Enhanced Tribological and Bacterial Resistance of Carbon Nanotube with Ceria- and Silver-Incorporated Hydroxyapatite Biocoating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Processing
2.2. Phase, Microstructural Characterization and Mechanical Characterization
2.3. Tribological Analysis of HA-Based Coatings
2.4. Wettability and Protein Adsorption
2.5. Antimicrobial Test
2.6. Cytocompatibility Test
3. Results
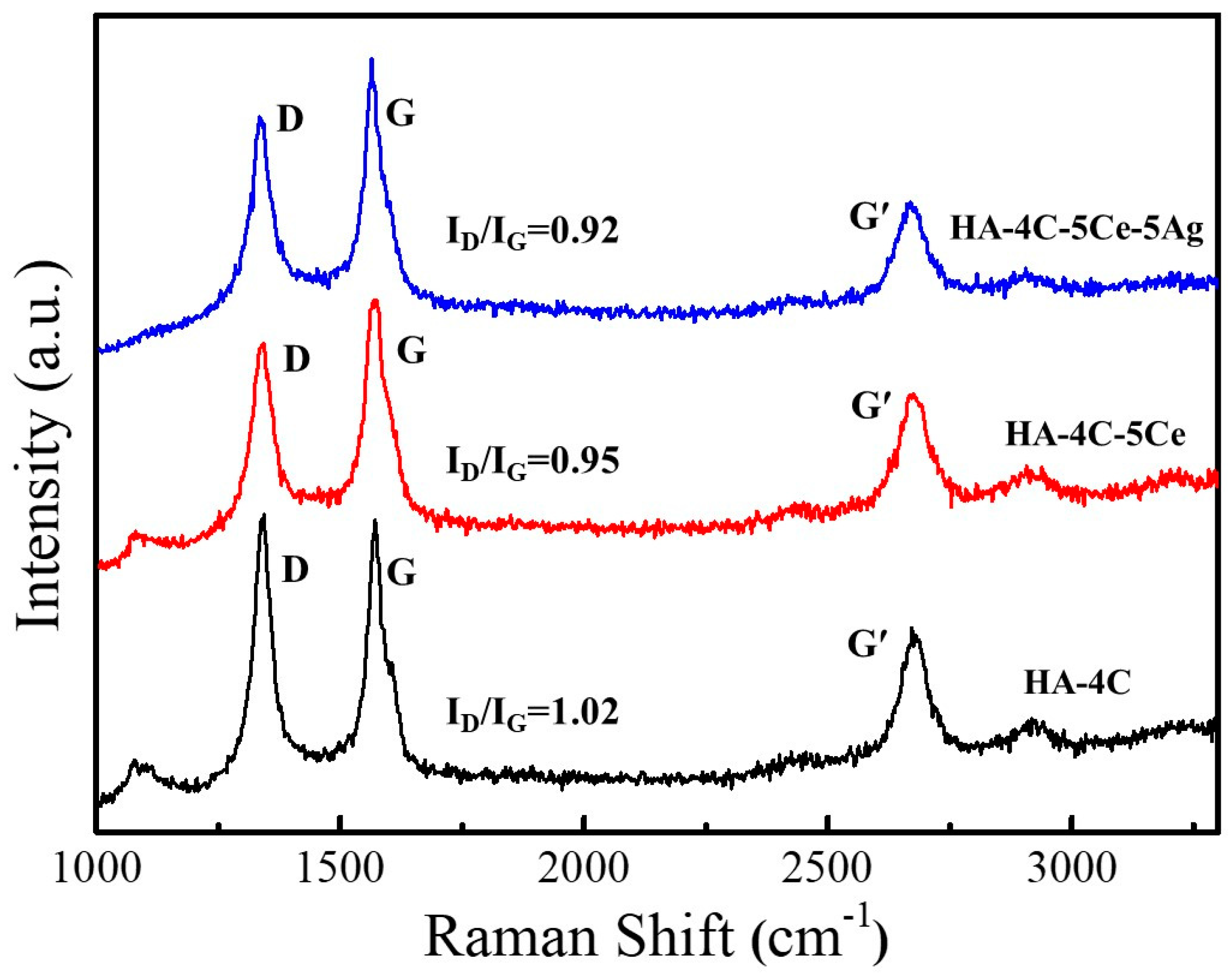
3.1. Phase Analysis
3.2. Microstructural Characterization and Mechanical Properties
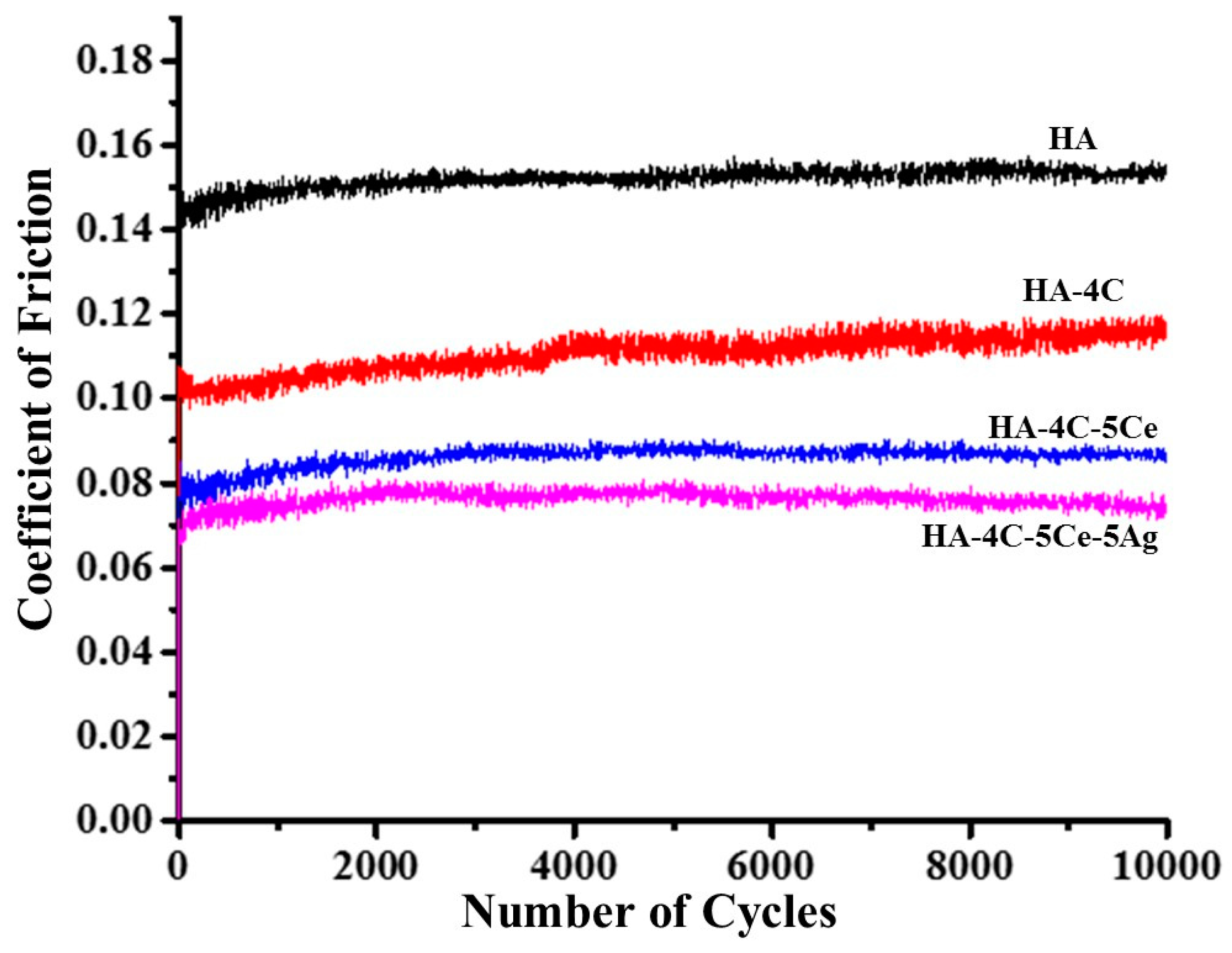
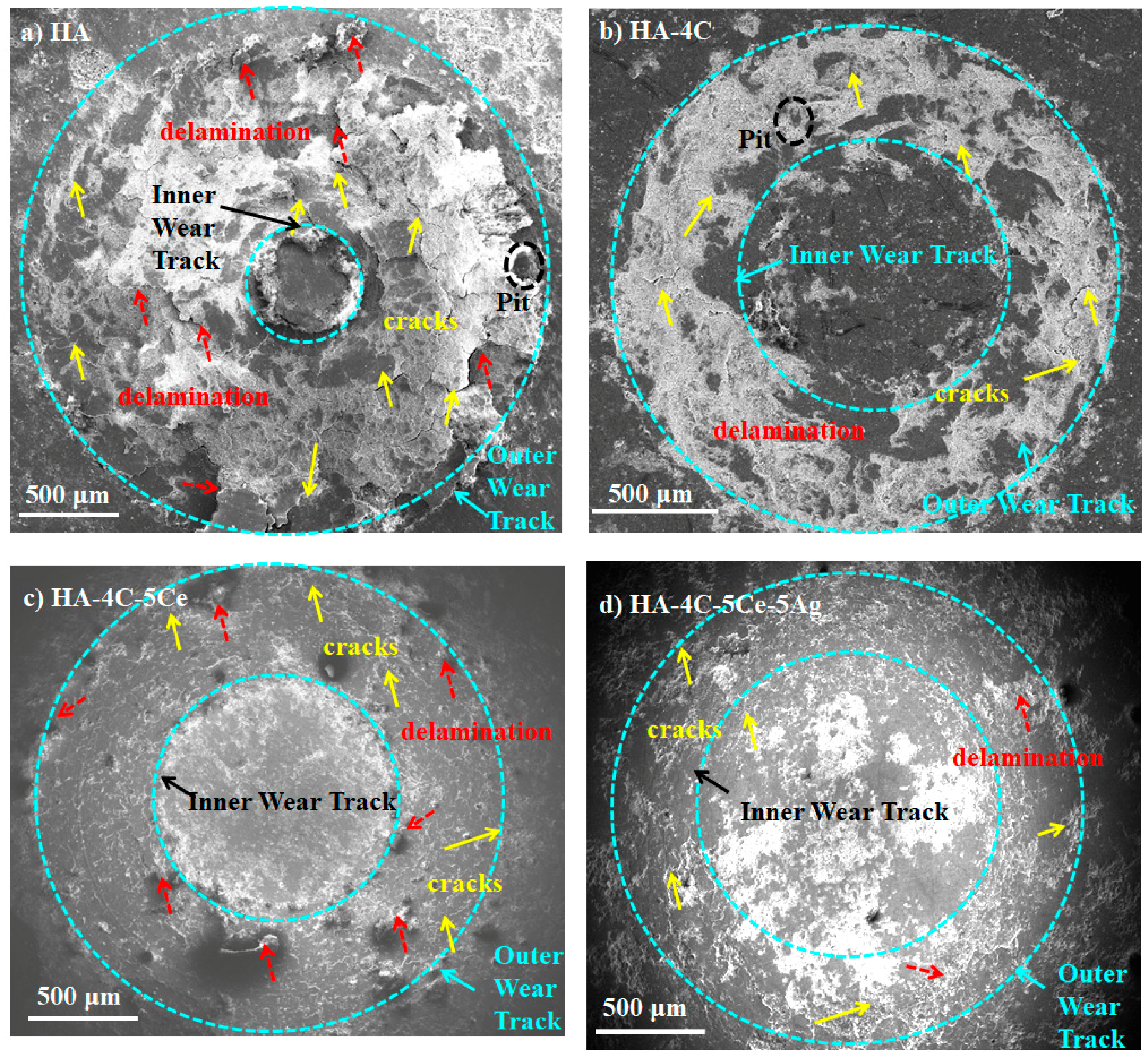
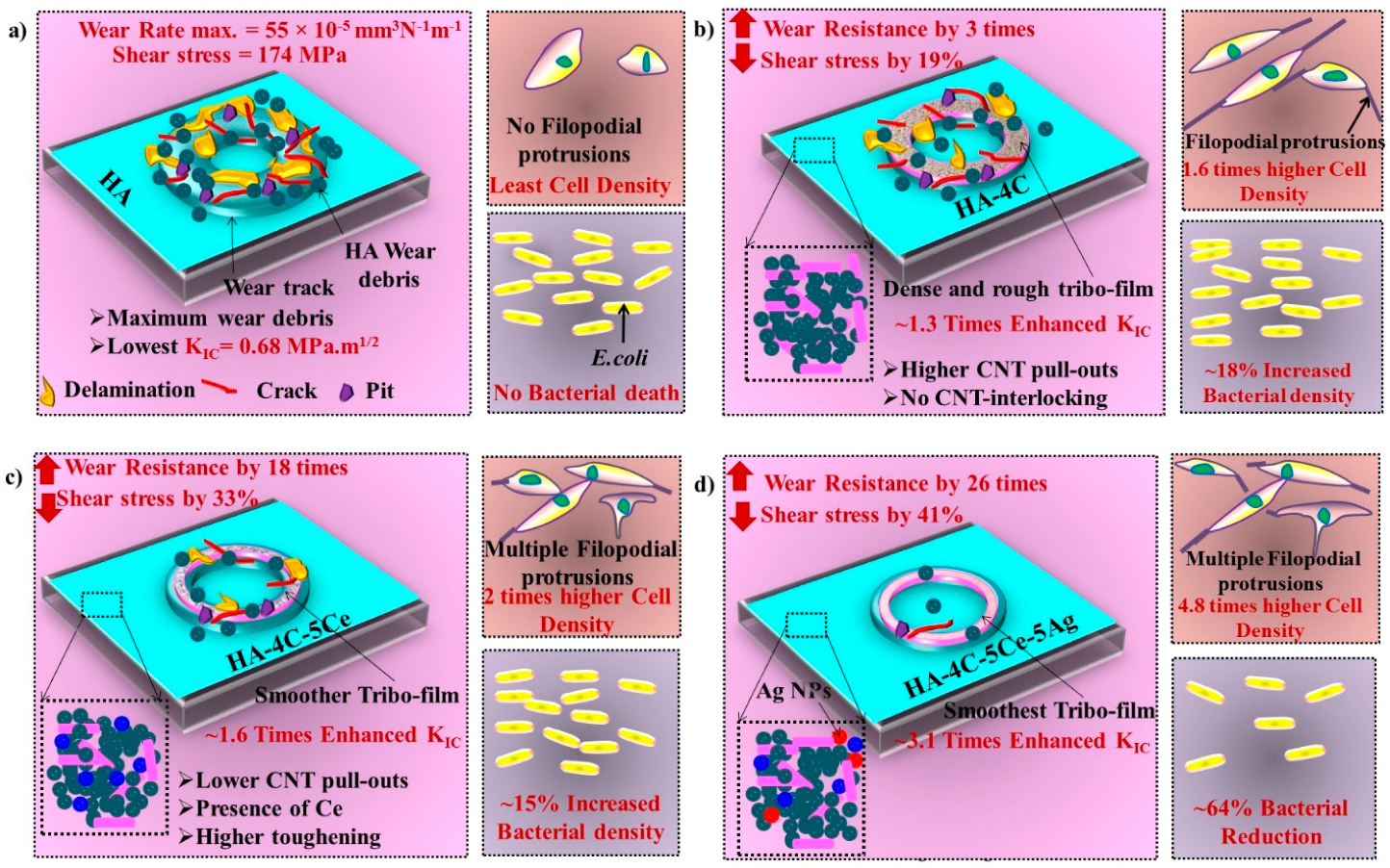
3.3. Tribological Studies of HA-Based Coatings
3.4. Wettability Studies and Its Role in Protein Adsorption of HA-Based Coatings
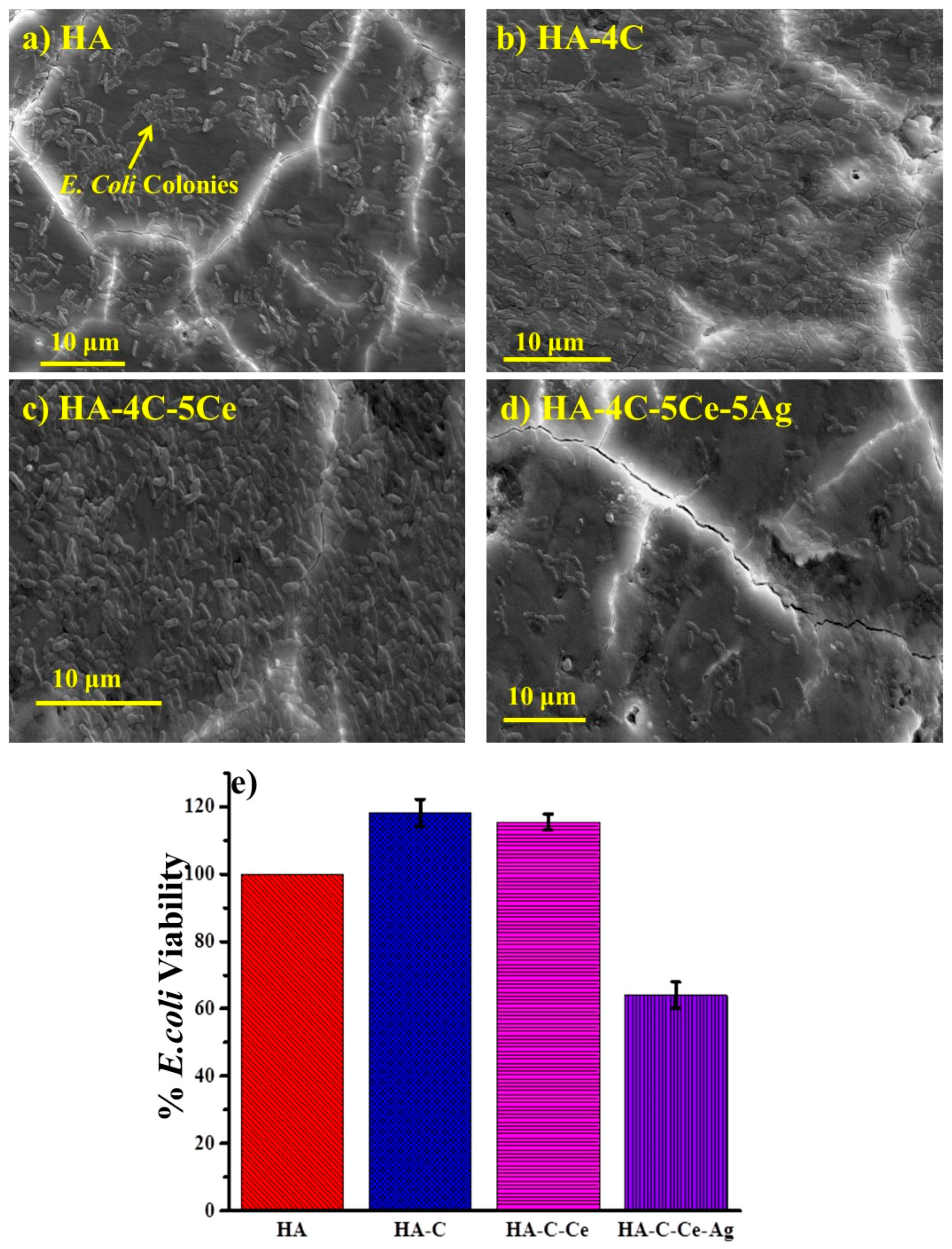
3.5. Antibacterial Efficacy of Silver-Reinforced HA Coating
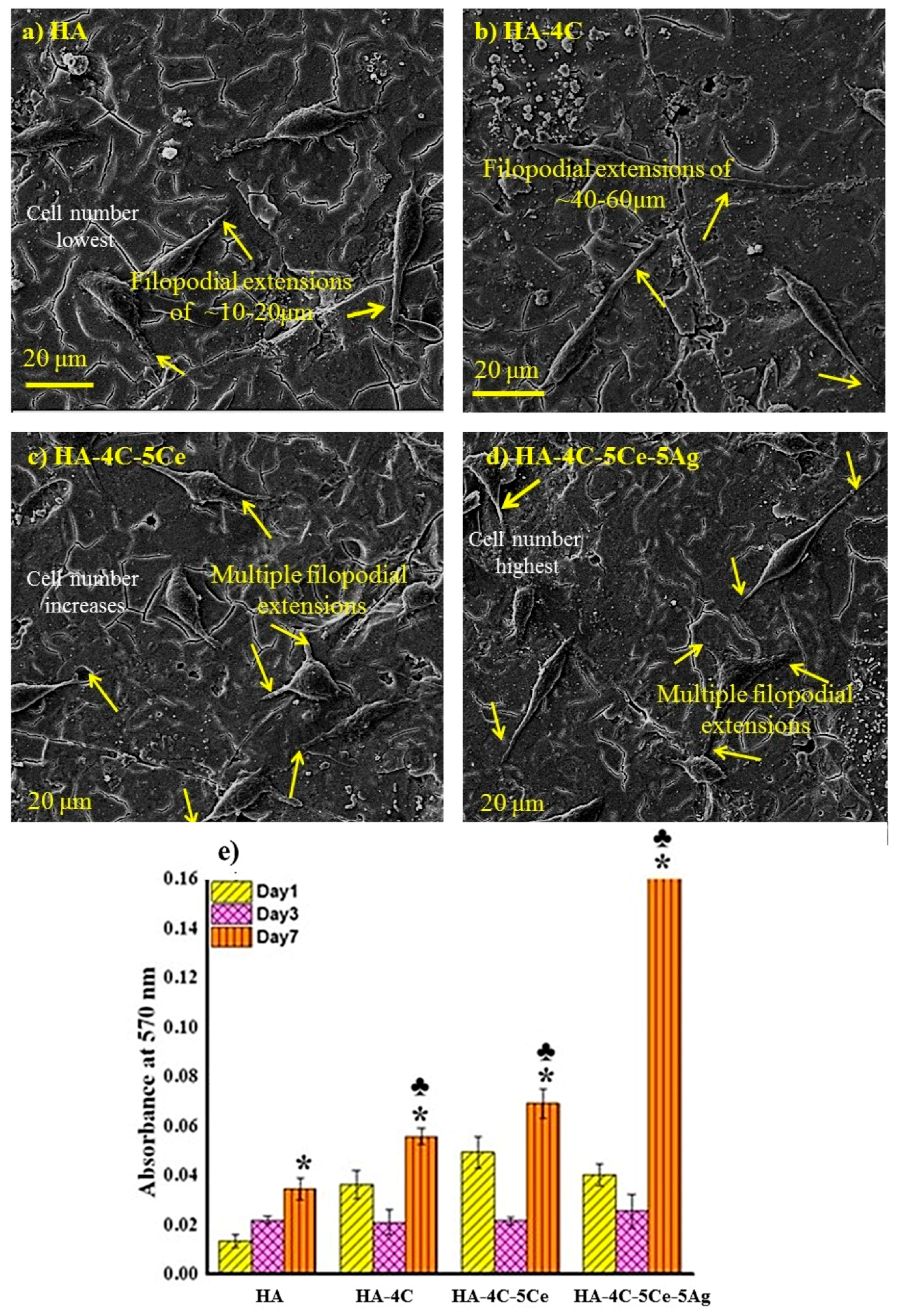
3.6. Cytocompatibilty Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Joshi, S.V.; Srivastava, M.P.; Pal, A.; Pal, S. Plasma spraying of biologically derived hydroxyapatite on implantable materials. J. Mater. Sci. Mater. Med. 1993, 4, 251–255. [Google Scholar] [CrossRef]
- Piattelli, A.; Trisi, P. A light and laser scanning microscopy study of bone/hydroxyapatite-coated titanium implants interface: Histochemical evidence of unmineralized material in humans. Biomed. Mater. Res. 1994, 28, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [PubMed]
- With, G.D.; Van Dijk, H.J.A.; Hattu, N.; Prijs, K. Preparation, micro-structure and mechanical properties of dense polycrystalline hydroxy-apatite. J. Mater. Sci. 1981, 16, 1592–1598. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Structural influence from calcium phosphate coatings and its possible effect on enhanced bone integration. Acta Odontol. Scand. 