Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis
Abstract
1. Introduction
1.1. Epidemiology
1.2. Conventional Treatment
2. Fluorescence Guided Surgery
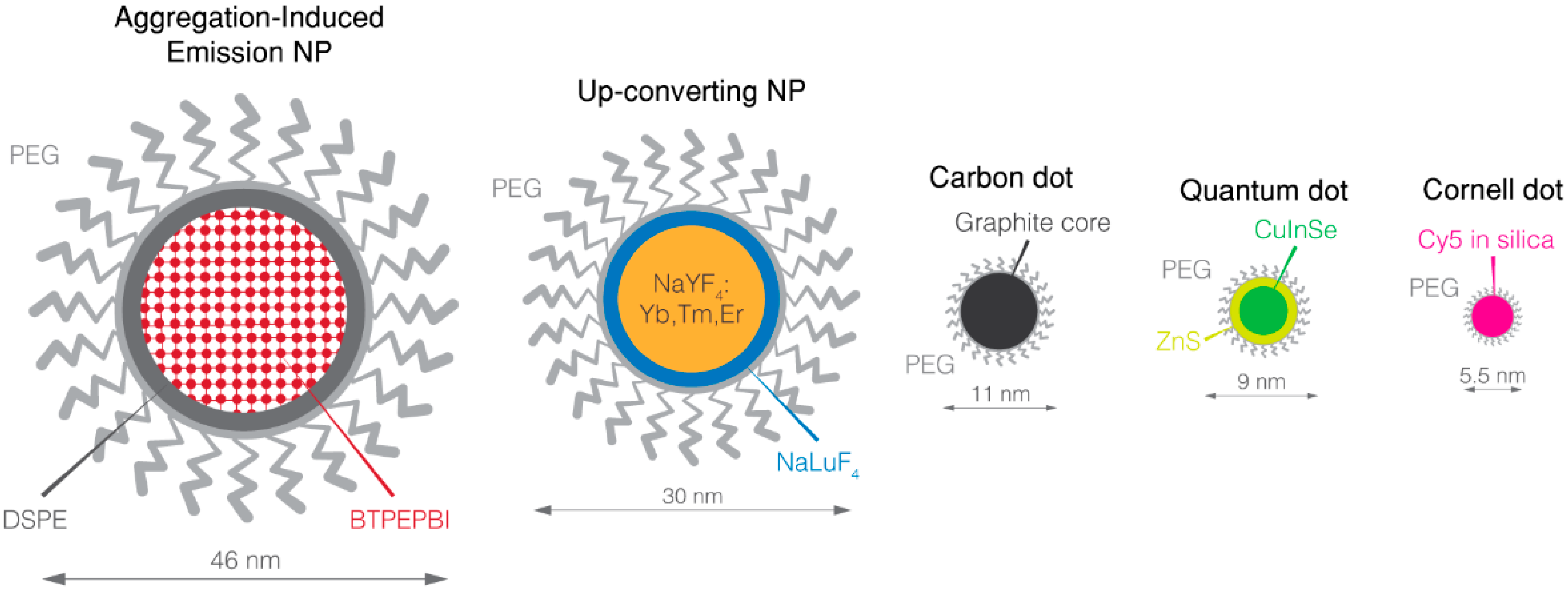
3. Overview of NIR Nanoparticles
3.1. Quantum Dots
3.2. Up-Converting Nanoparticles (UCNP)
3.3. Carbon Dots
3.4. Aggregation-Induced Emission Dyes
3.5. Silica-Encapsulated Dyes
4. Toward the Short-Wave Infrared
4.1. SWIR QD
4.2. Lanthanide Nanoparticles
4.3. Gold Nanoparticles
4.4. Carbon Nanoparticle
4.5. SWIR Fluorescent Organic Nanoparticles
5. NP Safety: A Major Concern
5.1. Urinary Excretion Is Mainly a Matter of Size
5.2. Rethinking of the Injection Route
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzi, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Cannistra, S.A. Cancer of the Ovary. N. Engl. J. Med. 2004, 351, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, L.A.; Huang, B.; Miller, R.W.; Tucker, T.; Goodrich, S.T.; Podzielinski, I.; DeSimone, C.P.; Ueland, F.R.; van Nagell, J.R.; Seamon, L.G. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012, 120, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhara, S.H.; Triveni, G.S.; Kumar, R. Imaging of peritoneal deposits in ovarian cancer: A pictorial review. World J. Radiol. 2016, 8, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Gallotta, V.; Romano, F.; Fanfani, F.; Rossitto, C.; Naldini, A.; Vigliotta, M.; Scambia, G. Peritoneal carcinosis of ovarian origin. World J. Gastrointest. Oncol. 2010, 2, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.T.; Parker, L.M.; Fuller, A.F. Role of cytoreductive surgical treatment in the management of advanced ovarian cancer. Cancer Treat. Rep. 1979, 63, 235–240. [Google Scholar] [PubMed]
- Sugarbaker, P.H. Peritoneal Carcinomatosis: Principles of Management; Springer Science & Business Media: Berlin, Germany, 1996; ISBN 978-0-7923-3727-0. [Google Scholar]
- Nougaret, S.; Addley, H.C.; Colombo, P.E.; Fujii, S.; Al Sharif, S.S.; Tirumani, S.H.; Jardon, K.; Sala, E.; Reinhold, C. Ovarian Carcinomatosis: How the Radiologist Can Help Plan the Surgical Approach. RadioGraphics 2012, 32, 1775–1800. [Google Scholar] [CrossRef] [PubMed]
- Forstner, R.; Meissnitzer, M.; Cunha, T.M. Update on Imaging of Ovarian Cancer. Curr. Radiol. Rep. 2016, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.M.; Albanese, E.F.; Miño, J.H.; Gómez, E.; Gómez, M.; Zandomeni, M.; Merlo, A.B. Peritoneal surface area: Measurements of 40 structures covered by peritoneum: Correlation between total peritoneal surface area and the surface calculated by formulas. Surg. Radiol. Anat. SRA 2009, 31, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzin. Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Pfisterer, J. Future options for first-line therapy of advanced ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2005, 15 (Suppl. 1), 42–50. [Google Scholar] [CrossRef] [PubMed]
- Weisberger, A.S.; Levine, B.; Storaasli, J.P. Use of Nitrogen Mustard in Treatment of Serous Effusions of Neoplastic Origin. J. Am. Med. Assoc. 1955, 159, 1704–1707. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.L.; Myers, C.E.; Bungay, P.M.; DeVita, V.T. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat. Rep. 1978, 62, 1–11. [Google Scholar] [PubMed]
- Torres, I.J.; Litterst, C.L.; Guarino, A.M. Transport of model compounds across the peritoneal membrane in the rat. Pharmacology 1978, 17, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Liu, P.Y.; Hannigan, E.V.; O’Toole, R.; Williams, S.D.; Young, J.A.; Franklin, E.W.; Clarke-Pearson, D.L.; Malviya, V.K.; DuBeshter, B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N. Engl. J. Med. 1996, 335, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.B.; Zimm, S.; Markman, M.; Abramson, I.S.; Cleary, S.; Lucas, W.E.; Weiss, R.J. Long-term survival of advanced refractory ovarian carcinoma patients with small-volume disease treated with intraperitoneal chemotherapy. J. Clin. Oncol. 1987, 5, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Ozols, R.F.; Gore, M.; Tropé, C.; Grenman, S. Intraperitoneal treatment and dose-intense therapy in ovarian cancer. Ann. Oncol. 1999, 10 (Suppl. 1), 59–64. [Google Scholar] [CrossRef] [PubMed]
