1. Introduction
In the last decade or so, there have been several works on synthesis strategies and determination of novel properties of crystalline semiconductor nanowires (NWs) [
1]. This intense research effort has been motivated by the fact that crystalline NWs have promising electronic, photonic and optoelectronic properties, making them ideal for a large number of applications. Their unique properties, as determined by their one-dimensional structure, make them attractive as sensors and in photovoltaic, and thermoelectric [
1] applications. Of the semiconducting NWs, the III-V group of semiconductors are promising and of these indium antimonide (
) is particularly interesting because it has the smallest direct energy band gap (0.17 eV at room temperature) [
2], and very high electron mobility (77,000 cm
V
s
) [
3]; making it an ideal material for use as field effective transistors and long-wavelength detectors [
4,
5]. Crystalline
(
c-
) NWs have been synthesized by various techniques such as pulsed electrodeposition technique [
2], pulsed-laser chemical vapor deposition [
6], molecular beam epitaxy [
7,
8], metal organic vapor phase epitaxy [
9], and chemical vapor deposition [
10,
11]. In most cases, growth of
c-
NWs follows the metal-catalyst assisted vapor-liquid-solid (VLS) growth mechanism [
1], and NW growth continues as long as
and
vapors are supplied to the molten alloy droplet.
Compared to crystalline semiconductor NWs, there have been limited investigations into amorphous NWs. This is most likely due to challenges in the synthesis of nanoscale amorphous materials [
12]. The two main categories of amorphous semiconductors are: chalcogenide glasses which include elements of VI and their compounds, and the tetrahedrally bonded semiconductors which include
,
, and amorphous III-V compounds [
13]. Though several techniques are available for the characterization of
c-
, experimental techniques that allow investigation of disordered amorphous
(
a-
) are very limited. It is, therefore, important to develop an effective strategy for the synthesis of
a-
NWs, where the amorphous phase and band structure can be tailored to enable the use of these NWs for energy storage and conversion applications [
12].
Amorphous semiconductors are in general characterized by the absence of long-range order, but they preserve the short-range order that is characteristic of the crystal [
14]. In the amorphous phase, the
-
bonds are longer (2.832
) and a large number of homopolar
-
and
-
bonds exist [
14]. This lack of long-range order in amorphous materials has led to limitations in understanding their intrinsic electronic properties. There are reports on instabilities in measured current in this class of material, caused by defect distribution on surface as well as surface morphology and charge trapping [
15,
16]. The electrical properties depend not just on device geometry and nature of barrier between material and electrodes, but also on the level of disorder in the system. This causes amorphous materials of the same type to have very different properties. The other notable difference is in the electron transport mechanism in crystalline and amorphous materials. In crystalline materials, electrons are driven across a potential energy barrier that exists between the two metal contacts and the semiconducor as their Fermi levels align. However, in many amorphous materials, the current has a power law dependence on bias (electric fields) [
17] and its magnitude is dependent on the defect type (Frenkel or Poole, charged or uncharged) and concentration.
Most III-V semiconductors like
have a tetrahedral bonding geometry involving
and
atoms, with a coordination number of four. This bonding geometry is maintained in
a-
, but with slight elongation of the interatomic distances [
14]. Because of the disorder in
a-
, the band edges lose their sharpness and there exists a large density of localized states that tails into the energy band gap [
18,
19]. The localized electronic states causes carrier scattering, resulting in low carrier mobility, and also induces charge trapping [
19,
20]. Despite the fact that amorphous semiconductors have a high concentration of defects, the limited number of electrically active charge carriers causes them to be poor electrical conductors [
13], with electrical resistivity values that are several orders of magnitude higher than the corresponding values in crystalline state. A similar difference is observed in the optical spectrum of amorphous materials, where the atomic disorder in the material causes the spectrum to be broadened with no sharp peaks. The X-ray diffraction pattern of amorphous semiconductors reveal no sharp spots, but shows intense diffusion rings or halos [
13]. It has been reported [
21] that the energy band gap of
a-
ranges from about 0.4 to 0.65 eV, a four times increase compared to the energy band gap of
c-
.
Synthesis of amorphous materials is generally low-cost and is carried out by relatively simple techniques. Due to absence of crystal quality constrains, such materials have structural flexibility and it becomes possible to construct materials with properties that cannot exist in the corresponding crystalline state [
19]. It is also possible to heat-treat amorphous materials and convert them into a crystalline state, thus providing an economically viable route for the synthesis of crystalline semiconductors [
12]. Since amorphous semiconductors are known to have high light absorption capability and high temperature coefficient of resistance, they are attractive in photoelectric and clean energy device applications [
12]. Amorphous bulk materials or films are typically grown by two mechanisms: (i) lower the temperature abruptly so that the source melt is frozen in what is called the ‘melt-quenched amorphous phase’; (ii) growth from a vapor phase using techniques like thermal evaporation or a chemical vapor deposition (CVD) [
13,
14]. Previous reports include deposition of
a-
film on various substrates by techniques such as flash evaporation on a cold substrate [
21] and by sputtering at low temperature [
22]. The studies validate the increases in energy band gap of
a-
film from 0.17 eV up to 0.65 eV, and a significantly higher electrical resistivity. In terms of low-dimensional
a-
structures, there are reports on the fabrication of high density near amorphous
NW arrays by electrodeposition method [
23], where the amorphous nature of the NWs is attributed to three factors: (i) high concentration of the deposition solution, (ii) high concentration of the complexing agents, and (iii) high deposition voltage. Under these circumstances, the high concentration ions have no choice but to follow the lower energy site to deposit and grow. In another work, an array of polycrystalline
NWs was fabricated by electrodeposition [
24], followed by two annealing steps: at 150
C and 450
C. The low temperature anneal enabled diffusion of
and
atoms in the
crystal and the high temperature anneal improved the crystalline quality of the NWs.
The process for synthesizing high quality
c-
NWs in a CVD system has been well documented [
10,
11,
25]. However, there has been no report on the growth of
a-
NWs and hence no appropriate growth mechanism has been established. In this paper, we report on a strategy to grow
a-
NWs in the CVD system. The lack of crystallinity in the as-grown NWs is attributed to the effect of a high temperature ramp-up rate. This work also highlights the effect of post-growth annealing treatment on the electronic and optical quality of the
a-
NWs.