2009, 67, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Manara, S.; Paolucci, F.; Palazzo, B.; Marcaccio, M.; Foresti, E.; Tosi, G.; Sabbatini, S.; Sabatino, P.; Altankov, G.; Roveri, N. Electrochemically-assisted deposition of biomimetic hydroxyapatite–collagen coatings on titanium plate. Inorg. Chim. Acta 2008, 361, 1634–1645. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Gale, D.; Yang, W.; Xiao, K.; Zhang, L.; Yin, Y.; Zreiqat, H. Novel sphene coatings on Ti-6Al-4V for orthopedic implants using sol-gel method. Acta Biomater. 2008, 4, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Shi, B.; Fairchild, A.; Cale, T. Applications of plasma coatings in artificial joints: An overview. Vacuum 2004, 73, 317–326. [Google Scholar] [CrossRef]
- Gotman, I. Characteristics of metals used in implants. J. Endourol. 1997, 6, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Fan, Y.B.; Gao, Y.; Hu, Q.H.; Wang, T.C. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials 2009, 30, 4590–4600. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayedhydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Chen, Y.; Gan, C.; Zhang, T.; Yu, G. Laser-surface-alloyed carbon nanotubes reinforced hydroxyapatite composite coatings. Appl. Phys. Lett. 2005, 86, 251905. [Google Scholar] [CrossRef]
- Hukovic, M.M.; Tkalcec, E.; Kwokal, A.; Piljac, J. An in vitro study of Ti and Ti-alloys coated with sol-gel derived hydroxyapatite coatings. Surf. Coat. Technol. 2003, 165, 40–50. [Google Scholar] [CrossRef]
- Liu, D.M.; Troczynski, T.; Tseng, W.J. Water based sol-gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Sarikaya, M.; Tamerler, C.; Jen, A.K.Y.; Schulten, K.; Baneyx, F. Molecular biomimetics: Nanotechnology through biology. Nat. Mater. 2003, 2, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kanhed, S.; Awasthi, S.; Goel, S.; Pandey, A.; Sharma, R.; Upadhyaya, A.; Balani, K. Porosity Distribution Affecting Mechanical and Biological Behaviour of Hydroxyapatite Bioceramic Composites. Ceram. Int. 2017, 43, 10442–10449. [Google Scholar] [CrossRef]