- Los, G.; Verdegaal, E.M.; Mutsaers, P.H.; McVie, J.G. Penetration of carboplatin and cisplatin into rat peritoneal tumor nodules after intraperitoneal chemotherapy. Cancer Chemother. Pharmacol. 1991, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Eoh, K.J.; Lee, J.Y.; Nam, E.J.; Kim, S.; Kim, Y.T.; Kim, S.W. Long-Term Survival Analysis of Intraperitoneal versus Intravenous Chemotherapy for Primary Ovarian Cancer and Comparison between Carboplatin- and Cisplatin-based Intraperitoneal Chemotherapy. J. Korean Med. Sci. 2017, 32, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Java, J.J.; Salani, R.; Armstrong, D.K.; Markman, M.; Herzog, T.; Monk, B.J.; Chan, J.K. Long-Term Survival Advantage and Prognostic Factors Associated With Intraperitoneal Chemotherapy Treatment in Advanced Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2015, 33, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Antoun, S.; Goharin, A.; Otmany, A.E.; Puizillout, J.M.; Lasser, P. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int. J. Surg. Investig. 2000, 1, 431–439. [Google Scholar] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Elattar, A.; Bryant, A.; Winter-Roach, B.A.; Hatem, M.; Naik, R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst. Rev. 2011, CD007565. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, W.J.; Bundy, B.N.; Thigpen, J.T.; Omura, G.A. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 1992, 47, 159–166. [Google Scholar] [CrossRef]
- Vermeulen, C.K.M.; Tadesse, W.; Timmermans, M.; Kruitwagen, R.F.P.M.; Walsh, T. Only complete tumour resection after neoadjuvant chemotherapy offers benefit over suboptimal debulking in advanced ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 219, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, A.; Lück, H.-J.; Meier, W.; Adams, H.-P.; Möbus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schröder, W.; et al. Arbeitsgemeinschaft Gynäkologische Onkologie Ovarian Cancer Study Group A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Lakhman, Y.; Akin, O.; Sohn, M.J.; Zheng, J.; Moskowitz, C.S.; Iyer, R.B.; Barakat, R.R.; Sabbatini, P.J.; Chi, D.S.; Hricak, H. Early postoperative CT as a prognostic biomarker in patients with advanced ovarian, tubal, and primary peritoneal cancer deemed optimally debulked at primary cytoreductive surgery. AJR Am. J. Roentgenol. 2012, 198, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Gildea, C.; Poole, J.; Meechan, D.; Nordin, A. Specialist surgery for ovarian cancer in England. Gynecol. Oncol. 2015, 138, 700–706. [Google Scholar] [CrossRef] [PubMed]
- DSouza, A.V.; Lin, H.; Henderson, E.R.; Samkoe, K.S.; Pogue, B.W. Review of fluorescence guided surgery systems: Identification of key performance capabilities beyond indocyanine green imaging. J. Biomed. Opt. 2016, 21, 080901. [Google Scholar] [CrossRef] [PubMed]
- Herly, L. Studies in Selective Differentiation of Tissues by Means of Filtered Ultraviolet Light. Cancer Res. 1944, 4, 227–231. [Google Scholar]
- Moore, G.E. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.E.; Peyton, W.T.; French, L.A.; Walker, W.W. The clinical use of fluorescein in neurosurgery. J. Neurosurg. 1948, 5, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L.D. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. (Wien) 2017, 159, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Leblond, F.; Davis, S.C.; Valdés, P.A.; Pogue, B.W. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J. Photochem. Photobiol. B 2010, 98, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Vendrell, M.; Das, R.; Chang, Y.-T. Development of photostable near-infrared cyanine dyes. Chem. Commun. Camb. Engl. 2010, 46, 7406–7408. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Nakamura, Y.; Makino, H.; Citterio, D.; Suzuki, K. Bright, color-tunable fluorescent dyes in the visible-near-infrared region. J. Am. Chem. Soc. 2008, 130, 1550–1551. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Yang, Y.; Chen, H. A unique class of near-infrared functional fluorescent dyes with carboxylic-acid-modulated fluorescence ON/OFF switching: Rational design, synthesis, optical properties, theoretical calculations, and applications for fluorescence imaging in living animals. J. Am. Chem. Soc. 2012, 134, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green: Historical Context, Current Applications, and Future Considerations. Surg. Innov. 2016, 23, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sevick-Muraca, E.M. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br. J. Radiol. 2014, 88, 20140547. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Morita, Y.; Sakaguchi, T.; Konno, H. Indocyanine Green-Related Transporters in Hepatocellular Carcinoma. SpringerLink 2016, 351–362. [Google Scholar] [CrossRef]
- Kosaka, N.; Mitsunaga, M.; Longmire, M.R.; Choyke, P.L.; Kobayashi, H. Near infrared fluorescence-guided real-time endoscopic detection of peritoneal ovarian cancer nodules using intravenously injected indocyanine green. Int. J. Cancer J. Int. Cancer 2011, 129, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Peters, A.A.W.; de Kroon, C.D.; Trimbos, J.B.M.Z.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The Value of Intraoperative Near-Infrared Fluorescence Imaging Based on Enhanced Permeability and Retention of Indocyanine Green: Feasibility and False-Positives in Ovarian Cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Choi, H.S.; Humblet, V.; Ohnishi, S.; Laurence, R.G.; Frangioni, J.V. Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery 2008, 144, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hoogstins, C.E.S.; Tummers, Q.R.J.G.; Gaarenstroom, K.N.; de Kroon, C.D.; Trimbos, J.B.M.Z.; Bosse, T.; Smit, V.T.H.B.M.; Vuyk, J.; van de Velde, C.J.H.; Cohen, A.F.; et al. A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin. Cancer Res. 2016, 22, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Hekman, M.; Rijpkema, M.; Oosterwijk, E.; Langenhuijsen, H.; Boerman, O.; Oyen, W.; Mulders, P. Intraoperative dual-modality imaging in clear cell renal cell carcinoma using Indium-111-DOTA-girentuximab-IRDye800CW. Eur. Urol. Suppl. 2017, 16, e1831. [Google Scholar] [CrossRef]
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.; Xie, Y.; Bae, S.; et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Rose, A. A Unified Approach to the Performance of Photographic Film, Television Pickup Tubes, and the Human Eye. J. Soc. Motion Pict. Eng. 1946, 47, 273–294. [Google Scholar] [CrossRef]
- Kusano, M.; Kokudo, N.; Toi, M.; Kaibori, M. ICG Fluorescence Imaging and Navigation Surgery; Springer: Berlin, Germany, 2016; ISBN 978-4-431-55528-5. [Google Scholar]
- Choi, H.S.; Frangioni, J.V. Nanoparticles for Biomedical Imaging: Fundamentals of Clinical Translation. Mol. Imaging 2010, 9, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, G.; Bouccara, S.; Tasso, M.; Francois, A.; Bezdetnaya, L.; Marchal, F.; Beaumont, M.; Pons, T. Multimodal Mn-doped I-III-VI quantum dots for near infrared fluorescence and magnetic resonance imaging: From synthesis to in vivo application. Nanoscale 2014, 6, 9264–9272. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Braun, G.B.; Qin, M.; Ruoslahti, E.; Sugahara, K.N. In vivo cation exchange in quantum dots for tumor-specific imaging. Nat. Commun. 2017, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, L.; Shen, B.; Chen, X.; Wang, L.; Wang, L.; Feng, W.; Huang, C.; Li, F. Amphiphilic PEGylated Lanthanide-Doped Upconversion Nanoparticles for Significantly Passive Accumulation in the Peritoneal Metastatic Carcinomatosis Models Following Intraperitoneal Administration. ACS Biomater. Sci. Eng. 2017, 3, 2176–2184. [Google Scholar] [CrossRef]