3. Result and Discussion
The experimental design and growth parameters were optimized to ensure large aerial coverage of the
substrate with
NWs. For a given thickness of the
film on the
substrate, our experiments show that NW growth occurs in a very narrow temperature range. For an
film of thickness in the range of 200–300 nm, the growth temperature for stoichiometric
NWs was determined to be in the range of about 550–570
C, and the as-grown NWs had diameters in the range of 200–350 nm. For an ∼100 nm thick
film, a growth temperature of 550–570
C resulted in non-stoichiometric
NWs with very high
content and relatively larger diameter (∼150 nm).
NWs grown by the technique described in this paper had diameters larger than the thickness of the seed film (
) . One possible reason for this could be the fact that III–
NWs are reported to generally show large diameters [
26] compared to other semiconductors. Moreover, the high temperature ramp-up rate, resulting in formation of large
islands also affects NW diameter. The average length of these NWs was ∼15
m. A more detailed explanation of the role of temperature ramp-up rate on the NW diameter is explained in the following paragraph that details the NW growth mechanism.
The role of sulfur in this work is that of a passivating agent [
27]. Sulfur has a low solubility in the molten
seeds. It also has a high vapor pressure, which implies that at the growth temperature it is driven out of the growth region. This is why an EDX spectrum of the NWs did not show the presence of any sulfur. The addition of sulfur was critical to the growth process. An alternative would be to grow in 100% H
, but high concentration of H
lowers the vapor pressure of
and so the resulting NWs are mostly just metallic
. Therefore, to maintain sufficient concentration of both
and
in the growing NW, it is important to have sulfur so that any trace of O
, can be removed from the growth region. To confirm that the NWs are purely
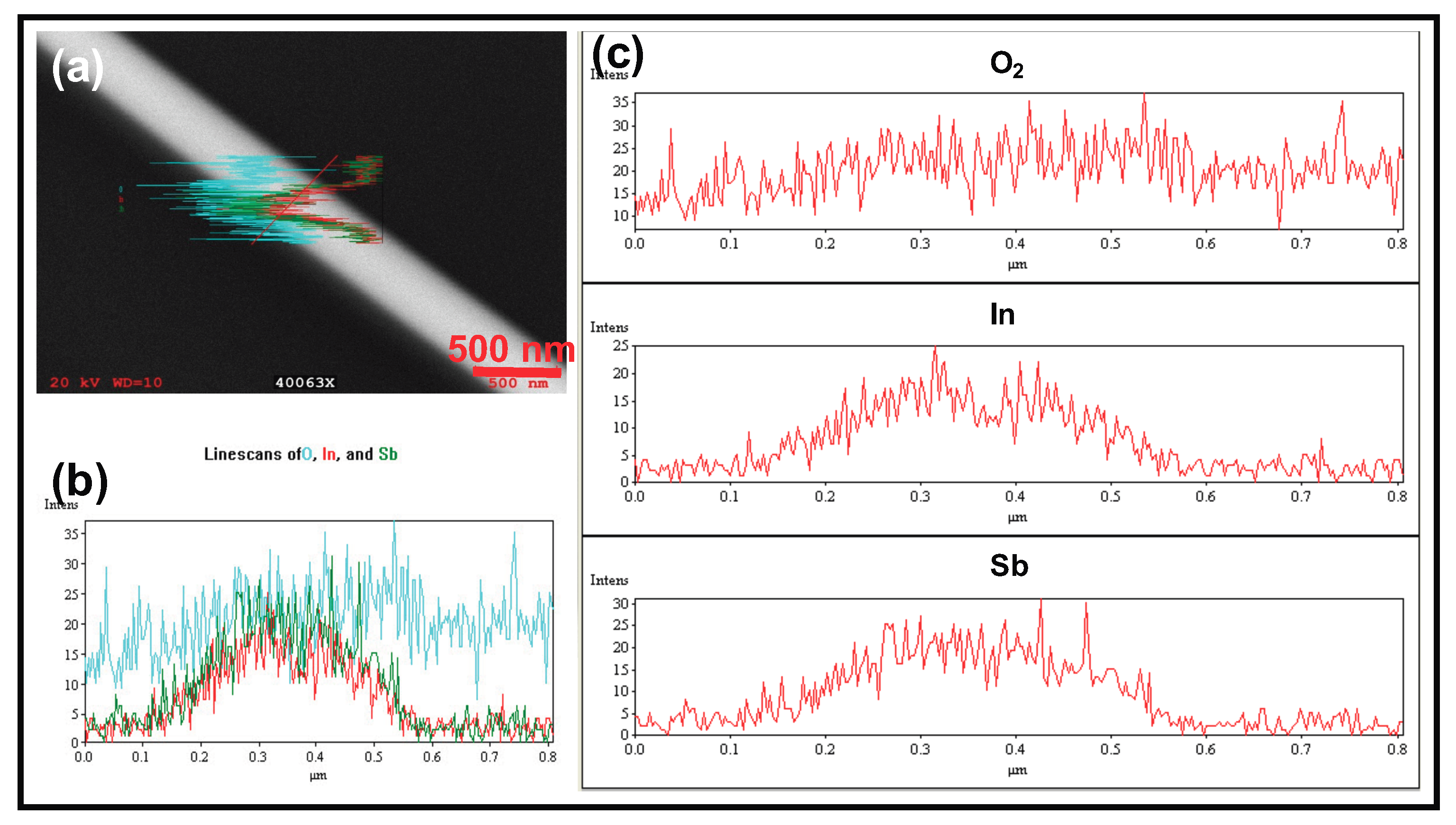
and there was no oxygen in the bulk of the NW, an EDX profile was obtained by performing a line scan in a direction perpendicular to the NW axis. As seen in
Figure 1, the
and
signals (red and green curves) show maximum intensity at the center of the NW. The O
signal (cyan) is relatively flat and has no characteristic feature across the width of the NW. Its presence is attributed to the fact that at 20 kV, the electron beam penetrates the
NW and the O
signal arises from the 200 nm thick SiO
layer that coats the
substrate.
