Preparation of Filtration Sorptive Materials from Nanofibers, Bicofibers, and Textile Adsorbents without Binders Employment
Abstract
:1. Introduction
2. Materials and Methods
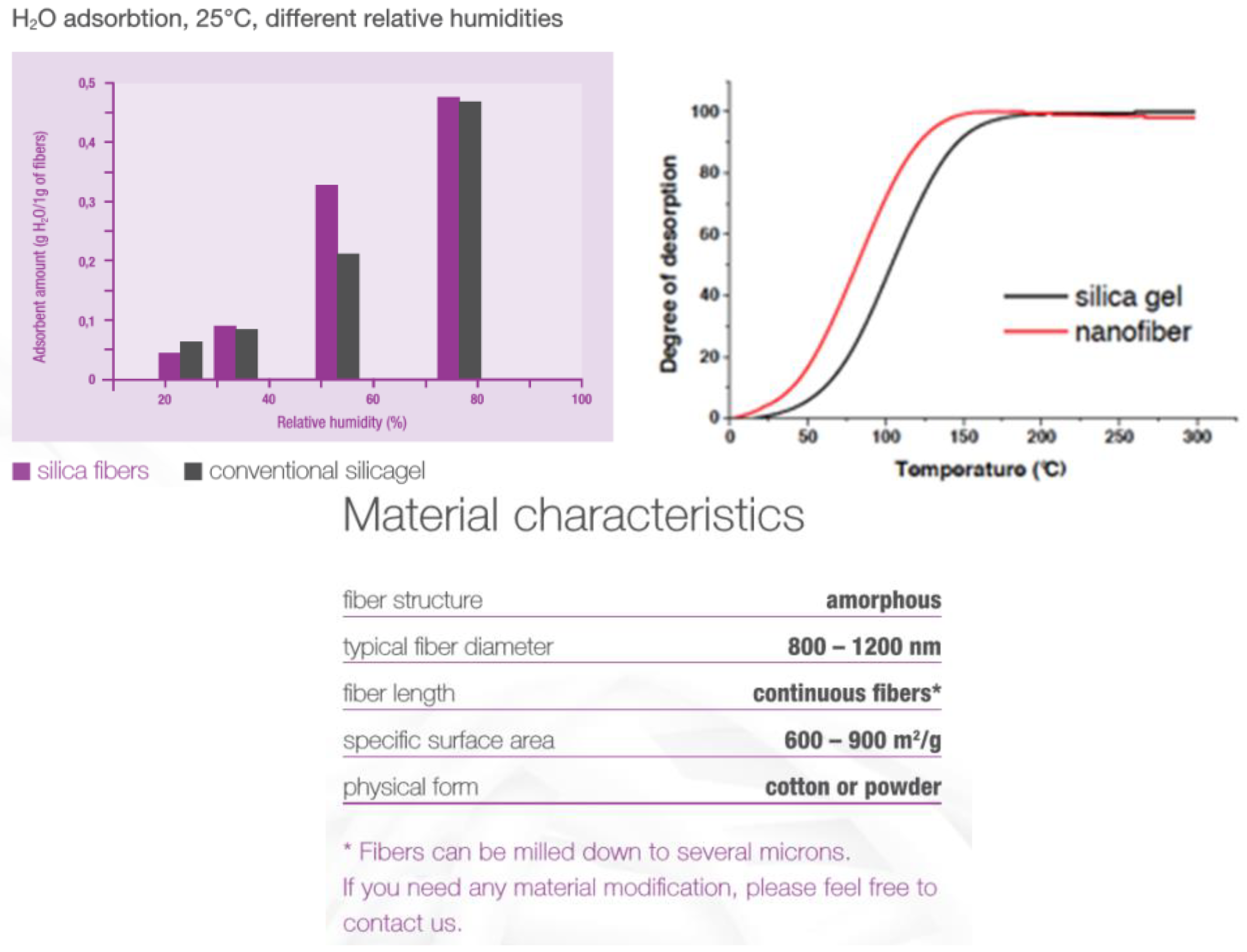
3. Results
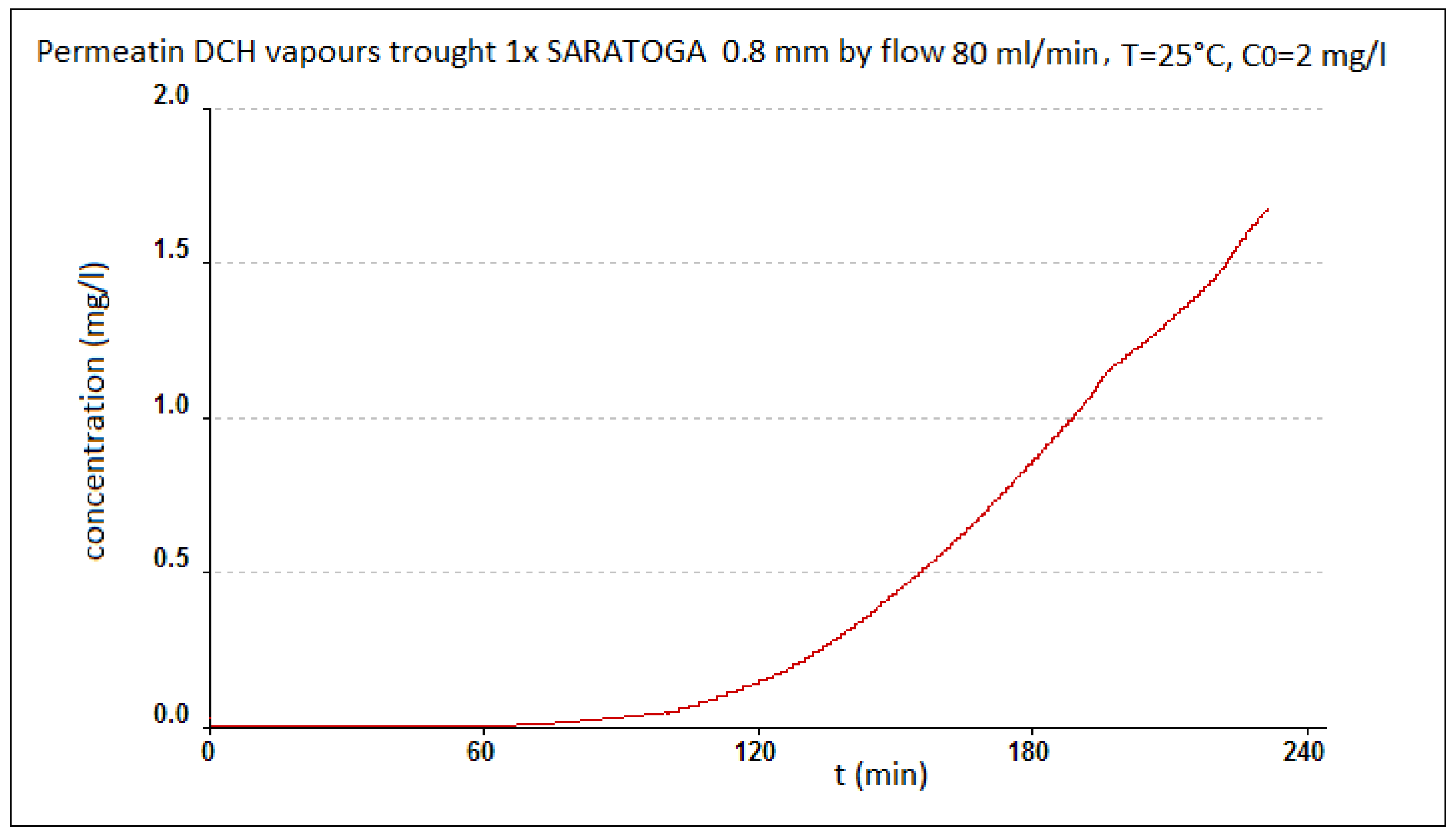
Principle of Measurement
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mrština, V.; Kučera, F. Vrstvená Filtračně-Absorpční Textilie. Available online: https://1url.cz/Rty4j (accessed on 11 June 2018).
- CSN EN 143. Respiratory Protective Devices—Particle Filter—Requirements, Testing, Marking; Český Normalizační Institut: Praha, Czech Republic, 2000. [Google Scholar]
- ČSN EN 13274-1. Respiratory Protective Devices—Methods of Test—Part 1: Determination of Inward Leakage and Total Inward Leakage; Český Normalizační Institut: Praha, Czech Republic, 2001. [Google Scholar]
- Ndaro, M.S.; Jin, X.; Chen, T.; Yu, C. Splitting of Islands-in-the-Sea Fibers (PA6/COPET) during Hydroentangling of Nonwovens. J. Eng. Fibers Fabr. 2007, 2, 1–9. [Google Scholar]
- Khavryuchenko, V.D.; Khavryuchenko, O.V.; Shkilnyy, A.I.; Stratiichuk, D.A.; Lisnyak, V.V. Characterization by SEM, TEM and Quantum-Chemical Simulations of the Spherical Carbon with Nitrogen (SCN) Active Carbon Produced by Thermal Decomposition of Poly(vinylpyridine-divinylbenzene) Copolymer. Materials 2009, 2, 1239–1251. [Google Scholar] [CrossRef] [Green Version]
- Kmochova, A.; Tichy, A.; Zarybnicka, L.; Sinkorova, Z.; Vavrova, J.; Rehacek, V.; Durisova, K.; Kubelkova, K.; Pejchal, J.; Kuca, K. Modulation of ionizing radiation-induced effects by NU7441, KU55933 and VE821 in peripheral blood lymphocytes. J. Appl. Biomed. 2016, 14, 19–24. [Google Scholar] [CrossRef]
- Atwater, M.A.; Mousavi, A.K.; Leseman, Z.; Phillips, J. Direct synthesis and characterization of a nonwoven structure comprised of carbon nanofibers. Carbon 2013, 57, 363–370. [Google Scholar] [CrossRef]