- Ko, H.Y.; Chang, Y.W.; Paramasivam, G.; Jeong, M.S.; Cho, S.; Kim, S. In vivo imaging of tumour bearing near-infrared fluorescence-emitting carbon nanodots derived from tire soot. Chem. Commun. Camb. Engl. 2013, 49, 10290–10292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, K.; Chen, S.; Qin, A.; Ding, D.; Zhang, S.; Liu, Y.; Liu, B.; Sun, J.Z.; Tang, B.Z. Aggregation-induced red-NIR emission organic nanoparticles as effective and photostable fluorescent probes for bioimaging. J. Mater. Chem. 2012, 22, 15128–15135. [Google Scholar] [CrossRef]
- Burns, A.A.; Vider, J.; Ow, H.; Herz, E.; Penate-Medina, O.; Baumgart, M.; Larson, S.M.; Wiesner, U.; Bradbury, M. Fluorescent silica nanoparticles with efficient urinary excretion for nanomedicine. Nano Lett. 2009, 9, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Ow, H.; Wiesner, U. Fluorescent core–shell silica nanoparticles: Towards “Lab on a Particle” architectures for nanobiotechnology. Chem. Soc. Rev. 2006, 35, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Landen, C.N.; Kim, T.-J.; Lin, Y.G.; Merritt, W.M.; Kamat, A.A.; Han, L.Y.; Spannuth, W.A.; Nick, A.M.; Jennnings, N.B.; Kinch, M.S.; et al. Tumor-Selective Response to Antibody-Mediated Targeting of αvβ3 Integrin in Ovarian Cancer. Neoplasia 2008, 10, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Zhang, L.; Ma, K.; Riegman, M.; Chen, F.; Ingold, I.; Conrad, M.; Turker, M.Z.; Gao, M.; Jiang, X.; et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat. Nanotechnol. 2016, 11, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, L.-M.; Delpech, F.; Nayral, C.; Lachaize, S.; Chaudret, B. New generation of magnetic and luminescent nanoparticles for in vivo real-time imaging. Interface Focus 2013, 3, 20120103. [Google Scholar] [CrossRef] [PubMed]
- Damalakiene, L.; Karabanovas, V.; Bagdonas, S.; Rotomskis, R. Fluorescence-Lifetime Imaging Microscopy for Visualization of Quantum Dots’ Endocytic Pathway. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Helle, M.; Cassette, E.; Bezdetnaya, L.; Pons, T.; Leroux, A.; Plénat, F.; Guillemin, F.; Dubertret, B.; Marchal, F. Visualisation of sentinel lymph node with indium-based near infrared emitting Quantum Dots in a murine metastatic breast cancer model. PLoS ONE 2012, 7, e44433. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Mu, Y.; Chen, Y.; Sun, H.; Han, S.; Wang, M.; Wang, H.; Li, Y.; Xu, Q.; Huang, P.; et al. Degradation of aqueous synthesized CdTe/ZnS quantum dots in mice: Differential blood kinetics and biodistribution of cadmium and tellurium. Part. Fibre Toxicol. 2013, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, G.; Sun, Z.; Mu, Y.; Han, S.; Xiao, Y.; Liu, N.; Wang, H.; Guo, C.; Shi, Z.; et al. Plasma kinetics and biodistribution of water-soluble CdTe quantum dots in mice: A comparison between Cd and Te. J. Nanopart. Res. 2011, 13, 5373. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, L.W.; Chang, H.; Yang, M.-H.; Yang, C.-S.; Lai, W.-H.; Chang, W.-H.; Lin, P. The chemical fate of the Cd/Se/Te-based quantum dot 705 in the biological system: Toxicity implications. Nanotechnology 2009, 20, 215101. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Cassette, E.; Helle, M.; Bezdetnaya, L.; Marchal, F.; Dubertret, B.; Pons, T. Design of new quantum dot materials for deep tissue infrared imaging. Adv. Drug Deliv. Rev. 2013, 65, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Duman, F.D.; Erkisa, M.; Khodadust, R.; Ari, F.; Ulukaya, E.; Acar, H.Y. Folic acid-conjugated cationic Ag2S quantum dots for optical imaging and selective doxorubicin delivery to HeLa cells. Nanomedicine 2017, 12, 2319–2333. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion Nanoparticles: Design, Nanochemistry, and Applications in Theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Su, Q.; Feng, W.; Li, F. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- González-Béjar, M.; Francés-Soriano, L.; Pérez-Prieto, J. Upconversion Nanoparticles for Bioimaging and Regenerative Medicine. Front. Bioeng. Biotechnol. 2016, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Suzuki, K.T. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ. Health Perspect. 