- Afzal, M.A.F.; Kalmodia, S.; Kesarwani, P.; Basu, B.; Balani, K. Bactericidal effect of silver-reinforced carbon nanotube and hydroxyapatite composites. J. Biomater. Appl. 2013, 27, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.A.; Kesarwani, P.; Reddy, K.M.; Kalmodia, S.; Basu, B.; Balani, K. Functionally graded hydroxyapatite-alumina-zirconia biocomposite:Synergy of toughness and biocompatibility. Mater. Sci. Eng. C 2012, 32, 1164–1173. [Google Scholar] [CrossRef]
- Lee, S.S.; Song, W.; Cho, M.; Puppala, H.L.; Nguyen, P.; Zhu, H.; Segatori, L.; Colvin, V.L. Antioxidant Properties of Cerium Oxide Nanocrystals as a Function of Nanocrystal Diameter and Surface Coating. ACS Nano 2013, 7, 9693–9703. [Google Scholar] [CrossRef] [PubMed]
- Herkendell, K.; Shukla, V.R.; Patel, A.K.; Balani, K. Domination of volumetric toughening by silver nanoparticles over interfacial strengthening of carbon nanotubes in bactericidal hydroxyapatite biocomposite. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 34, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Midha, S.; Sharma, R.; Maurya, R.; Nigam, V.; Ghosh, S.; Balani, K. Antioxidant and Antibacterial Property of Hydroxyapatite Biocomposites. Mater. Sci. Eng. C 2017. [Google Scholar] [CrossRef]
- Mullins, L.P.; Bruzzi, M.S.; McHugh, P.E. Measurement of the microstructural tracture toughness of cortical bone using indentation fracture. J. Biomech. 2007, 40, 3285–3288. [Google Scholar] [CrossRef] [PubMed]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.M.; Gong, F.; Liu, P.; Tran, T.Q. Advanced Fabrication and Properties of Aligned Carbon Nanotube Composites: Experiments and Modeling. In Carbon Nanotubes-Current Progress of their Polymer Composites; Berber, M.R., Hafez, I.H., Eds.; InTech Open: San Francisco, CA, USA, 2016. [Google Scholar]