A notable feature of the experimental design is the high temperature ramp-up rate. The source temperature was increased at a rate of 75
C/min, until it reached the set point of 560
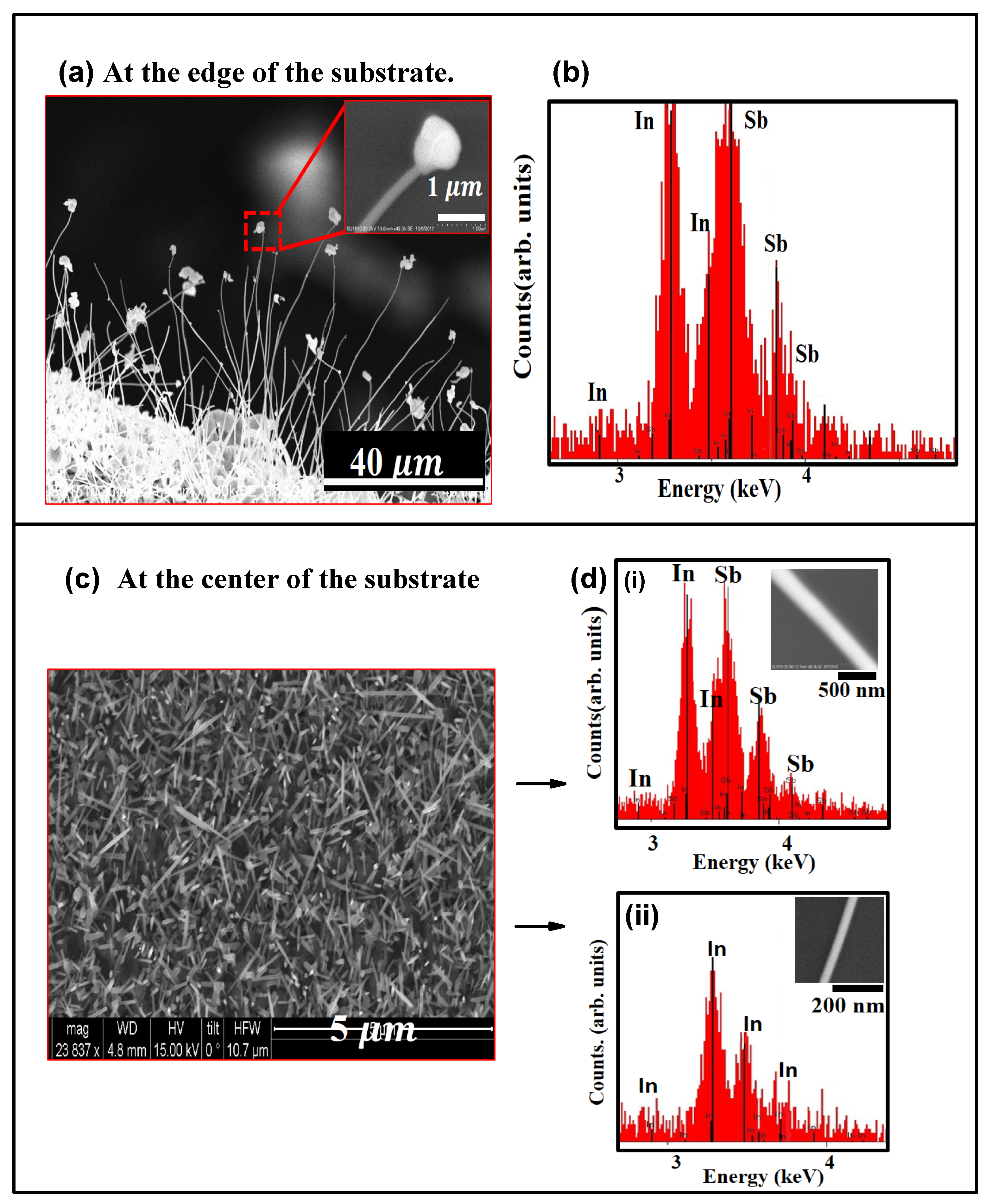
C. At such high temperature ramp up rates, the NW growth mechanism is most likely modified. Though growth most likely follows the VLS mechanism, it is possible that the high temperature ramp-rate affects the crystallinity of the growing NWs. SEM images depicting the morphology of the as-grown structures at the edge and at the center of the substrate is shown in
Figure 2a,c respectively. During the initial stage of growth, as the temperature was raised up to the set point at the high rate of 75
C/min, the 200 nm thick
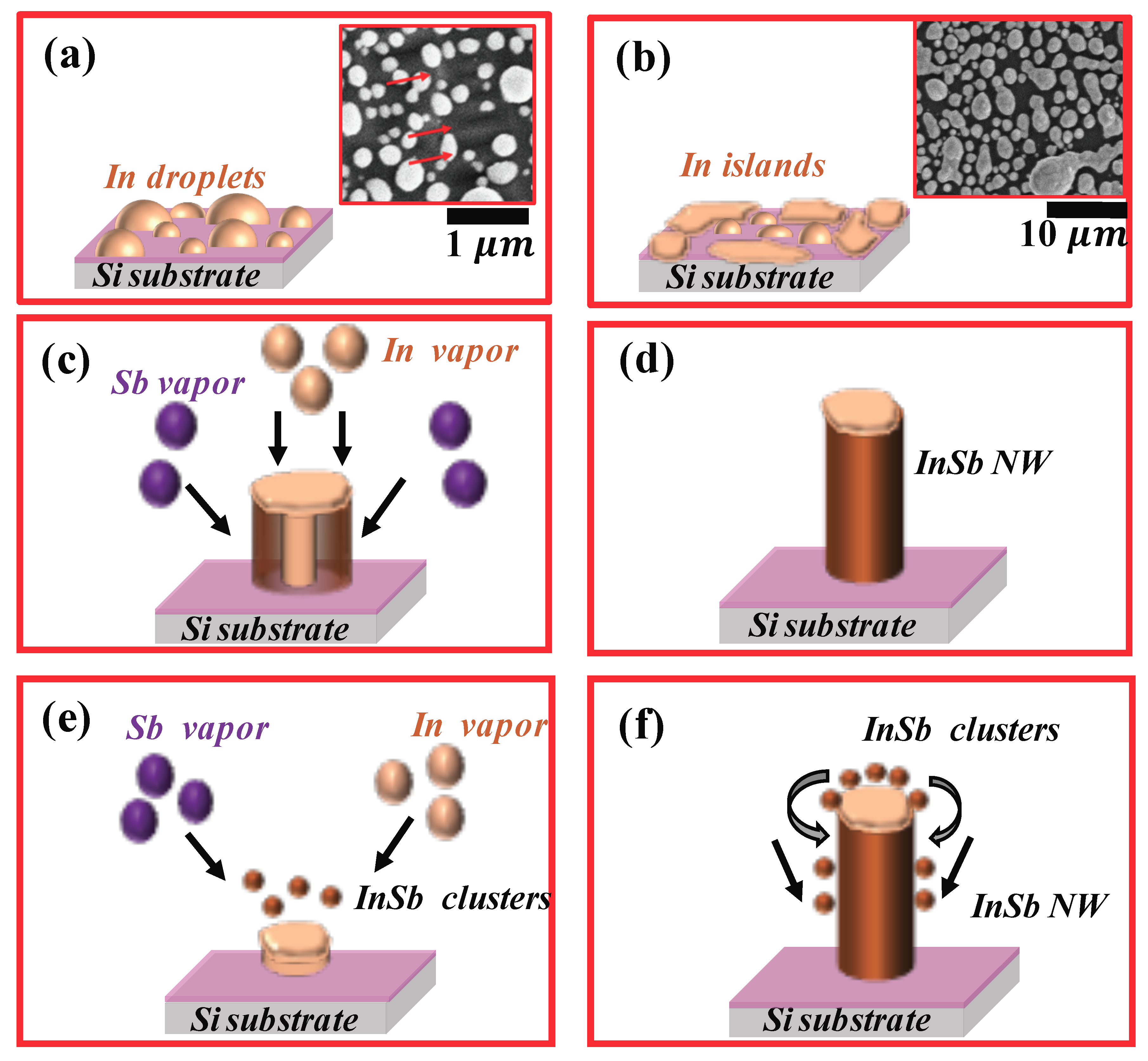
film breaks up into droplets. These droplets are not uniform in size and as seen in
Figure 3a, the droplets range in diameter from about 100 nm to about 300 nm. As the temperature continues to increase, the In droplets increase in size by coalescing with other droplets nearby. These droplets have sufficient energy to migrate to the edge of the substrate where they are eventually immobilized as seen in
Figure 3b. Therefore, the
substrate has relatively large sized In-islands at the edges and smaller sized droplets at the center of the substrate. As seen in the SEM image shown in
Figure 2a, the edges of the
substrate has a high density of
NWs. The NWs grow off small nucleating sites on islands or clusters of