- Suzuki, M. Activated carbon fiber: Fundamentals and applications. Carbon 1994, 32, 577–586. [Google Scholar] [CrossRef]
- Che, G.; Lakshmi, B.B.; Martin, C.R.; Fisher, E.R. Chemical Vapor Deposition Based Synthesis of Carbon Nanotubes and Nanofibers Using a Template Method. Chem. Mater. 1998, 10, 260–267. [Google Scholar] [CrossRef]
- Mosteanu, D.; Barsan, G.; Otrisal, P.; Giurgiu, L.; Oancea, R. Obtaining the Volatile Oils from Wormwood and Tarragon Plants by a new Microwave Hydrodistillation Method. Rev. Chim. 2017, 68, 2499–2502. [Google Scholar]
- Kalebek, N.A.; Babaarslan, O. Non-Woven Fabrics: Fiber Selection for the Production of Nonwovens; Jeon, H.-Y., Ed.; Inha University: Inha, Korea, 2016; Chapter 1; pp. 1–32. ISBN 978-953-51-2271-5. [Google Scholar]
- Obšel, V.; Buk, J.; Kuchař, A.; Kučera, F. A Filtration and Filtration-Sorption Plane Formation. Available online: https://1url.cz/Wty4d (accessed on 11 June 2018).
- Turbak, A.F. Nonwovens: Theory, Process, Performance, and Testing; TAPPI Press: Atlanta, GA, USA, 1993; 256p, ISBN 978-089-852-265-5. [Google Scholar]
- Horova, I.; Kolacek, J.; Vopatova, K. Full bandwidth matrix selectors for gradient kernel density estimate. Comput. Stat. Data Anal. 2013, 57, 364–376. [Google Scholar] [CrossRef]
- ČSN EN 16523-1. Determination of Material Resistance to Permeation by Chemicals—Part 1: Permeation by Liquid Chemical under Conditions of Continuous Contact; European Committee for Standardization: Brussel, Belgium, 2015. [Google Scholar]
- (CSN) EN 16523-2. Determination of Material Resistance to Permeation by Chemicals—Part 1: Permeation by Gaseous Chemicals under Conditions of Continuous Contact; European Committee for Standardization: Brussel, Belgium, 2015. [Google Scholar]
- Kudryavtseva, L.Y.; Shkrabo, M.L.; Ivakhnyuk, G.K.; Bolkunov, O.A.; Malinin, V.R. Preparation and properties of activated carbon fabrics for gas-protecting respirators. Russ. J. Appl. Chem. 1998, 71, 1155–1159. [Google Scholar]
