Kim, K.; Adams, R.A.; Kim, P.J.; Arora, A.; Martinez, E.; Youngblood, J.P.; Pol, V.G. Li-ion storage in an amorphous, solid, spheroidal carbon anode produced by dry-autoclaving of coffee oil. Carbon 2018, 133, 62–68. [Google Scholar] [CrossRef]
- Kim, K.; Lim, D.G.; Han, C.W.; Osswald, S.; Ortalan, J.P.; Youngblood, J.P.; Pol, V.G. Tailored Carbon Anodes Derived from Biomass for Sodium-Ion Storage. ACS Sustain. Chem. Eng. 2017, 5, 8720–8728. [Google Scholar] [CrossRef]
- Drewett, N.E.; Aldous, I.M.; Zou, J.L.; Hardwick, L.J. In situ Raman spectroscopic analysis of the lithiation and sodiation of antimony microparticles. Electrochim. Acta 2017, 247, 296–305. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, S.; Ji, X.; Jiang, Y.; Zhou, J.; Liu, M. Investigations into the origin of pseudocapacitive behavior of Mn3O4 electrodes using in operando Raman spectroscopy. J. Mater. Chem. A 2015, 3, 7338–7344. [Google Scholar] [CrossRef]
- Julien, C.; Massot, M.; Baddour-Hadjean, R.; Franger, S.; Bach, S.; Pereira-Ramos, J. Raman spectra of birnessite manganese dioxides. Solid State Ion. 2003, 159, 345–356. [Google Scholar] [CrossRef]


| References | Synthesis Method | Initial Capacity (Current Density) | Cycle Retention | RateCapacity | Electrode |
|---|---|---|---|---|---|
| Chen [8] | KMnO4 + HCl Hydrothermal | 1119 mAh g−1 (100 mA g−1) | 96% (100 cycles) | 1000 mAh g−1 (200 mA g−1) | Anode |
| Vernardou [1] | KMnO4 + Nitric Acid Hydrothermal | 943 mAh g−1 | N/A | N/A | Anode |
| Walanda [20] | Hydrothermal of Mn2O3 in H2SO4 | ~1100 mAh g−1 (20 mA g−1) | N/A | N/A | Anode |
| Li [17] | MnSO4 + H2SO4 Hydrothermal | 750 mAh g−1 (270 mA g−1) | 64% (40 cycles) | N/A | Anode |
| Dai [18] | MnSO4 + H2SO4 Ozone Oxidation | 200 mAh g−1 (20 mA g−1) | 82.5% (20 cycles) | N/A | Cathode |
| Abuzeid [5] | KMnO4 + H2SO4 green-like synthesis method | 236 mAh g−1 (20 mA g−1) | 73% (20 cycles) | 75 mAh g−1 (200 mA g−1) | Cathode |
| Our Study | KMnO4 + NH4OH Stirring and precipitation | 800 mAh g−1 (50 mA g−1) | 91% (200 cycles) | 600 mAh g−1 (200 mA g−1) | Anode |
| 250 mAh g−1 (50 mA g−1) | 93% (200 cycles) | 100 mAh g−1 (200 mA g−1) | Cathode |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A.; Pol, V.G. Basic Medium Heterogeneous Solution Synthesis of α-MnO2 Nanoflakes as an Anode or Cathode in Half Cell Configuration (vs. Lithium) of Li-Ion Batteries. Nanomaterials 2018, 8, 608. https://doi.org/10.3390/nano8080608
Kim K, Daniel G, Kessler VG, Seisenbaeva GA, Pol VG. Basic Medium Heterogeneous Solution Synthesis of α-MnO2 Nanoflakes as an Anode or Cathode in Half Cell Configuration (vs. Lithium) of Li-Ion Batteries. Nanomaterials. 2018; 8(8):608. https://doi.org/10.3390/nano8080608
Chicago/Turabian StyleKim, Kyungho, Geoffrey Daniel, Vadim G. Kessler, Gulaim A. Seisenbaeva, and Vilas G. Pol. 2018. "Basic Medium Heterogeneous Solution Synthesis of α-MnO2 Nanoflakes as an Anode or Cathode in Half Cell Configuration (vs. Lithium) of Li-Ion Batteries" Nanomaterials 8, no. 8: 608. https://doi.org/10.3390/nano8080608
APA StyleKim, K., Daniel, G., Kessler, V. G., Seisenbaeva, G. A., & Pol, V. G. (2018). Basic Medium Heterogeneous Solution Synthesis of α-MnO2 Nanoflakes as an Anode or Cathode in Half Cell Configuration (vs. Lithium) of Li-Ion Batteries. Nanomaterials, 8(8), 608. https://doi.org/10.3390/nano8080608