1996, 104, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-H.; Liu, R.-S. Advanced sensing, imaging, and therapy nanoplatforms based on Nd3+-doped nanoparticle composites exhibiting upconversion induced by 808 nm near-infrared light. Nanoscale 2017, 9, 18153–18168. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.T.; Koo, K.H.; Park, J.S. Toxicological Evaluations of Rare Earths and Their Health Impacts to Workers: A Literature Review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, S.-T.; Wang, X.; Luo, P.G.; Liu, J.-H.; Sahu, S.; Liu, Y.; Sun, Y.-P. Competitive Performance of Carbon “Quantum” Dots in Optical Bioimaging. Theranostics 2012, 2, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, J.A.; Pramod, K. Artful and multifaceted applications of carbon dot in biomedicine. J. Control. Release 2018, 269, 302–321. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.; Ji, S.; Liu, Q.; Ding, D.; Zhao, D.; Liu, B. Long wavelength excitable near-infrared fluorescent nanoparticles with aggregation-induced emission characteristics for image-guided tumor resection. Chem. Sci. 2017, 8, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Helle, M.; Rampazzo, E.; Monchanin, M.; Marchal, F.; Guillemin, F.; Bonacchi, S.; Salis, F.; Prodi, L.; Bezdetnaya, L. Surface Chemistry Architecture of Silica Nanoparticles Determine the Efficiency of in Vivo Fluorescence Lymph Node Mapping. ACS Nano 2013, 7, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Elsaesser, A.; Howard, C.V. Toxicology of nanoparticles. Adv. Drug Deliv. Rev. 2012, 64, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sawada, K.; Kimura, T. Potential of Integrin Inhibitors for Treating Ovarian Cancer: A Literature Review. Cancers 2017, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kenny, H.A.; Jagadeeswaran, S.; Zillhardt, M.R.; Montag, A.G.; Kistner, E.; Yamada, S.D.; Mitra, A.K.; Lengyel, E. β3-Integrin Expression on Tumor Cells Inhibits Tumor Progression, Reduces Metastasis, and Is Associated with a Favorable Prognosis in Patients with Ovarian Cancer. Am. J. Pathol. 2009, 175, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.T.; Kim, S.; Nakayama, A.; Stott, N.E.; Bawendi, M.G.; Frangioni, J.V. Selection of quantum dot wavelengths for biomedical assays and imaging. Mol. Imaging 2003, 2, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, R.J.; Goldstein, S.D.; Singh, J.; Safar, B.; Banerjee, A.; Ahuja, N. Automated diagnosis of colon cancer using hyperspectral sensing. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1897. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Mingozzi, M.; Higgins, L.M.; Ganapathy, V.; Zevon, M.; Riman, R.E.; Roth, C.M.; Moghe, P.V.; Pierce, M.C. Small Animal Imaging Platform for Quantitative Assessment of Short-Wave Infrared-Emitting Contrast Agents; International Society for Optics and Photonics: Washington, DC, USA, 2015; Volume 9311, p. 93110T. [Google Scholar]
- Hong, G.; Robinson, J.T.; Zhang, Y.; Diao, S.; Antaris, A.L.; Wang, Q.; Dai, H. In Vivo Fluorescence Imaging with Ag2S Quantum Dots in the Second Near-Infrared Region. Angew. Chem. Int. Ed. 2012, 51, 9818–9821. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Chang, J.; Antaris, A.L.; Chen, C.; Zhang, B.; Zhao, S.; Atochin, D.N.; Huang, P.L.; Andreasson, K.I.; et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics 2014, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Salo, D.; Kim, D.M.; Komarov, S.; Tai, Y.-C.; Berezin, M.Y. Penetration depth of photons in biological tissues from hyperspectral imaging in shortwave infrared in transmission and reflection geometries. J. Biomed. Opt. 2016, 21, 126006. [Google Scholar] [CrossRef] [PubMed]