- Schonfeld, M.; Schonfeld, R. Methods for Producing Spherical Activated Carbon. Available online: https://1url.cz/utyD9 (accessed on 12 June 2018).
- Lee, T.; Ooi, C.H.; Othman, R.; Yeoh, F. Activated carbon fiber—The Hybrid of Carbon Fiber and Activated Carbon. Rev. Adv. Mater. Sci. 2014, 36, 119–136. [Google Scholar]
- Hayashi, T.; Lee, T.G.; Hazelwood, M.; Hedrick, E.; Biswas, P. Characterization of Activated Carbon Fiber Filters for Pressure Drop, Submicrometer Particulate Collection, and Mercury Capture. J. Air Waste Manag. Assoc. 2000, 50, 922–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargavi, R.; Kadirvelu, K.; Kumar, N.S. Vapor Phase Adsorption of Homologous Aliphatic Ketones on Activated Spherical Carbon. Int. J. Environ. Sci. 2011, 71, 1155–1159. [Google Scholar]
- Otrisal, P.; Florus, S.; Svorc, L.; Barsan, G.; Mosteanu, D. A New Colorimetric Assay for Determination of Selected Toxic Vapors and Liquids Permeation through Barrier Materials Using the Minitest Device. Rev. Plast. 2017, 54, 748–751. [Google Scholar]
- Karkalic, R.M.; Ivankovic, N.D.; Jovanovic, D.B.; Markovic, S.M.; Indjic, D.R.; Micovic, M.D.; Kovacevic, B.V. Dynamic adsorption characteristics of thin layered activated charcoal materials used in chemical protective overgarments. Indian J. Fibre Text. Res. 2016, 41, 402–410. [Google Scholar]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 1, 938–947. [Google Scholar] [CrossRef]
- Hoskova-Mayerova, S.; Maturo, A. Algebraic hyperstructures and social relations. Ital. J. Pure Appl. Math. 2018, 39, 701–709. [Google Scholar]
- Macák, J.M.; Hromádko, L.; Bulánek, R.; Koudelková, E.; Buk, J.; Tejkl, M. Process for Preparing Submicron Fibers of Amorphous Silica and Submicron Fibers of Amorphous Silica Prepared in Such a Manner. Available online: https://1url.cz/qtyDc (accessed on 12 June 2018).