- Khoshnevis, H.; MyoMint, S.; Yedinak, E.; Tran, T.Q.; Zahoush, A.; Youssefi, M.; Pasquali, M.; Duong, H.M. Super high-rate fabrication of high-purity carbon nanotube aerogels from floating catalyst method for oil spill cleaning. Chem. Phys. Lett. 2018, 693, 146–151. [Google Scholar] [CrossRef]
- Rajesh, R.; Senthilkumar, N.; Hariharasubramanian, A.; Ravichandran, Y.D. Review on hydroxyapatite-carbon nanotube composites and some of their Applications. Int. J. Pharm. Pharm. Sci. 2012, 4, 23–27. [Google Scholar]
- Chlopek, J.; Czajkowska, B.; Szaraniec, B.; Frackowiak, E.; Szostak, K.; Beguin, F. In vitro studies of carbon nanotubes biocompatibility. Carbon 2006, 44, 1106–1111. [Google Scholar] [CrossRef]
- Zanello, L.P.; Zhao, B.; Hu, H.; Haddon, R.C. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006, 6, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Usui, Y.; Aoki, K.; Narita, N.; Murakami, N.; Nakamura, I.; Nakamura, K. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small 2008, 4, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Singh, V.; Keshri, A.K.; Seal, S.; Agarwal, A. Carbon nanotube toughened hydroxyapatite by spark plasma sin-tering: Microstructural evolution and multi-scale tribological properties. Carbon 2010, 48, 3103–3120. [Google Scholar] [CrossRef]
- Balani, K.; Lahiri, D.; Keshri, A.K.; Bakshi, S.R.; Tercero, J.E.; Agarwal, A. The Nano-scratch Behavior of Biocompatible Hydroxyapatite Reinforced with Aluminum Oxide and Carbon Nanotubes. JOM J. Miner. Met. Mater. Soc. TMS 2009, 61, 63–66. [Google Scholar] [CrossRef]
- Rouahi, M.; Champion, E.; Hardouin, P.; Anselme, K. Quantitative kinetic analysis of gene expression during human osteoblastic adhesion on orthopedic materials. Biomaterials 2006, 27, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.Y.; Chueh, S.C.; Wang, Y.J. Microspheres of hydroxyapatite/reconstituted collagen as supports for osteoblast cell growth. Biomaterials 1999, 20, 1931–1936. [Google Scholar] [CrossRef]