and most of them were found to have a deformed bulky tip, as seen in the inset of
Figure 2a. As shown in the EDX spectrum of
Figure 2b, the NWs are stoichiometric
with an
:
ratio of 1:1. Contrary to the deformed tips on NWs growing along the substrate edge, it was found that majority of the NWs on the body of the substrate did not have a tip. This is not in violation of the VLS NW growth mechanism, since NW growth was enabled by a self-seeded mechanism and it is possible that
was consumed during the growth process. An SEM image of the NWs grown at the center of the
substrate (
Figure 2c), shows that the NWs have different diameters, attributed to non-uniformity in the seed sizes (
Figure 3a). It was also found that NWs with relatively large diameters (d > 200 nm) were mostly
(
Figure 2d-i), while the thinner NWs at the center of the substrate (d < 100 nm) were mostly pure
In, as seen in the EDX spectrum of
Figure 2d-ii.
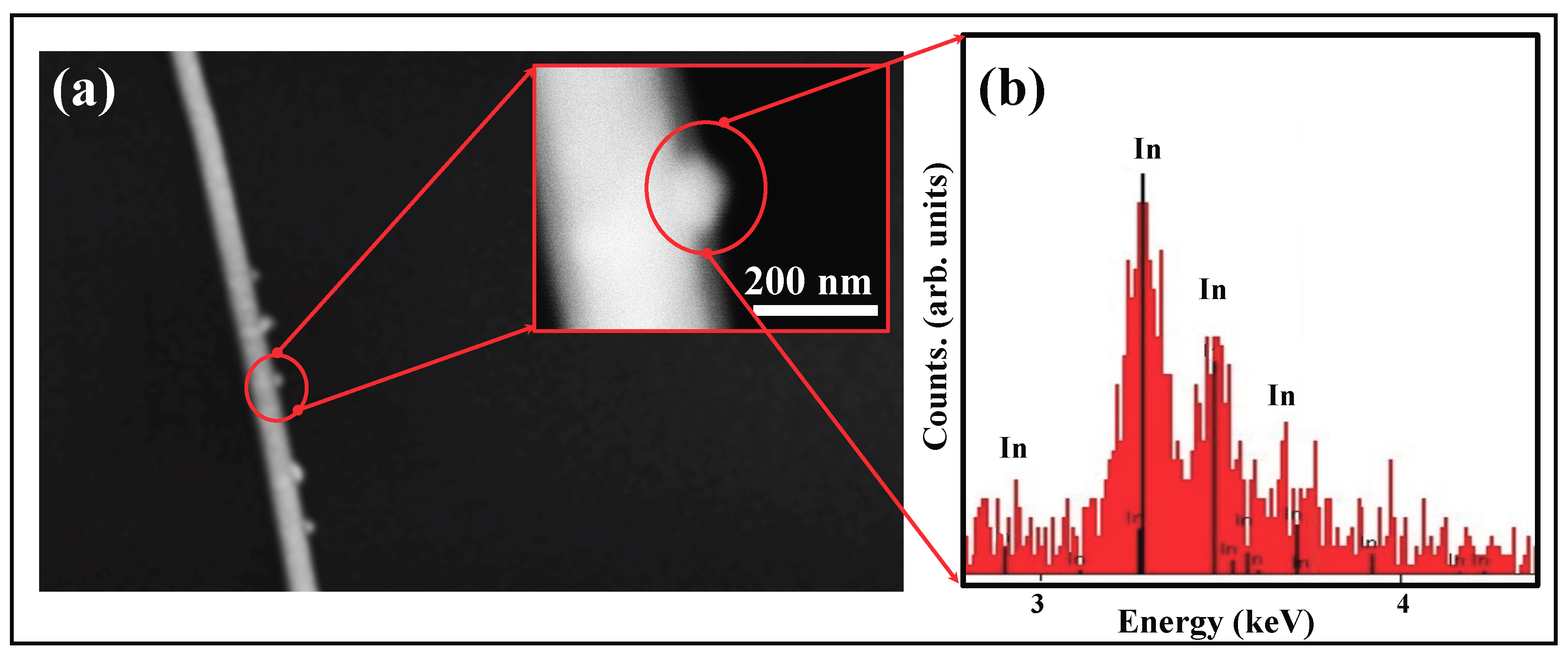
Another significant feature of some of the NWs is shown in
Figure 4a, where metallic In precipitates out of the growing NW and is present as `nodules’ on the edge of the NW. As seen in the EDX spectrum (
Figure 4b), these nodules are pure
. Such precipitates of the metal catalyst along the NW edges have been reported by other groups. Their appearance is attributed to evaporation of the element
from the source and subsequent condensation along the NW edges, following the mechanism discussed in Refs. [
29,
30].