- ČSN EN 149+A1 83 2225. Respiratory Protective Devices—Filtering Half Masks to Protect against Particles—Requirements, Testing, Marking; Český Normalizační Institut: Praha, Czech Republic, 2009. [Google Scholar]
- Li, W.; Linag, C.; Zhou, W.; Qiu, J.; Sun, G.; Xin, Q. Preparation and Characterization of Multiwalled Carbon Nanotube-Supported Platinum for Cathode Catalysts of Direct Methanol Fuel Cells. J. Phys. Chem. B 2003, 107, 6292–6299. [Google Scholar] [CrossRef] [Green Version]
- Hajipour, M.H.; Fromm, K.M.; Ashkarran, A.A.; Aiba, S.; Aberasturi, D.J.A.; Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.Z.; Chen, W.Y.; Wu, C.Y.; Chen, Y.C. Potent antibacterial nanoparticles for pathogenic bacteria. ACS Appl. Mater. Interfaces 2015, 7, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Guzman, G.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balanay, J.A.; Bartolucci, A.A.; Lungu, C.T. Adsorption characteristics of activated carbon fibers (ACFs) for toluene: Application in respiratory protection. J. Occup. Environ. Hyg. 2014, 33, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Balanay, J.A.; Floyd, E.L.; Lungu, C.T. Breakthrough Curves for Toluene Adsorption on Different Types of Activated Carbon Fibers: Application in Respiratory Protection. Ann. Occup. Hyg. 2014, 59, 481–490. [Google Scholar] [PubMed] [Green Version]
- Míša, M.; Obšel, V. System for Measuring Permeation of Gases and Vapors through Barrier Membranes. Available online: https://1url.cz/Bty4h (accessed on 11 June 2018).
- ČSN EN 13274-2 (83 2205). Respiratory Protective Devices—Methods of Test—Part 2: Practical Performance Tests; Český Normalizační Institut: Praha, Czech Republic, 2001. [Google Scholar]