- Ramires, P.A.; Romito, A.; Cosentino, F.; Milella, E. The influence of titania/hydroxyapatite composite coatings on in vitro osteoblasts behaviour. Biomaterials 2001, 22, 1467–1474. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, S.K.; Agarwal, A.K. Tribological studies of epoxy composites with solid and liquid fillers. Tribol. Int. 2017, 105, 27–36. [Google Scholar] [CrossRef]
- Murab, S.; Chameettachal, S.; Ghosh, S. Establishment of an in vitro monolayer model of macular corneal distrophy. Lab. Investig. Nat. Publ. Group 2016, 96, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Lawn, B.R.; Anstis, G.R.; Chantikul, P.; Marshall, D.B. A critical evaluation of indentation techniques for measuring fracture toughness: I, direct crack measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar]
- Nath, S.; Kalmodia, S.; Basu, B. Densification, phase stability and in vitro biocompatibility property of hydroxyapatite-10 wt% silver composites. J. Mater. Sci. Mater. Med. 2010, 21, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.M.; Francis Rose, L.R. The martensitic transformation in ceramics-its role in transformation toughening. Prog. Mater. Sci. 2002, 47, 463–557. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Zhang, C.; Nieto, A.; Agarwal, A. Ultrathin graphene tribofilm formation during wear of Al2O3-graphene composites. Nanomater. Energy 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Pandey, A.; Nigam, V.K.; Balani, K. Effect of Ceria and Silver Reinforcement on Multi-length Scale Tribology of Hydroxyapatite. Wear 2017. under review. [Google Scholar]
- Wu, P.Q.; Chen, H.; Stappen, M.V.; Stals, L.; Celisa, J.-P. Comparison of fretting wear of uncoated and PVD TiN coated high-speed steel under different testing conditions. Surf. Coat. Technol. 2000, 127, 114–119. [Google Scholar] [CrossRef]
- Balani, K.; Harimkar, S.P.; Keshri, A.; Chen, Y.; Dahotre, N.B.; Agarwal, A. Multiscale wear of plasma-sprayed carbon-nanotube-reinforced aluminum oxide nanocomposite coating. Acta Mater. 2008, 56, 5984–5994. [Google Scholar] [CrossRef]