To explain the amorphous nature of the as-grown NWs, we consider the situation where as growth progresses, the temperature increases very rapidly to the growth temperature. The droplets receive incoming flux of
and
to reach the stage of supersaturation, from which the
solid phase precipitates out. The transformation from the liquid to solid phase requires some level of cooling of the molten droplet. As the solid NW phase begins to separate out from the highly disordered molten alloy, the droplet is cooled. Heat (q) is taken away from the droplet at the rate
[
31], and the temperature (T) of the molten droplet decreases at a rate
[
31] where
is heat capacity. The cooling rate of the molten alloy droplet determined by (dT/dt) is an indicator of the crystalline quality of the growing NW. The physical, chemical and thermodynamic condition of the molten droplet during this solidification phase will determine the arrangement of the atoms in the growing NW. The criteria for NW growth as expressed by the inequality equation between chemical potentials,
[
32] requires that the Gibbs free energy for a solid becomes less than that of the liquid (
<
). The difference in the Gibbs free energy provides the driving force for the liquid to solid phase transformation and is expressed as
, where H is enthalpy of fusion and S is entropy in the system. At the temperature T, as the nucleating droplet begins to precipitate the solid phase as a NW, the arrangement of atoms in the nuclei is critical. The relatively high entropy of the nucleating site implies that there are movements of atoms in the tetrahedral arrangements, causing bonding and compositional changes in the growing NW. This rapid movement of
and
atoms in the solidifying (cooling) molten liquid is attributed to the high temperature ramp-rate and is the most likely cause for the amorphous nature of the
NW.
The exact mechanism wherein the
a-
NWs solidifies from a molten catalyst is not very evident. A simple schematic of the
NW growth mechanism for the experimental conditions described above is shown in
Figure 3. The process could follow either of the mechanisms discussed below and is based on the growth mechanism cited for synthesis of a-SiO
NWs [
28]. The first possible growth mechanism is based on the fact that due to the high volume of metallic
in the seed, an In NW initially precipitates out of the molten seed. As the temperature is rapidly raised to the growth temperature,
vapors impinge on the growing NW leading to antimonidization of the
NW. In this mechanism, the initially formed highly metallic
core is antimonidized; the antimonidization process continues as long as the
NW core exists. A simple schematic of the process is shown in
Figure 3c,d There is a high possibility that antimonidization of an
