Single-Step Direct Hydrothermal Growth of NiMoO4 Nanostructured Thin Film on Stainless Steel for Supercapacitor Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. NiMoO4 Nanostructure Growth Process
2.2. Materials Characterization
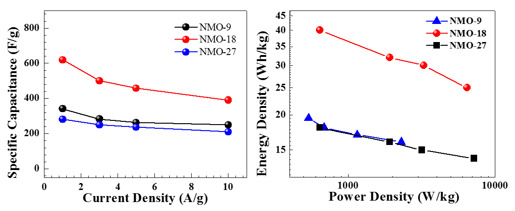
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Liban, D.J.; Saleh, M.A.; Berkson, Z.J.; El-Kady, M.F.; Hwang, J.Y.; Mohamed, N.; Wixtrom, A.I.; Titarenko, E.; Shao, Y.; McCarthy, K.; et al. A molecular cross-linking approach for hybrid metal oxides. Nat. Mater. 2018, 17, 341–348. [Google Scholar]
- Cheng, F.; Shen, J.; Peng, B.; Pan, Y.; Tao, Z.; Chen, J. Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 2011, 3, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Pang, H.; Lou, X.W. Facile synthesis of mesoporous Ni0.3Co2.7O4 hierarchical structures for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 3619–3626. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese–cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Ma, J.Z.; Zhang, L.L.; Guo, P.Z.; Jiang, J.W.; Zhao, X.S. Template synthesis of tubular ruthenium oxides for supercapacitor applications. J. Phys. Chem. C 2010, 114, 13608–13613. [Google Scholar] [CrossRef]
- Wang, Q.H.; Jiao, L.F.; Du, H.M.; Peng, W.X.; Han, Y.; Song, D.W.; Si, Y.C.; Wang, Y.J.; Yuan, H.T. Novel flower-like CoS hierarchitectures: One-pot synthesis and electrochemical properties. J. Mater. Chem. 2011, 21, 327–329. [Google Scholar] [CrossRef]
- Zhou, L.; He, Y.; Jia, C.; Pavlinek, V.; Saha, P.; Cheng, Q. Construction of hierarchical CuO/Cu2O@NiCo2S4 nanowire arrays on copper foam for high performance supercapacitor electrodes. Nanomaterials 2017, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Yang, F.; Zhao, Y.L.; Xu, X.; Xu, L.; Luo, Y.Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Kang, L.; Kong, L.B.; Lu, C.; Ma, X.J.; Li, X.M.; Luo, Y.C. Facile synthesis of NiMoO4·xH2O nanorods as a positive electrode material for supercapacitors. RSC Adv. 2013, 3, 6472–6478. [Google Scholar] [CrossRef]
- Guan, B.K.; Hu, L.L.; Zhang, G.H.; Guo, D.; Fu, T.; Li, J.D.; Duan, H.G.; Li, Q.H. Facile synthesis of ZnWO4 nanowall arrays on Ni foam for high performance supercapacitors. RSC Adv. 2014, 4, 4212–4217. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, P.; Zhang, H.; Yu, X.; Zhu, J.; Li, Q.; Wang, T. NiMoO4 nanowires supported on Ni foam as novel advanced electrodes for supercapacitors. J. Mater. Chem. A 2013, 7, 9024–9027. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Wu, H.B.; Zhang, C.; Lou, X.W. Controlled synthesis of hierarchical CoxMn3−xO4 array micro-/nanostructures with tunable morphology and composition as integrated electrodes for lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2664–2671. [Google Scholar] [CrossRef]
- Shaijumon, M.M.; Perre, E.; Daffos, B.; Taberna, P.L.; Tarascon, J.M.; Simon, P. Nanoarchitectured 3D cathodes for Li-Ion microbatteries. Adv. Mater. 2010, 22, 4978–4981. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, L.; Wu, H.B.; Madhavi, S.; Lou, X.W. Controlled growth of NiMoO4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1401172. [Google Scholar] [CrossRef]