- De Nicola, F.; Castrucci, P.; Scarselli, M.; Nanni, F.; Cacciotti, I.; De Crescenzi, M. Super-hydrophobic multi-walled carbon nanotube coatings for stainless steel. Nanotechnology 2015, 26, 145701. [Google Scholar] [CrossRef] [PubMed]
- Azimi, G.; Dhiman, R.; Hyuk-Min, K.; Paxton, A.T.; Varanasi, K.K. Hydrophobicity of Rare-earth Oxide Ceramics. Nat. Mater. 2013, 12, 315–320. [Google Scholar]
- Tsukuma, K.; Shimada, M. Strength, Fracture Toughness and Vickers Hardness of CeO2 Stabilized Tetragonal ZrO2 Polycrystals. J. Mater. Sci. 1985, 20, 1178–1184. [Google Scholar] [CrossRef]
- Giovambattista, N.; Debenedetti, P.G.; Rossky, P.J. Effect of Surface Polarity on Water Contact Angle and Interfacial Hydration Structure. J. Phys. Chem. B 2007, 111, 9581–9587. [Google Scholar] [CrossRef] [PubMed]
- Pratik, K.C.; Nammari, A.; Ashton, T.S.; Adjei, I.M. Saturated pool boiling heat transfer from vertically oriented silicon surfaces modified with foam-like hexagonal boron nitride nanomaterials. Int. J. Heat Mass Transf. 2016, 95, 964–971. [Google Scholar] [CrossRef]
- Tanaka, M.; Motomura, T.; Kawada, M.; Anzai, T.; Kasori, Y.; Shiroya, T.; Shimura, K.; Onishi, M.; Mochizuki, A. Blood compatible aspects of poly(2-methoxyethylacrylate) (PMEA)-relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials 2000, 21, 1471–1481. [Google Scholar] [CrossRef]
- Wertz, C.F.; Santore, M.M. Effect of Surface Hydrophobicity on Adsorption and Relaxation Kinetics of Albumin and Fibrinogen:Single-Species and Competitive Behavior. Langmuir 2001, 17, 3006–3016. [Google Scholar] [CrossRef]
- Ravindran, A.; Singh, A.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Studies on interaction of colloidal Ag nanoparticles with Bovine Serum Albumin (BSA). Colloids Surf. B Biointerfaces 2010, 76, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Norde, W. My voyage of discovery to proteins in flatland … and beyond. Colloids Surf. B Biointerfaces 2008, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of Serum Albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Adsorption of S-S containing proteins on a colloidal silver surface studied by surface-enhanced Raman spectroscopy. Appl. Spectrosc. 