NW core accounts for the synthesis of
a-
NW, because when the
vapor pressure is reduced, the resulting NWs are pure metallic
(
Figure 2d-ii). A second possible mechanism that results in the formation of
a-
NWs is the case where the
does not penetrate the
droplet. Instead,
clusters form over the molten In droplet. Due to the low solubility of
in
, the
clusters slip to the lower hemisphere of the molten catalyst and begins to grow as a 1-D NW. Continuous supply of
clusters then support the growth of an
a-
NW as shown in
Figure 3e,f.
Raman spectrum of
c-
is well documented and is known to be characterized by TO phonon 179.8 cm
and LO phonon modes 190.8 cm
[
6,
10,
33,
34,
35,
36]. The amorphous nature of a single as-grown
NW was first verified by Raman spectroscopy studies at room temperature (
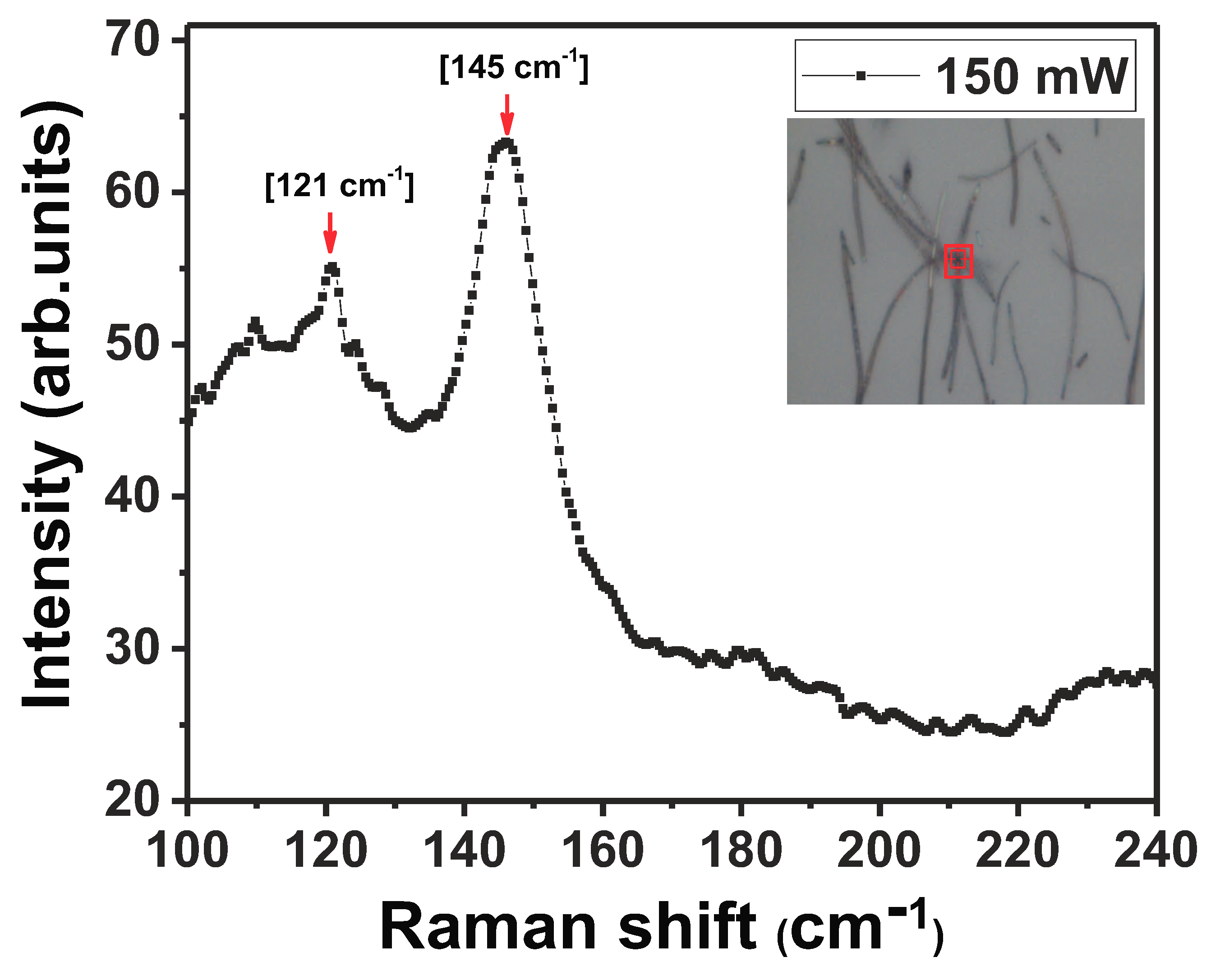
Figure 5). The stoichiometric NW (In:46.9 wt%, Sb: 53.1 wt%) exhibited a Raman spectrum with a relatively wide peak at low frequency, centered around ∼145 cm
. This peak has been attributed to a high density of homopolar
-
bonds that are found in
a-
[
37,
38,
39]. The aggregation of
-
defects is attributed to growth conditions which include NW growth in an
-rich environment and a high temperature ramp-up rate. Additionally, a shoulder peak was detected around 120 cm
. This peak was observed in several amorphous semiconductors like
a-
,
a-
,
a-
and
a-
[
39,
40], and is reported to occur near the LA branch of a crystal. To study the effect of laser light on the amorphous nature of the as-grown
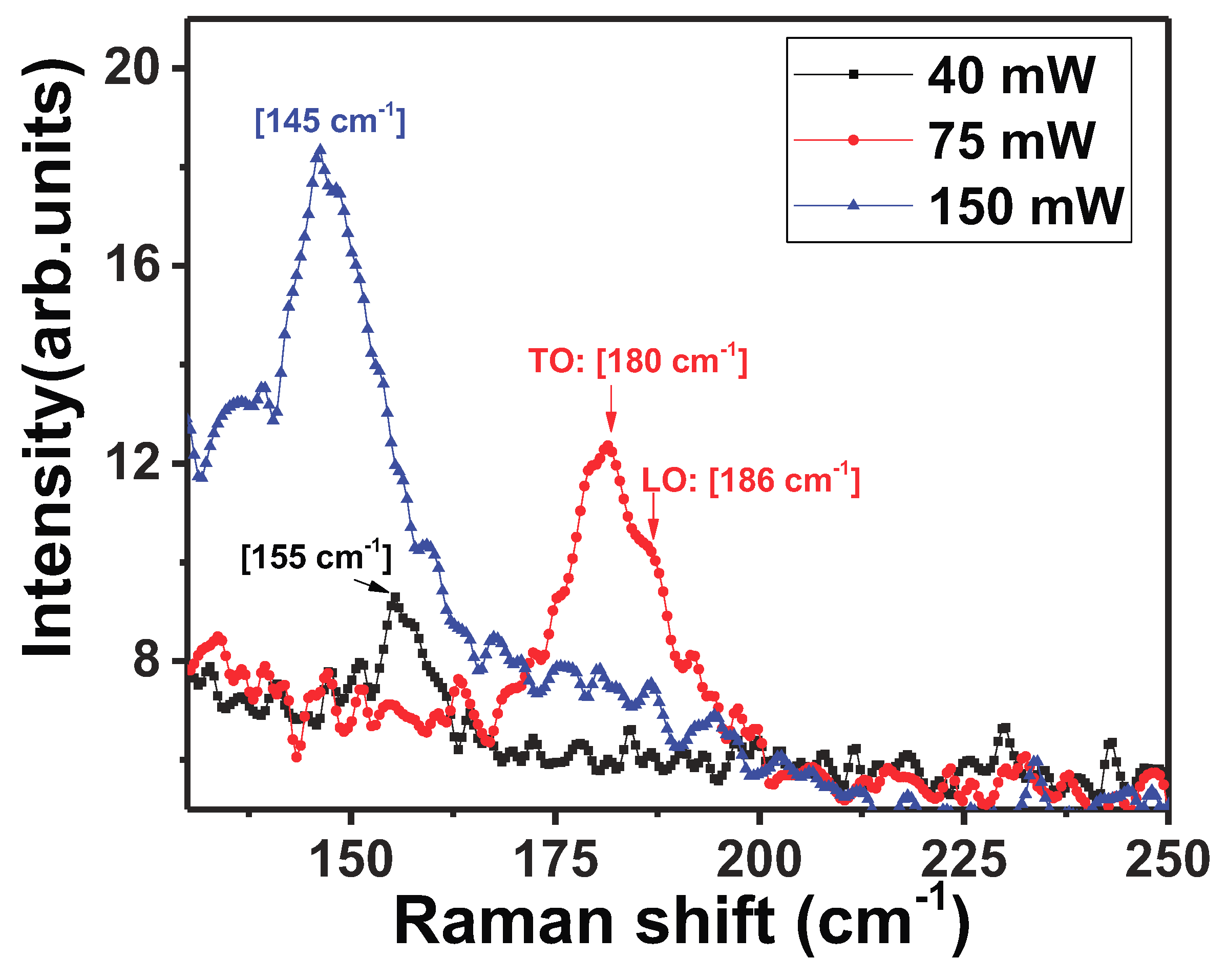
NWs, the evolution of the Raman spectrum as a function of laser power was studied at room temperature, by measuring the Raman spectrum at the same spot on the NW for three different laser intensities. The results are shown in
Figure 6. The broad peak around ∼155 cm
, attributed to the presence of
-
bonds is evident at the low laser power of 40 mW. The laser beam was focused at a spot on the NW for 60 s. As the laser power was increased to 75 mW, the peak attributed to
c-
at 181 cm
(TO phonon) and at 186 cm
(LO phonon) appear as shown in
Figure 6. The observation of these peaks indicates that the laser light induced localized crystallization of the area hit by the laser beam. Further increase in the laser intensity causes the crystalline peaks at 181 cm
and 186 cm
to disappear and the peak attributed to
a-
re-appears around 145 cm
. It is thus evident that with increasing laser power, structural defects of the
-
type form, returning the crystal to its amorphous state. The slight shift (about 10 cm
) in the position of the peaks in
a-
is most likely induced by stress in the highly defective material. Laser light intensity is thus shown to cause radiation-stimulated diffusion of defects in
a-
.