- ČSN EN 13274-3 (83 2205). Respiratory Protective Devices—Methods of Test—Part 3: Determination of Breathing Resistance; Český Normalizační Institut: Praha, Czech Republic, 2001. [Google Scholar]
- ČSN EN 149 (83 2225). Respiratory Protective Devices—Filtering Half Masks to Protect against Particles—Requirements, Testing, Marking; Český Normalizační Institut: Praha, Czech Republic, 2002. [Google Scholar]
- SRPS EN 60068-2-38 (34 5791). Environmental Testing—Part 2-38: Tests—Test Z/AD: Composite Temperature/Humidity Cyclic Test; Serbian Normalization Institute: Beograd, Serbia, 2010. [Google Scholar]


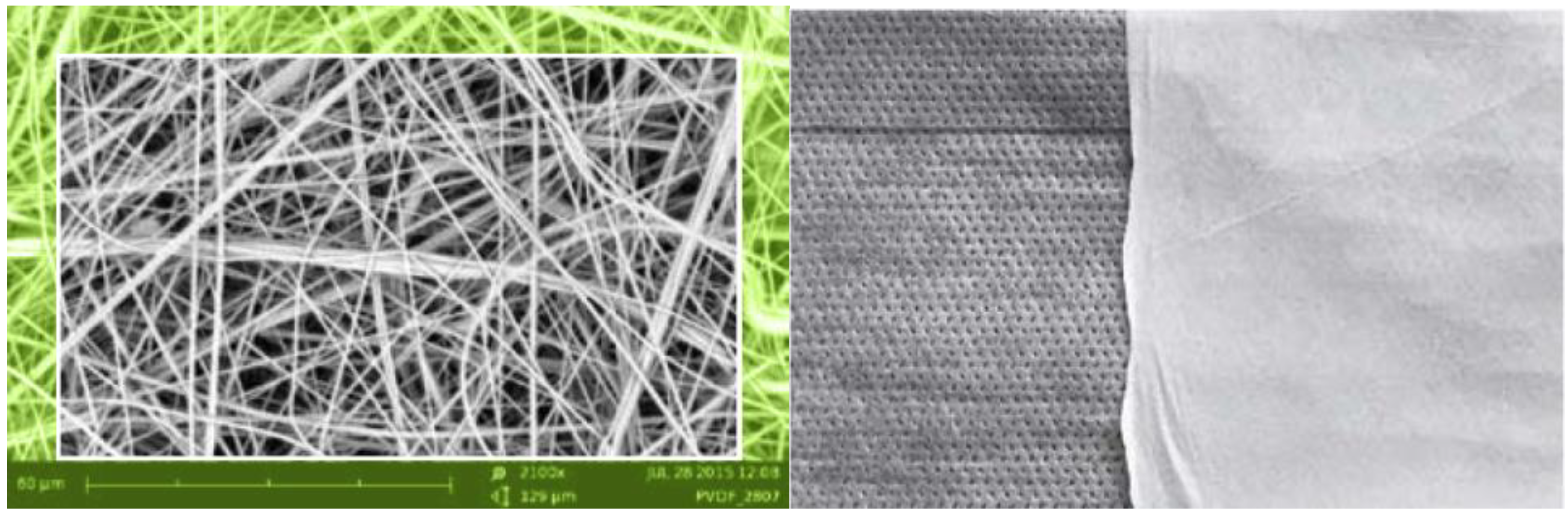

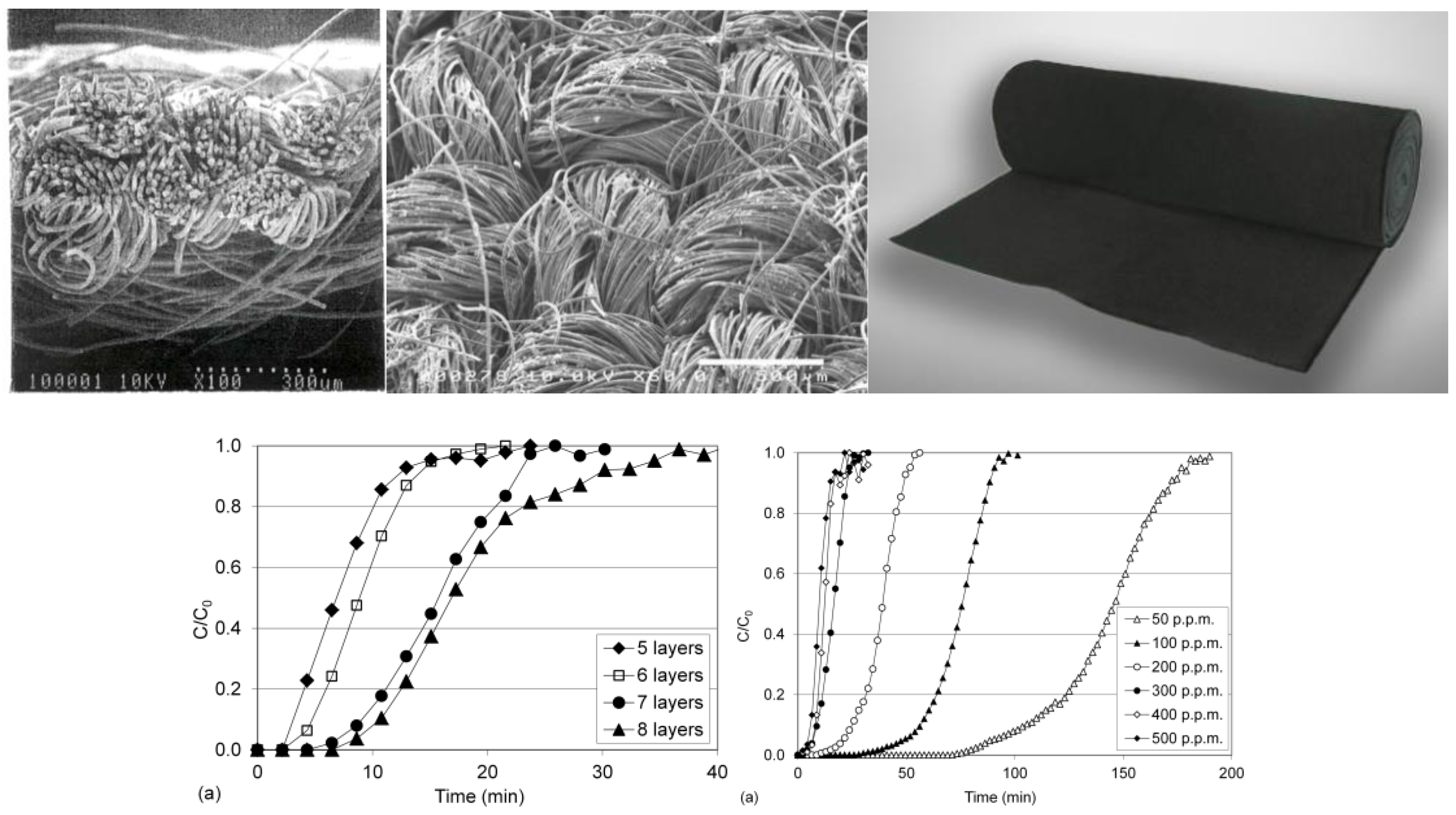

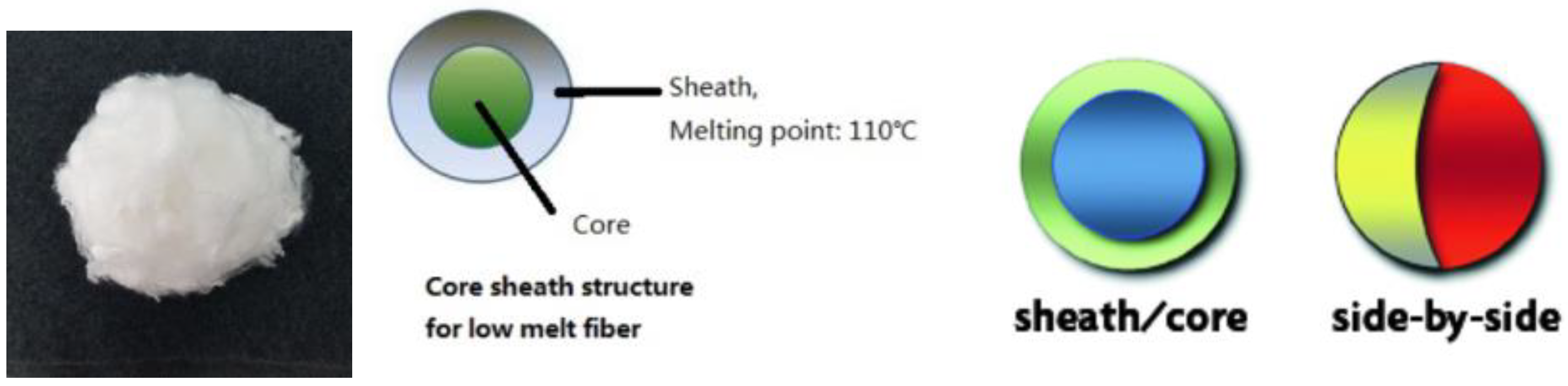








| Article No. | Weight | Thickness | Specific Surface Area | Typical Size/Roll | |
|---|---|---|---|---|---|
| Width | Length | ||||
| (g/m2) | (mm) | (m2/g) | (cm) | (m) | |
| ACC5092-10 | 200 | 0.65 | >800 | 115 | 35 |
| ACC5092-15 | 170 | 0.60 | >1300 | 110 | 35 |
| ACC5092-20 | 135 | 0.55 | >1800 | 105 | 35 |
| Testing Samples NANO for Water Filtration | ||
|---|---|---|
| Sample No. | Sample Specification Materials, Time, and Pressing Temperature | Results |
| VI | Nonwoven textile (NT) BICO Vigona (VIG) + NANO PVDF 140 °C, 30 s | Best |
| I | NT STRUTO (Jilana) JIL + NANO PA6 + NT STRUTO JIL 140 °C, 30 s | Good |
| IV | NANO PVDF + adsprption carbon textile (ACT) KYNOL + NT BICO VIG 140 °C, 30 s | Good |
| IX | NT BICO VIG + NANO PVDF + NT BICO VIG 140 °C, 30 s, lesser pressure | Good |