2004, 58, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Thill, A.; Zeyons, O.L.; Spalla, O.; Chauvat, F.; Rose, J.M.; Auffan, M.L.; Flank, A.M. Cytotoxicity of CeO2 Nanoparticles for Escherichia coli. Physico-Chemical Insight of the Cytotoxicity Mechanism. Environ. Sci. Technol. 2006, 40, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.K.; Sarkar, D. Study of BSA protein adsorption/release on hydroxyapatite nanoparticles. Appl. Surf. Sci. 2013, 286, 99–103. [Google Scholar] [CrossRef]
- Liu, X.; Lim, J.Y.; Donahue, H.J.; Dhurjati, R.; Mastro, A.M.; Vogler, E.A. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials 2007, 28, 4535–4550. [Google Scholar] [CrossRef] [PubMed]
- Jowsey, J.; Rowland, R.E.; Marshall, J.H. The deposition of the rare earths in bone. Radiat. Res. 1958, 8, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, D.; Finney, B.; Wilkinson, W.; Kemp, P. Novel regulatory aspects of the extracellular Ca2+-sensing receptor, CaR. Pflüg. Arch. Eur. J. Phys. 2009, 458, 1007–1022. [Google Scholar] [CrossRef] [PubMed]


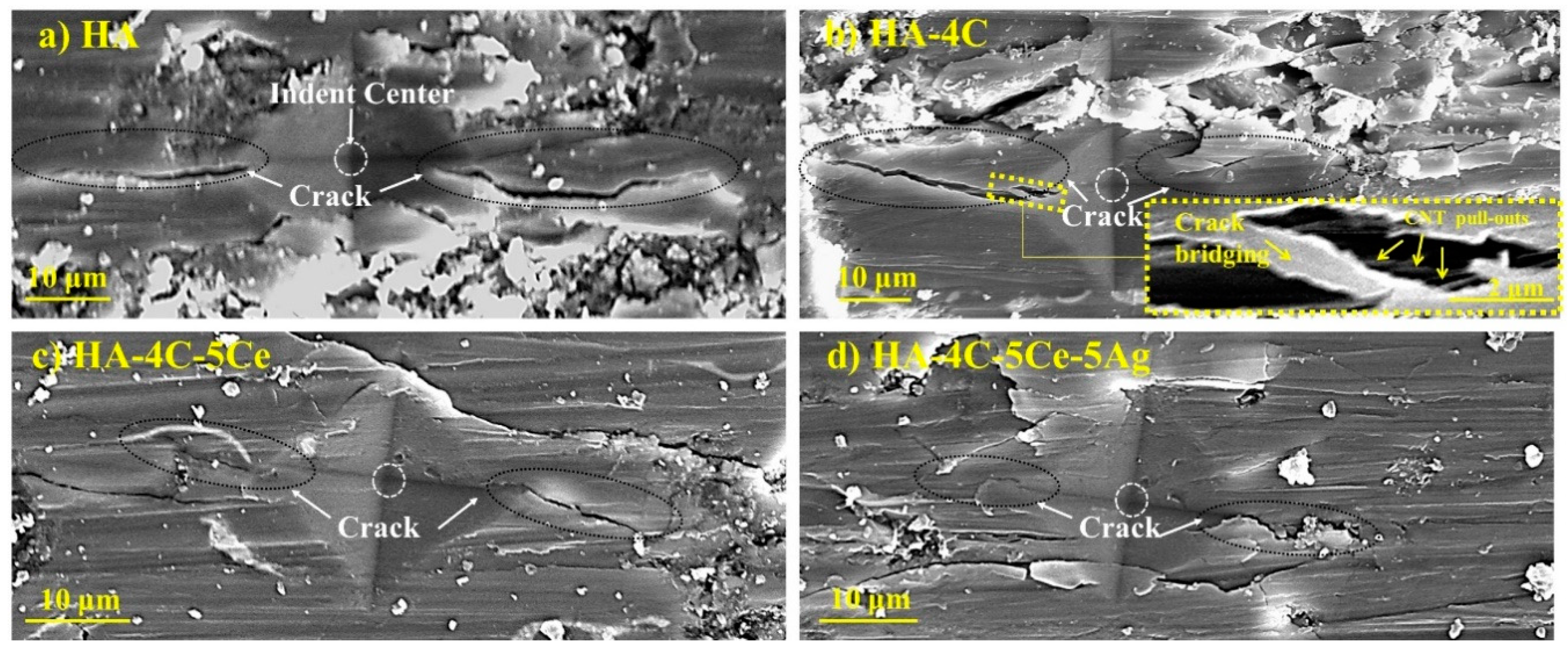
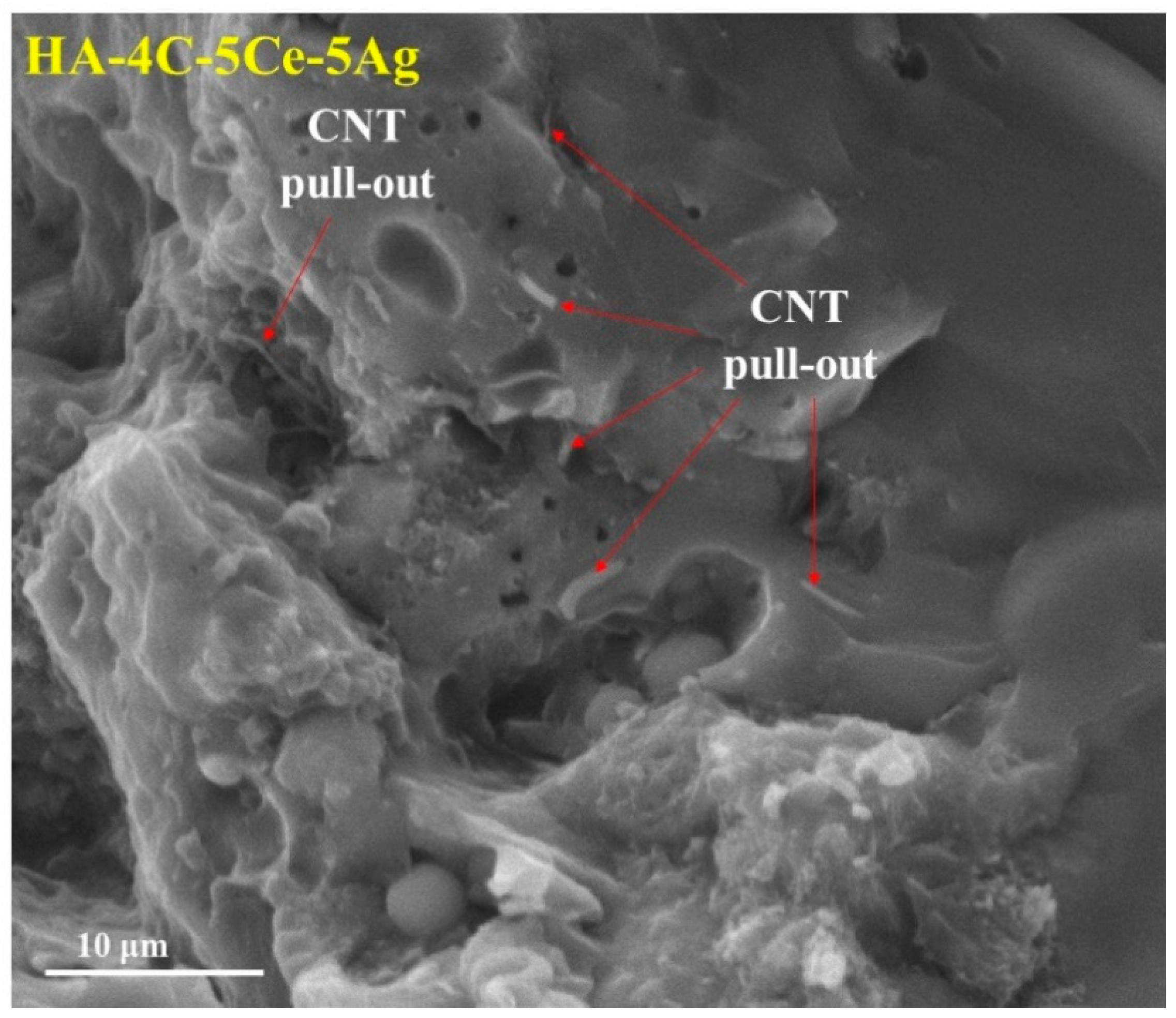

| Plasma Sprayed Coatings | Abbreviations | H (GPa) | E/H | KIC (MPa·m1/2) |
|---|---|---|---|---|
| Hydroxyapatite | HA | 1.88 ± 0.88 | 44 | 0.68 |
| Hydroxyapatite-CNT | HA-4C | 3.95 ± 0.96 | 41.8 | 0.89 |
| Hydroxyapatite-CNT-CeO2 | HA-4C-5Ce | 4.13 ± 0.54 | 41.4 | 1.12 |
| Hydroxyapatite-CNT-CeO2-Ag | HA-4C-5Ce-5Ag | 5.85 ± 0.85 | 29.3 | 2.12 |
| Sample | COF | WR (×10−6 mm3·N−1·m−1) | Roughness after Wear (µm) | E* (GPa) | HCD (µm) | τ (MPa) | Wear Resistance |
|---|---|---|---|---|---|---|---|