To ascertain the possibility that thermally activated defect diffusion is also a possibility,
a-
NWs were subjected to post-growth heat treatments and their electronic quality was assessed by analyzing their current-voltage behavior. In the first set of experiments, as-grown
a-
NWs were annealed in a tube furnace for 4 hrs under flow of Ar gas at 150
C. Raman spectrum showed that the NWs retained their amorphous nature. In the second set of experiments, the as-grown
a-
NWs were annealed in an inert environment at 150
C for 18 hrs. Current-voltage response of the three devices [Device A (as-grown
a-
NWs); Device B (
a-
NWs after 4 hrs of thermal anneal at 150
C); and Device C (
a-
NWs after 18 hrs of thermal anneal at 150
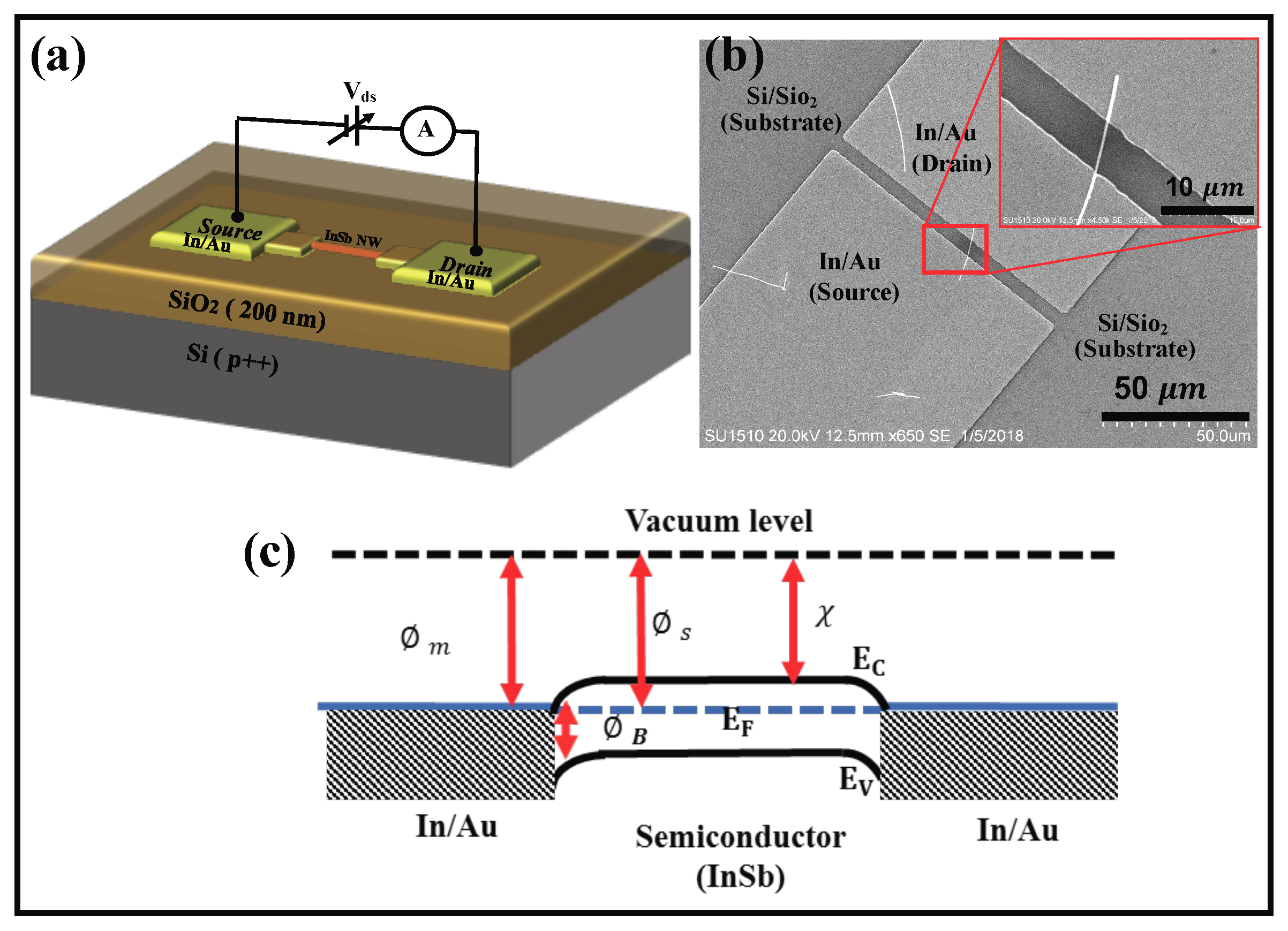
C)] was analyzed. The device schematic is shown in
Figure 7a.