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Starosolski, Z.; Bhavane, R.; Ghaghada, K.B.; Vasudevan, S.A.; Kaay, A.; Annapragada, A. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLoS ONE 2017, 12, e0187563. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hu, Z.; Zhu, S.; Ma, R.; Ma, H.; Ma, Z.; Wan, H.; Zhu, T.; Jiang, Z.; Liu, W.; et al. Donor Engineering for NIR-II Molecular Fluorophores with Enhanced Fluorescent Performance. J. Am. Chem. Soc. 2018, 140, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-N.; Jiang, P.; Zhang, Z.-L.; Zhu, D.-L.; Tian, Z.-Q.; Pang, D.-W. Ag2Se Quantum Dots with Tunable Emission in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2013, 5, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Bruns, O.T.; Bischof, T.S.; Harris, D.K.; Franke, D.; Shi, Y.; Riedemann, L.; Bartelt, A.; Jaworski, F.B.; Carr, J.A.; Rowlands, C.J.; et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017, 1, 0056. [Google Scholar] [CrossRef] [PubMed]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Montana, D.M.; Wei, H.; Cordero, J.M.; Schneider, M.; Le Guével, X.; Chen, O.; Bruns, O.T.; Bawendi, M.G. Shortwave Infrared in Vivo Imaging with Gold Nanoclusters. Nano Lett. 2017, 17, 6330–6334. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Bagley, A.F.; Na, Y.J.; Birrer, M.J.; Bhatia, S.N.; Belcher, A.M. Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes. Proc. Natl. Acad. Sci. USA 2014, 111, 13948–13953. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Sun, C.; Zebibula, A.; Zhang, H.; Kwok, R.T.K.; Zhao, X.; Xi, W.; Lam, J.W.Y.; Qian, J.; Tang, B.Z. Real-Time and High-Resolution Bioimaging with Bright Aggregation-Induced Emission Dots in Short-Wave Infrared Region. Adv. Mater. Deerfield Beach Fla 2018, 30, e1706856. [Google Scholar] [CrossRef] [PubMed]
- Zevon, M.; Ganapathy, V.; Kantamneni, H.; Mingozzi, M.; Kim, P.; Adler, D.; Sheng, Y.; Tan, M.C.; Pierce, M.; Riman, R.E.; et al. CXCR-4 Targeted, Short Wave Infrared (SWIR) Emitting Nanoprobes for Enhanced Deep Tissue Imaging and Micrometastatic Lesion Detection. Small Weinh. Bergstr. Ger. 2015, 11, 6347–6357. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Dang, X.; Huang, X.; Muzumdar, M.D.; Xu, E.S.; Bardhan, N.M.; Song, H.; Qi, R.; Yu, Y.; Li, T.; et al. Early tumor detection afforded by in vivo imaging of near-infrared II fluorescence. Biomaterials 2017, 134, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.; Nunez, R.; Szklaruk, J.; Erwin, W.; Madoff, D.C.; Gupta, S.; Ahrar, K.; Wallace, M.J.; Cohen, A.; Coldwell, D.M.; et al. Yttrium-90 microsphere therapy for hepatic malignancy: Devices, indications, technical considerations, and potential complications. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc 2005, 25 (Suppl. 1), S41–S55. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-F.; Chen, L.-D.; Li, M.; Xie, M.-Y.; Zhou, L.; Li, Y.; Wang, Q.-Q. Highly Efficient Fluorescence of NdF3/SiO2 Core/Shell Nanoparticles and the Applications for in vivo NIR Detection. Adv. Mater. 2008, 20, 4118–4123. [Google Scholar] [CrossRef]
- Saito, N.; Haniu, H.; Usui, Y.; Aoki, K.; Hara, K.; Takanashi, S.; Shimizu, M.; Narita, N.; Okamoto, M.; Kobayashi, S.; et al. Safe Clinical Use of Carbon Nanotubes as Innovative Biomaterials. Chem. Rev. 2014, 114, 6040–6079. [Google Scholar] [CrossRef] [PubMed]
- Sanginario, A.; Miccoli, B.; Demarchi, D. Carbon Nanotubes as an Effective Opportunity for Cancer Diagnosis and Treatment. Biosensors 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Sgarzi, M.; Talmon, Y.; et al. Cancer-cell-targeted theranostic cubosomes. Langmuir ACS J. Surf. Colloids 2014, 30, 6228–6236. [Google Scholar] [CrossRef] [PubMed]
- Saei, A.A.; Yazdani, M.; Lohse, S.E.; Bakhtiary, Z.; Serpooshan, V.; Ghavami, M.; Asadian, M.; Mashaghi, S.; Dreaden, E.C.; Mashaghi, A.; et al. Nanoparticle Surface Functionality Dictates Cellular and Systemic Toxicity. Chem. Mater. 2017, 29, 6578–6595. [Google Scholar] [CrossRef]
- Pourmand, A.; Abdollahi, M. Current Opinion on Nanotoxicology. DARU J. Pharm. Sci. 2012, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, V.; Sekhar, S.; Manoharan, N. Safety and Risk Associated with Nanoparticles—A Review. J. Miner. Mater. Charact. Eng. 2010, 9, 455–459. [Google Scholar] [CrossRef]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.A.; Dai, Q.; Sarsons, C.D.; Chen, J.; Rocheleau, J.V.; Hwang, D.M.; Zheng, G.; Cramb, D.T.; Rinker, K.D.; Chan, W.C.W. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl. Acad. Sci. USA 2016, 113, E1142–E1151. [Google Scholar] [CrossRef] [PubMed]