| HA | 0.16 ± 0.01 | 176.1 ± 7.0 | 12 | 77.1 | 66.3 | 173.7 | Base |
| HA-4C | 0.11 ± 0.01 | 54.7 ± 5.2 | 10 | 98.5 | 61.1 | 140.6 | 3.2 times |
| HA-4C-5Ce | 0.09 ± 0.01 | 9.7 ± 0.8 | 6 | 100.3 | 61.7 | 115 | 18.1 times |
| HA-4C-5Ce-5Ag | 0.08 ± 0.01 | 6.8 ± 0.2 | 5 | 99.5 | 61.9 | 102.9 | 25.9 times |
| Sample | Contact Angle (Degree) | Protein Adsorption (μg/mm2) | Day 1 Cell Density Improvement |
|---|---|---|---|
| HA |  74.0° ± 1.8 | 2.1 ± 0.1 | 1 |
| HA-4C |  104.0° ± 1.1 | 3.4 ± 0.1 * | 2.7 times |
| HA-4C-5Ce |  112.0° ± 1.3 | 5.0 ± 0.1 * | 3.7 times |
| HA-4C-5Ce-5Ag |  97.0° ± 2.0 | ±0.1 * | 3.0 times |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.; Patel, A.K.; S., A.; Kumar, V.; Sharma, R.K.; Kanhed, S.; Nigam, V.K.; Keshri, A.; Agarwal, A.; Balani, K. Enhanced Tribological and Bacterial Resistance of Carbon Nanotube with Ceria- and Silver-Incorporated Hydroxyapatite Biocoating. Nanomaterials 2018, 8, 363. https://doi.org/10.3390/nano8060363
Pandey A, Patel AK, S. A, Kumar V, Sharma RK, Kanhed S, Nigam VK, Keshri A, Agarwal A, Balani K. Enhanced Tribological and Bacterial Resistance of Carbon Nanotube with Ceria- and Silver-Incorporated Hydroxyapatite Biocoating. Nanomaterials. 2018; 8(6):363. https://doi.org/10.3390/nano8060363
Chicago/Turabian StylePandey, Aditi, Anup Kumar Patel, Ariharan S., Vikram Kumar, Rajeev Kumar Sharma, Satish Kanhed, Vinod Kumar Nigam, Anup Keshri, Arvind Agarwal, and Kantesh Balani. 2018. "Enhanced Tribological and Bacterial Resistance of Carbon Nanotube with Ceria- and Silver-Incorporated Hydroxyapatite Biocoating" Nanomaterials 8, no. 6: 363. https://doi.org/10.3390/nano8060363
APA StylePandey, A., Patel, A. K., S., A., Kumar, V., Sharma, R. K., Kanhed, S., Nigam, V. K., Keshri, A., Agarwal, A., & Balani, K. (2018). Enhanced Tribological and Bacterial Resistance of Carbon Nanotube with Ceria- and Silver-Incorporated Hydroxyapatite Biocoating. Nanomaterials, 8(6), 363. https://doi.org/10.3390/nano8060363