Figure 7b is an SEM image of the contacted NW; inset shows an enlarged view of the channel that contained a single
NW. The channel length was determined to be 5
m. The magnitude of the current through the device depends on the characteristic of the metal-semiconductor contact and the intrinsic properties of the
a-
. Due to the device geometry, the effective area of the metal contact with the NW is ≈10
m
, corresponding to the total metal-semiconductor contact area. Since the two contacts to the NW are the same (
/
contacts), a simplified energy band diagram of the metal-nanowire-metal contact in the absence of any applied bias is as shown in
Figure 7c, where the
a-
is considered to be n-type. Considering that the metal contact is
/
(defined by a work function of 5.1 <
< 4.09), the metal-semiconductor contact is characterized by the case where the metal work function is less than that of the semiconductor. Though the work function of
a-
is not documented, assuming a work function close to
c-
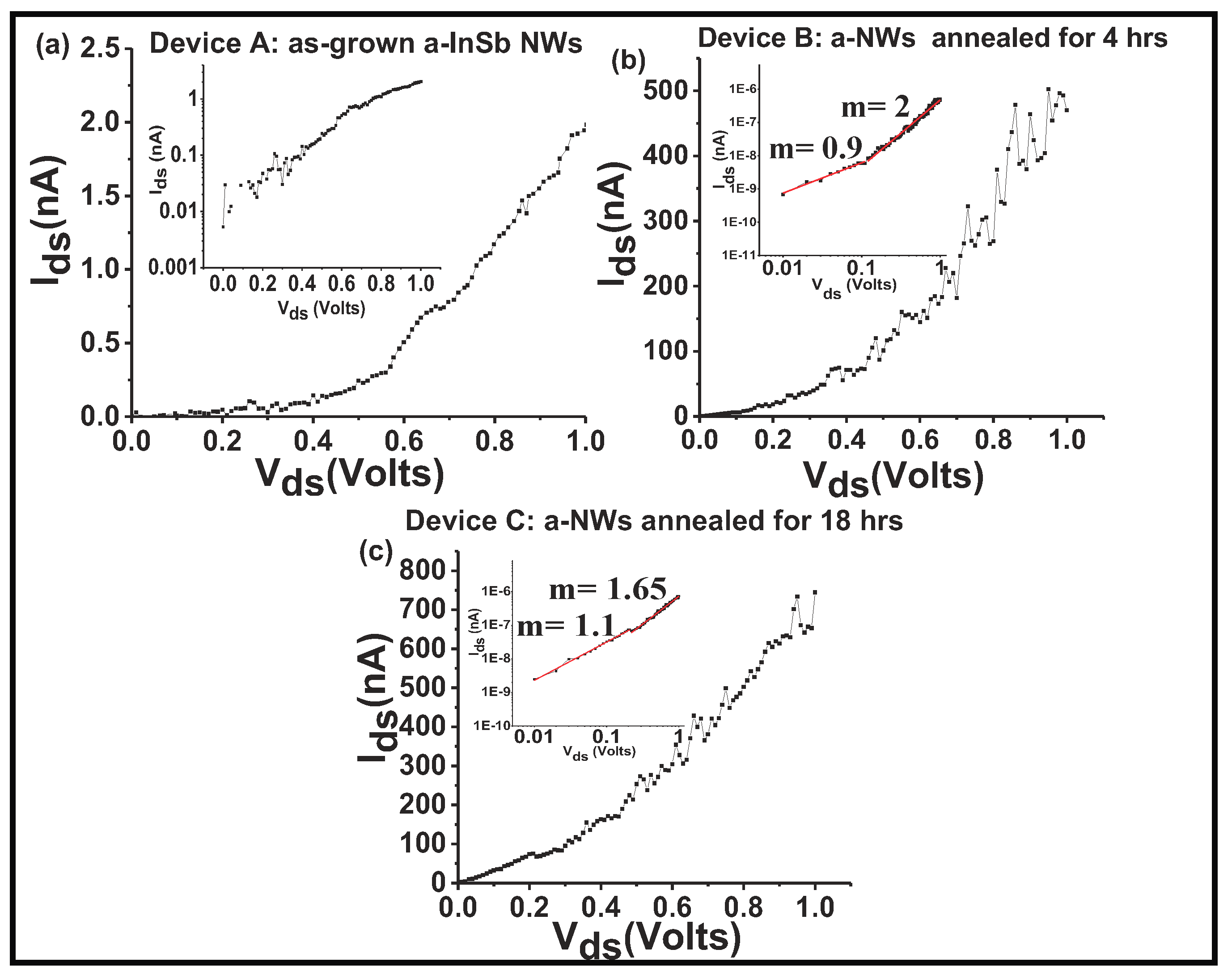
(4.57 eV), it is possible that the barrier height at both the interfaces are negative and the contacts are ohmic. The magnitude of the current through the device would therefore be determined by the resistance of the nanowire. To validate the hypothesis that the contacts are ohmic, the non-linear I–V plots (
Figure 8a–c) recorded at room temperature were analyzed. The I–V data for device A (as-grown NWs) was first studied to assess a possible Schottky diode behavior by creating a semi-log plot of the I–V curve. For a Schottky diode behavior, the semi-log plot of I–V should exhibit linear characteristics. The non-linearity of the I–V curve (shown in the inset of
Figure 8a) confirms that the metal-semiconductor contact is not a Schottky contact. Moreover, the ideality factor (
n), estimated from the fitting parameter q/nkT gives n much grater than 100, whereas
for an ideal Schottky junction. It was hence concluded that the non-linearity in the I–V curves is not due to current through a Schottky junction.
There are three fundamental carrier injection processes: (i) conduction by thermionic emission, (ii) conduction by space-charge-limited-current (SCLC), and (iii) Fowler-Nordheim (F-N) tunneling induced conduction. To ascertain a power dependence, a log-log plot of the I-V curves was analyzed (
Figure 8b,c). For all three devices, in the low-bias regime the I-V plot is linear (m ≈ 1), which implies that in the voltage ranges below ≈ 0.4 V, the conduction mechanism is related to thermionic emission type of conduction, and for all three devices, in the low-bias regime, I ∝ V and the device obeys Ohm’s law. The linear fit to the log-log plots for all three devices for bias above 0.4 V shows values of slope varying from 3.1 to 1.6, indicating that in this relatively high-bias regime, I ∝
, with m decreasing as the NW device is annealed for longer durations. For the as-grown NW device,
m = 3.2, while for NW that was annealed for 4 hrs the value of m decreases to 2 and for the NW that was annealed for 18 hours, m decreases further to 1.6. The high values of m are consistent with the space-charge-limited current (SCLC) conduction model [
20,
41]. A possible explanation for different transport mechanisms in the low and high bias regimes and for the changing m is the existence of a high density of defects at the metal-semiconductor interface. These defects can trap injected electrons and increase the electric field at the interface. After 18 hrs of annealing, the 50% reduction in the value of m indicates that the NW is becoming more crystalline and the conduction becoming more ohmic. A comparison of the NW resistivity shows that the as-grown sample has a high resistivity of ∼19 × 10
± 4 ×
cm. For the
NWs that were annealed at 150
C for 4 hrs, the resistivity drops to about 7 ± 2
cm and the NW that was annealed for 18 hrs has a resistivity of 2 ± 0.2
cm. These values are about 10 times higher than that reported for
c-
NWs that have a resistivity of about 0.3
cm [
42]. These results indicate that the electrical properties of the
a-
NWs are related to the quality of the NWs and also to the presence of defect states. At low bias fields, the mechanism of carrier transport is found to be via thermionic field emission. However, at high bias, the interface defect states modify the charge transport mechanism and conduction occurs via the SCLC.