| X | Adsorption textile (AT) of the Filtration protective suit (hereinafter “FOP”) + NT BICO VIG + NANO PVDF 140 °C, 30 s | Best |
| Vll | AT FOP + NANO PVDF + AT FOP 140 °C 30 s | Good |
| V | AT FOP + NANO PVDF 140 °C, 30 s, lesser pressure | Bad |
| Effectivity of Paraffin Oil Aerosol Capture 0.4 μm at a Flow Rate of 30 L/min | |||||
| Sample | Permeation coefficient KP (%) | Average | |||
| 1 | 99.38 | 99.56 | 100.00 | 99.74 | 99.78 |
| 2 | 99.08 | 99.04 | 99.55 | 99.00 | 99.30 |
| 3 | 80.34 | 80.34 | 92.02 | 88.53 | 86.96 |
| Permeability at a Pressure of 140 Pa with an Area of 150 cm2 | |||||
| Sample | Flow (L/min) | ||||
| 1 | 99.30 | 93.60 | 84.30 | 95.90 | 93.28 |
| 2 | 202.00 | 205.00 | 244.00 | 206.00 | 214.28 |
| 3 | 450.00 | 479.00 | 478.00 | 468.25 | |
| Pressure Loss at Flow Rate of 200 L/min through the Material Surface 150 cm2 | |||||
| Sample | Pa | ||||
| 1 | 82.1 | 87.3 | 91.5 | 85.2 | 86.5 |
| 2 | 133.5 | 154.7 | 137.6 | 141.1 | 141.7 |
| 3 | 211.2 | 217.6 | 205.7 | 223.5 | 214.5 |
| Measured Part: Respirator | Number of Particles Surroundings | Number of Particles in Respirator | Fit Factor (Fc) | Uncertainty (extensive) 0.2·Fc |
|---|---|---|---|---|
| DEZA 3/1 + NT BICO FIB + nano PA6 | 33,571 | 348 | 97 | 19.4 |
| DEZA 3/2 + NT BICO FIB + nano PA-6 | 37,214 | 747 | 50 | 10 |
| DEZA 3/3 + NT BICO FIB + nano PA-6 | 37,143 | 706 | 48 | 9.6 |
| DEZA 3/4 + NT BICO FIB + nano PA-6 | 31,900 | 550 | 57 | 11.4 |
| DEZA 3/5 + NT BICO FIB+ nano PA-6 | 34,714 | 684 | 51 | 10.2 |
| NANOVIA 1 + NT BICO FIB + NVA nano | 35,100 | 1587 | 21 | 4.2 |
| NANOVIA 2 + NT BICO FIB + NVA nano | 35,429 | 2344 | 14 | 2.8 |
| NANOVIA 3 + NT BICO FIB + NVA nano | 36,800 | 1650 | 21 | 4.2 |