- Polo, E.; Collado, M.; Pelaz, B.; del Pino, P. Advances toward More Efficient Targeted Delivery of Nanoparticles in Vivo: Understanding Interactions between Nanoparticles and Cells. ACS Nano 2017, 11, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jiang, X.; Das, A.; Zhou, Q.; Yu, M.; Jin, R.; Zheng, J. Glomerular barrier behaves as an atomically precise bandpass filter in a sub-nanometre regime. Nat. Nanotechnol. 2017, 12, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Marshall, A.F.; Gambhir, S.S.; Rao, J. Facile Synthesis, Silanization and Biodistribution of Biocompatible Quantum Dots. Small Weinh. Bergstr. Ger. 2010, 6, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xu, D.; Wan, Q.; Liu, M.; Tian, J.; Huang, Q.; Deng, F.; Wen, Y.; Zhang, X.; Wei, Y. Construction of biodegradable and biocompatible AIE-active fluorescent polymeric nanoparticles by Ce(IV)/HNO3 redox polymerization in aqueous solution. Mater. Sci. Eng. C 2017, 78, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, I.I.; Kapralov, A.A.; Michael, Z.P.; Burkert, S.C.; Shurin, M.R.; Star, A.; Shvedova, A.A.; Kagan, V.E. Enzymatic Oxidative Biodegradation of Nanoparticles: Mechanisms, Significance and Applications. Toxicol. Appl. Pharmacol. 2016, 299, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Hoshino, A.; Yamamoto, K.; Tilley, R.D. Water-Soluble Photoluminescent Silicon Quantum Dots. Angew. Chem. Int. Ed. 2005, 44, 4550–4554. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Tsang, C.H.A.; Zhang, Z.; Zhang, M.; Wong, N.; Zapien, J.A.; Shan, Y.; Lee, S.-T. A polyoxometalate-assisted electrochemical method for silicon nanostructures preparation: From quantum dots to nanowires. J. Am. Chem. Soc. 2007, 129, 5326–5327. [Google Scholar] [CrossRef] [PubMed]
- Erogbogbo, F.; Yong, K.-T.; Roy, I.; Xu, G.; Prasad, P.N.; Swihart, M.T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Erogbogbo, F.; Yong, K.-T.; Ye, L.; Liu, J.; Hu, R.; Chen, H.; Hu, Y.; Yang, Y.; Yang, J.; et al. Assessing Clinical Prospects of Silicon Quantum Dots: Studies in Mice and Monkeys. ACS Nano 2013, 7, 7303–7310. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.; Herrero, M.A.; Venner, K.; Bianco, A.; Prato, M.; Kostarelos, K. Carbon-Nanotube Shape and Individualization Critical for Renal Excretion. Small 2008, 4, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Colby, A.H.; Berry, S.M.; Moran, A.M.; Pasion, K.A.; Liu, R.; Colson, Y.L.; Ruiz-Opazo, N.; Grinstaff, M.W.; Herrera, V.L.M. Highly Specific and Sensitive Fluorescent Nanoprobes for Image-Guided Resection of Sub-Millimeter Peritoneal Tumors. ACS Nano 2017, 11, 1466–1477. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; Tung, C.-H. Lessons learned from imaging mouse ovarian tumors: The route of probe injection makes a difference. Quant. Imaging Med. Surg. 2014, 4, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Parungo, C.P.; Soybel, D.I.; Colson, Y.L.; Kim, S.-W.; Ohnishi, S.; De Grand, A.M.; Laurence, R.G.; Soltesz, E.G.; Chen, F.Y.; Cohn, L.H.; et al. Lymphatic Drainage of the Peritoneal Space: A Pattern Dependent on Bowel Lymphatics. Ann. Surg. Oncol. 2007, 14, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Itoh, K.; Yaoi, T.; Tozawa, T.; Yoshikawa, Y.; Yasui, H.; Kanamura, N.; Hoshino, A.; Manabe, N.; Yamamoto, K.; et al. Organ distribution of quantum dots after intraperitoneal administration, with special reference to area-specific distribution in the brain. Nanotechnology 2010, 21, 335103. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Im, H.-Y.; Seo, J.-E.; Hasan, M.; Woo, K.; Kwon, O.-S. Acute toxicity and tissue distribution of CdSe/CdS-MPA quantum dots after repeated intraperitoneal injection to mice. J. Appl. Toxicol. 2013, 33, 940–950. [Google Scholar] [CrossRef] [PubMed]

| Spectra | Near-Infrared | ||||||
|---|---|---|---|---|---|---|---|