- Cai, D.; Wang, D.; Liu, B.; Wang, Y.; Liu, Y.; Wang, L.; Li, H.; Huang, H.; Li, Q.; Wang, T. Comparison of the electrochemical performance of NiMoO4 nanorods and hierarchical nanospheres for supercapacitor applications. ACS Appl. Mater. Interface 2013, 5, 12905–12910. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, S.; Chen, Y.; Gao, P.; Zhu, C.; Yang, P.; Qi, L. Hierarchical nanosheet-based NiMoO4 nanotubes: Synthesis and high supercapacitor performance. J. Mater. Chem. A 2015, 3, 739–745. [Google Scholar] [CrossRef]
- Teeraphat, W.; Manickam, M.S.; Sudip, C.; Dan, L.; Shafiullah, G.M.; Robert, D.A.; Rajeev, A. Effect of transition metal cations on stability enhancement for molybdate-based hybrid supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 17977–17991. [Google Scholar]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for high-performance supercapacitor applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Chen, J.S.; Ng, M.F.; Wu, H.B.; Zhang, L.; Lou, X.W. Synthesis of phase-pure SnO2 nanosheets with different organized structures and their lithium storage properties. CrystEngComm 2012, 14, 5133–5136. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Lou, X.W. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.H.; Chen, Y.J.; Zhang, M.; Hu, L.L.; Lei, D.N.; Lu, B.G.; Li, Q.H.; Wang, Y.G.; Chen, L.B.; Wang, T.H. β-Cobalt sulfide nanoparticles decorated graphene composite electrodes for high capacity and power supercapacitors. Nanoscale 2012, 4, 7810–7816. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Fan, Z.J.; Sun, W.; Ning, G.Q.; Wei, T.; Zhang, Q.; Zhang, R.F.; Zhi, L.J.; Wei, F. Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Charles, C.L.M.; Jung, S.; Jonas, C.P.; Thomas, F.J. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar]
- Qian, H.; Jinlong, L.; Tongxiang, L.; Chen, W. In situ synthesis of mesoporous NiMoO4 on ball milled graphene for high performance supercapacitors. J. Electrochem. Soc. 2017, 164, E173–E179. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Beidaghi, M.; Campos, J.W.; Dennison, C.R.; Kumbur, E.C.; Gogotsi, Y. A high performance pseudocapacitive suspension electrode for the electrochemical flow capacitor. Electrochim. Acta 2013, 111, 888–897. [Google Scholar] [CrossRef]
- Han, D.D.; Xu, P.C.; Jing, X.Y.; Wang, J.; Yang, P.P.; Shen, Q.H.; Liu, J.Y.; Song, D.L.; Gao, Z.; Zhang, M.L. Trisodium citrate assisted synthesis of hierarchical NiO nanospheres with improved supercapacitor performance. J. Power Sources 2013, 235, 45–53. [Google Scholar] [CrossRef]
- Yu, G.H.; Hu, L.B.; Vosgueritchian, M.; Wang, H.L.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z.N. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Brousse, T.; B’elanger, D.; Longd, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Owusu, K.A.; Qu, L.; Li, J.; Wang, Z.; Zhao, K.; Yang, C.; Hercule, K.M.; Lin, C.; Shi, C.; Wei, Q.; et al. Low-crystalline iron oxide hydroxide nanoparticle anode for high-performance supercapacitors. Nat. Commun. 2017, 8, 14264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.C.; Kong, L.B.; Lu, C.; Ma, X.J.; Li, X.M.; Luo, Y.C.; Kang, L. Design and synthesis of CoMoO4–NiMoO4·xH2O bundles with improved electrochemical properties for supercapacitors. J. Mater. Chem. A 2013, 1, 1380–1387. [Google Scholar] [CrossRef]