| DEZA 3/1 + AT FOP − outer site | 50,829 | 1176 | 42 | 8.4 |
| DEZA 3/2 + AT FOP − outer site | 53,343 | 786 | 67 | 13.4 |
| NT BICO FIB + AT FOP − outer site | 55,400 | 2596 | 20 | 4 |
| NT BICO FIB + AT FOP − outer site | 51,343 | 3344 | 14 | 2.8 |
| RESPIMASK − 12 | 42,914 | 1089 | 6.3 | 1.3 |
| DEZA 3/1 + AUT | 37,386 | 547 | 67 | 13.4 |
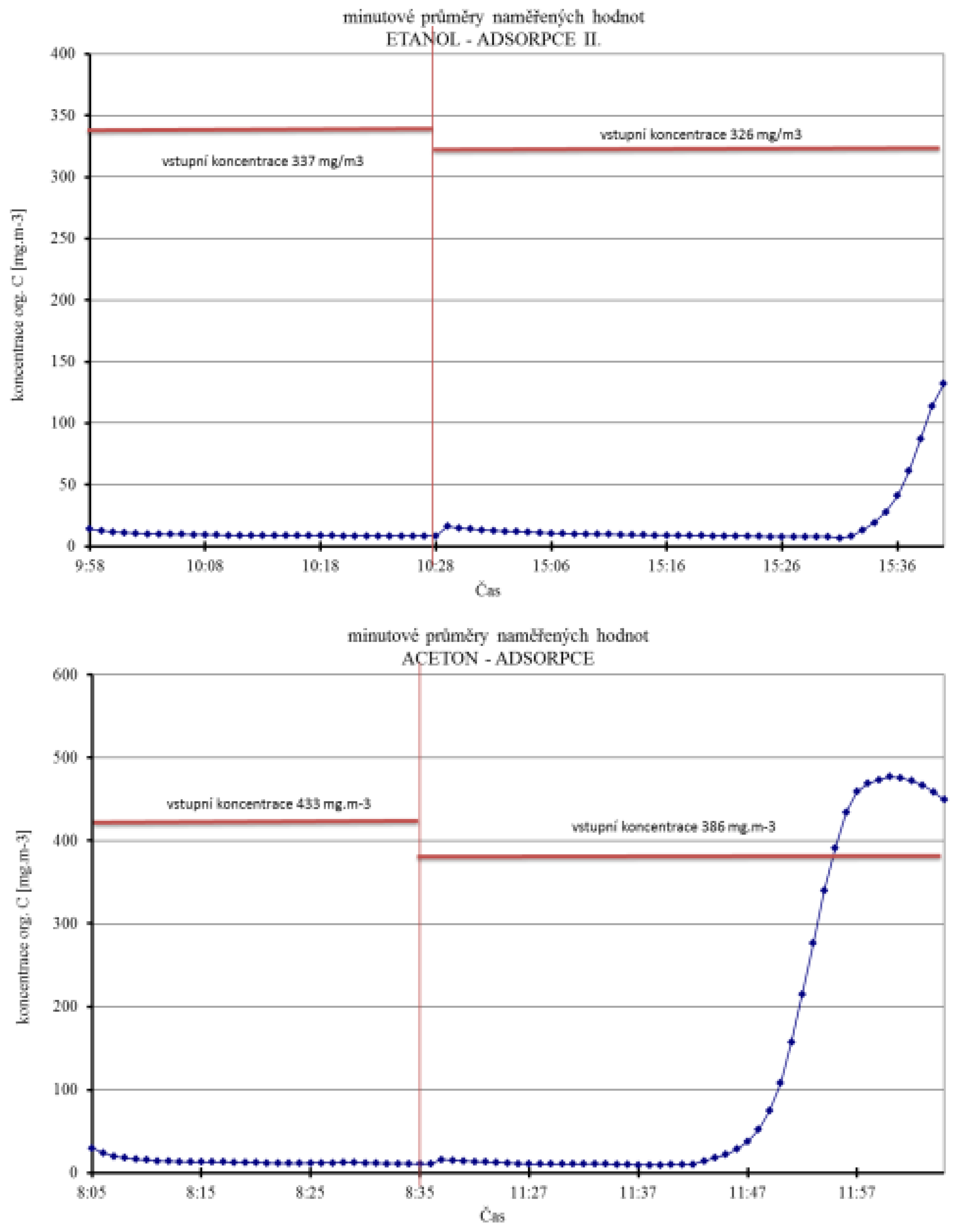
| Chemical Compounds | Heptane I | Heptane II | Ethanol I | Cyclohexanone | Acetone | Ethanol II | Heptane III |
|---|---|---|---|---|---|---|---|
| Input Concentration (ppm) | 1350 | 380 | 530 | 780 | 255 | 206.5 | 300 |
| Output Measured Concentration (mg/m3) | 10 | 3 | 28 | 3 | 13 | 10 | 54 |
| Conversion to a Given Substance | 1.2 | 1.2 | 2.41 | 1.36 | 1.61 | 2.41 | 1.2 |
| Time until Breakthrough (min) | 7 | 24 | 21 | 80 | 53 | 66 | 10 |
| Flow-input (L/min) | 2 | 1.6 | 4 | 1.4 | 3.5 | 3.4 | 3.2 |
| Convert to mg/m3 | 2168.6 | 610.4 | 851.4 | 1253.0 | 409.6 | 331.7 | 481.9 |
| Flow-input (L/min) | 0.6 | 0.43 | 0.6 | 0.54 | 0.55 | 0.6 | 0.56 |
| Desorption within Concentration (mg/m3) | 6826 | 2293 | 4556 | 3071 | 3348 | 2758 | 1870 |
| Efficiency | 9.5 | 99.5 | 96.7 | 99.8 | 96.8 | 97.0 | 88.8 |
| Time until Breakthrough | 7.0 | 24.0 | 21.0 | 80.0 | 53.0 | 66.0 | 10.0 |
| Force amounts | 14.0 | 38.4 | 84.0 | 112.0 | 185.5 | 224.4 | 32.0 |
| Amount of sorbed Substance | 36.4 | 28.1 | 172.4 | 190.9 | 122.3 | 179.4 | 18.5 |
| Conversion to 1 g of Sorbent | 8.7 | 6.7 | 41.0 | 45.4 | 29.1 | 42.7 | 4.4 |
| Degree of Concentration | 3.1 | 3.8 | 5.4 | 2.5 | 8.2 | 8.3 | 3.9 |
| Flow rate | 3.3 | 3.7 | 6.7 | 2.6 | 6.4 | 5.7 | 5.7 |
| Grade | |