| Name | ICG | OTL-38 | Quantum Dots | UCNP | Carbon Dot | AIE NP | Cornell Dots |
| Component | C43H47N2NaO6S2 | C61H63N9O17S4/4Na | CuInSe/ZnS(Mn) ZnSeHg | Yb, Tm, Er doped NaYF4 nanocrystal/NaLuF4 shell | Graphite core | Organic core | Cyanine 5 core and silica shell |
| Size (nm) | − | − | 9.0 (CuInSe/ZnS(Mn) 6.6 (ZnSeHg) | 30 | 11 | 46 | 5.5 |
| Coating | − | − | PEG | PEG | PEG | PEG | PEG |
| Targeting | − | Folate | iRGD | − | − | Folate | cRGD |
| Excretion | Hepatobiliary | Hepatobiliary | − | − | − | Hepatobiliary | Renal |
| Multimodality | − | − | MRI (Mn) | − | − | − | PET (124I) |
| Photostability | Low | Low | High | High | High | High | High |
| Excitation (nm) | 805 | 774 | 690 (CuInSe) 785 (ZnSeHg) | 980 (multiphotonic) | 633 | 635 | 650 |
| Emission peak (nm) | 835 | 794 | 685(CuInSe/ZnS(Mn)) >800 (ZnSeHg) | 800 | >710 | 810–815 | 670 |
| SBR of i.p. tumor | 2 ± 1 | 4.4 | 12 | >5 | − | 7.2 | − |
| Results in vivo | − | − | − | Passive accumulation in peritoneal tumors following i.p. injection | SBR ≈ 2 in subcutaneously injected matrigel | Allow the detection of sub-millimetric peritoneal tumors | − |
| Clinical | Low specificity | Improved cytoreduction | − | − | − | − | Preferential uptake of Cornell dots at the site of the disease, in vivo stability and safety |
| Reference | [47,48,49] | [50] | [56,57] | [58] | [59] | [60] | [61,62,63,64,65] |
| Spectra | Short-Wave Infrared | ||||||
|---|---|---|---|---|---|---|---|
| Name | IR-1050 | ICG | Quantum Dot | Lanthanide NP | Gold NP | Phage Stabilized SWCNT | AIE NP |
| Component | C41H40BCl3F4N2 | C43H47N2NaO6S2 | Ag2S InAs | NaYF4 Yb:Ln core doped with rare-earth NaYF4 shell | Gold | Pure carbon nanotube | Organic core |
| Size (nm) | − | − | 3.0–4.0 (Ag2S) 4.5 (InAs) | 9.0–11 | 1.6 | 880 × 6.5 * | 33 |
| Coating | − | − | PEG | Polymeric coating by poly(ethylene oxide) | Lipoic acid-based sulfobetaine | Phage M13 | Pluronic |
| Targeting | − | − | − | Folate | − | SPARC-Binding peptide | − |
| Excretion | Hepatobiliary | Hepatobiliary | Hepatobiliary (Ag2S) | − | Renal | − | − |
| Multimodality | − | − | − | − | − | − | − |
| Photostability | Low | Low | High | High | High | High | High |
| Excitation (nm) | 790 | 805 | 808 | 980 | 808 | 808 | 630 |
| Emission peak (nm) | 1050 | 835 | 1125 (Ag2S) 1080–1330 (InAs) | 1185 (Ho doped) 1310 (Pr doped) 1475 (Tm doped) 1525 (Er doped) | 800–1400 | 1000 – 1300 | 808 |
| SBR of i.p. tumor | − | − | 14 (Ag2Se) | >3 | − | 8 | − |
| Results in vivo | − | − | i.v. injected Ag2S QDs passively accumulate in subcutaneous. murine tumor with a ratio of 10% ID/g tumors | i.p. injected lanthanide NPs accumulate, with or without targeting, in i.p. tumors from ovarian cancer OVCAR8 cell line | − | Effective imaging of peritoneal tumors after i.p. injection, with higher resection rate, especially for sub-millimetric nodules | SBR is 33 at the depth of 150 µm in mouse brain vasculature following i.v. injection |
| Clinical | − | − | − | − | − | − | − |
| Reference | [100] | [100] | [93,102,103] | [104] | [105] | [106] | [107] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangeolle, T.; Yakavets, I.; Marchal, S.; Debayle, M.; Pons, T.; Bezdetnaya, L.; Marchal, F. Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis. Nanomaterials 2018, 8, 572. https://doi.org/10.3390/nano8080572
Mangeolle T, Yakavets I, Marchal S, Debayle M, Pons T, Bezdetnaya L, Marchal F. Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis. Nanomaterials. 2018; 8(8):572. https://doi.org/10.3390/nano8080572
Chicago/Turabian StyleMangeolle, Tristan, Ilya Yakavets, Sophie Marchal, Manon Debayle, Thomas Pons, Lina Bezdetnaya, and Frédéric Marchal. 2018. "Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis" Nanomaterials 8, no. 8: 572. https://doi.org/10.3390/nano8080572
APA StyleMangeolle, T., Yakavets, I., Marchal, S., Debayle, M., Pons, T., Bezdetnaya, L., & Marchal, F. (2018). Fluorescent Nanoparticles for the Guided Surgery of Ovarian Peritoneal Carcinomatosis. Nanomaterials, 8(8), 572. https://doi.org/10.3390/nano8080572