- He, Y.M.; Chen, W.J.; Li, X.D.; Zhang, Z.X.; Fu, J.C.; Zhao, C.H.; Xie, E.Q. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.R.; Miura, N. Electrochemically deposited nanowhiskers of nickel oxide as a high-power pseudocapacitive electrode. Appl. Phys. Lett. 2004, 85, 4199–4201. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Polleux, J.; Dunn, B.; Tolbert, S.H. Templated nanocrystal-based porous TiO2 films for next-generation electrochemical capacitors. J. Am. Chem. Soc. 2009, 131, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.P.; Jow, T.R. High energy and high power density electrochemical capacitors. J. Power Sources. 1996, 62, 155–159. [Google Scholar] [CrossRef]
- Wang, H.L.; Gao, Q.M.; Jiang, L. Facile approach to prepare nickel cobaltite nanowire materials for supercapacitors. Small 2011, 7, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, C.Z.; Sun, T.; Ma, J. High-performance supercapacitor material based on Ni(OH)2 nanowire-MnO2 nanoflakes core–shell nanostructures. Chem. Commun. 2012, 48, 2606–2608. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Sundaram, M.M. Electrochemical synthesis of polyaniline crosslinked NiMoO4 nanofibre dendrites for energy storage devices. New J. Chem. 2016, 40, 7456–7464. [Google Scholar] [CrossRef]
- Barmi, M.J.; Minakshi, M. Tuning the redox properties of the nanostructured CoMoO4 electrode: effects of surfactant content and synthesis temperature. ChemPlusChem 2016, 81, 964–977. [Google Scholar] [CrossRef]
- Nan, H.; Ma, W.; Gu, Z.; Geng, B.; Zhang, X. Hierarchical NiMn2O4@CNT nanocomposites for high-performance asymmetric supercapacitors. RSC Adv. 2015, 5, 24607–24614. [Google Scholar] [CrossRef]
- Sugimoto, W.; Iwata, H.; Yasunaga, Y.; Murakami, Y.; Takasu, Y. Preparation of ruthenic acid nanosheets and utilization of its interlayer surface for electrochemical energy storage. Angew. Chem. Int. Ed. 2003, 42, 4092–4096. [Google Scholar] [CrossRef] [PubMed]
- Toupin, M.; Brousse, T.; Be’langer, D. Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]







| S. No. | NiMoO4 Electrode Type | Substrate | Substrate Type | Super Capacitance (F/g) | Reference |
|---|---|---|---|---|---|
| 1. | Nanorods | Ni-Foam | Reactive | 1136 | [16] |
| 2. | Nanorods | Ni-Foam | Reactive | 944 | [17] |
| 3. | Nanotubes | Ni-Foam | Reactive | 864 | [18] |
| 4. | Nanoneedles | Carbon | Non-reactive | 412 | [19] |
| 5. | Nanograins | Stainless Steel | Non-reactive | 619 | This work |
| S. No. | Electrode Material | Substrate | Specific Capacitance (F/g) | Energy Density (Wh/kg) | Reference |
|---|---|---|---|---|---|
| 1. | PANI/PTSa/cross linked NiMoO4 | Graphite | 1300 | 60 | [39] |
| 2. | NiMoO4 CoMoO4 MnMoO4 | Carbon | 412 240 120 | 35 15 10 | [19] |
| 3. | CoMoO4 | Carbon | 79 | 21 | [40] |
| 4. | NiMn2O4/CNT | Ni | 151 | 60.69 | [41] |
| 5. | RuO2 | - | 658 | - | [42] |
| 6. | MnO2 | - | 1380 | - | [43] |
| 7. | NiMoO4 | SS | 619 | 40 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannan, V.; Kim, H.-J.; Park, H.-C.; Kim, H.-S. Single-Step Direct Hydrothermal Growth of NiMoO4 Nanostructured Thin Film on Stainless Steel for Supercapacitor Electrodes. Nanomaterials 2018, 8, 563. https://doi.org/10.3390/nano8080563
Kannan V, Kim H-J, Park H-C, Kim H-S. Single-Step Direct Hydrothermal Growth of NiMoO4 Nanostructured Thin Film on Stainless Steel for Supercapacitor Electrodes. Nanomaterials. 2018; 8(8):563. https://doi.org/10.3390/nano8080563
Chicago/Turabian StyleKannan, V., Hyun-Jung Kim, Hyun-Chang Park, and Hyun-Seok Kim. 2018. "Single-Step Direct Hydrothermal Growth of NiMoO4 Nanostructured Thin Film on Stainless Steel for Supercapacitor Electrodes" Nanomaterials 8, no. 8: 563. https://doi.org/10.3390/nano8080563