|---|---|
| 0 | No growth apparent under a nominal magnification of 50×. |
| 1 | Traces of growth plainly visible under the microscope. |
| 2a | Sparse growth visible to the naked eye and/or under the microscope scattered only or localized to a few places covering all together not more than 5% of the test surface. |
| 2b | Growth plainly visible to the naked eye and/or under the microscope, distributed more or less homogenously on many places covering all together not more than 25% of the test surface. |
| 3 | Growth plainly visible to the naked eye and covering more than 25% of the test surface. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otrisal, P.; Obsel, V.; Buk, J.; Svorc, L. Preparation of Filtration Sorptive Materials from Nanofibers, Bicofibers, and Textile Adsorbents without Binders Employment. Nanomaterials 2018, 8, 564. https://doi.org/10.3390/nano8080564
Otrisal P, Obsel V, Buk J, Svorc L. Preparation of Filtration Sorptive Materials from Nanofibers, Bicofibers, and Textile Adsorbents without Binders Employment. Nanomaterials. 2018; 8(8):564. https://doi.org/10.3390/nano8080564
Chicago/Turabian StyleOtrisal, Pavel, Vladimir Obsel, Jan Buk, and Lubomír Svorc. 2018. "Preparation of Filtration Sorptive Materials from Nanofibers, Bicofibers, and Textile Adsorbents without Binders Employment" Nanomaterials 8, no. 8: 564. https://doi.org/10.3390/nano8080564
APA StyleOtrisal, P., Obsel, V., Buk, J., & Svorc, L. (2018). Preparation of Filtration Sorptive Materials from Nanofibers, Bicofibers, and Textile Adsorbents without Binders Employment. Nanomaterials, 8(8), 564. https://doi.org/10.3390/nano8080564






