Self-Assembled Ag-Cu2O Nanocomposite Films at Air-Liquid Interfaces for Surface-Enhanced Raman Scattering and Electrochemical Detection of H2O2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Ag-Cu2O Nanocomposite Films
2.3. SERS Measurements
2.4. Electrochemical Experiments
2.5. Characterizations
3. Results and Discussion
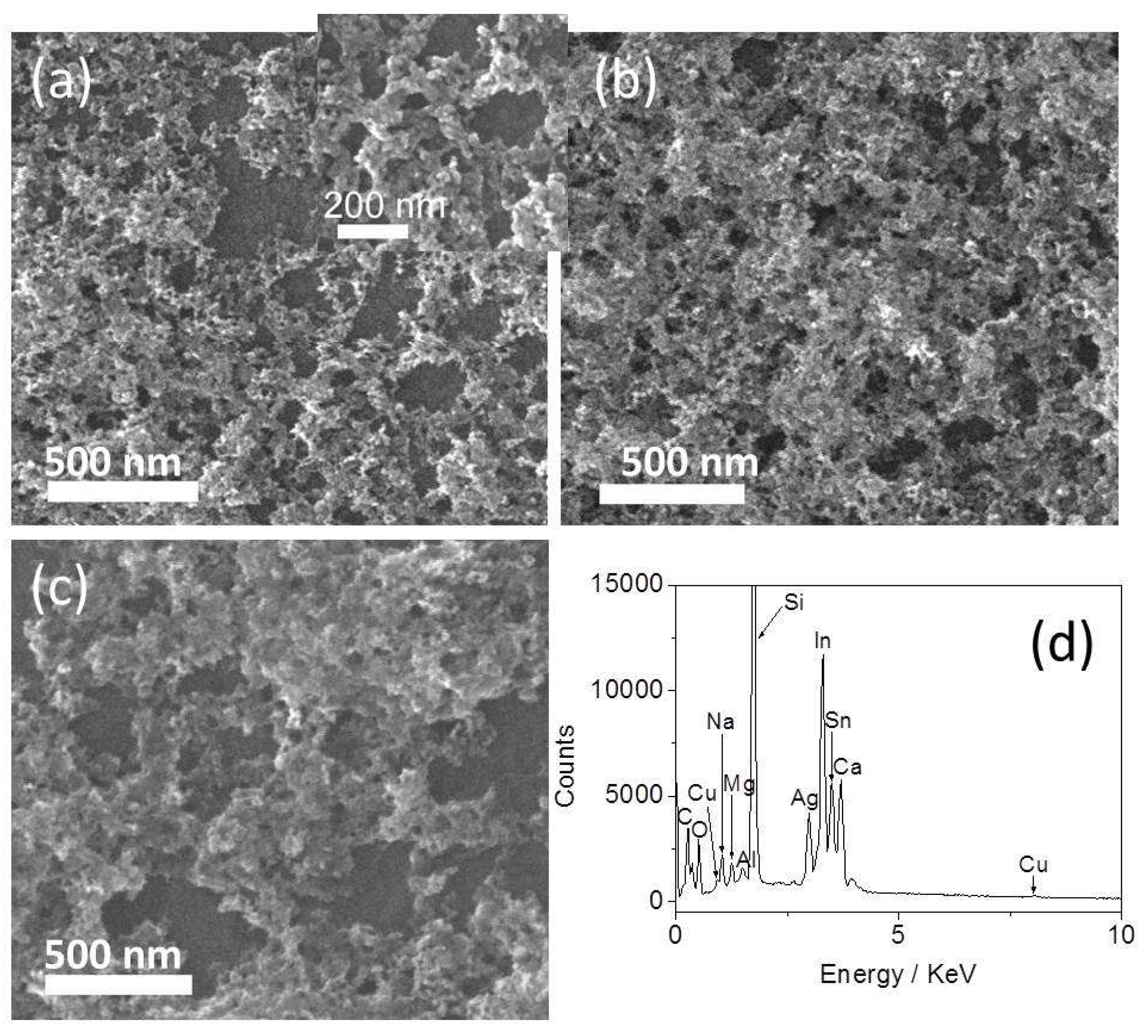
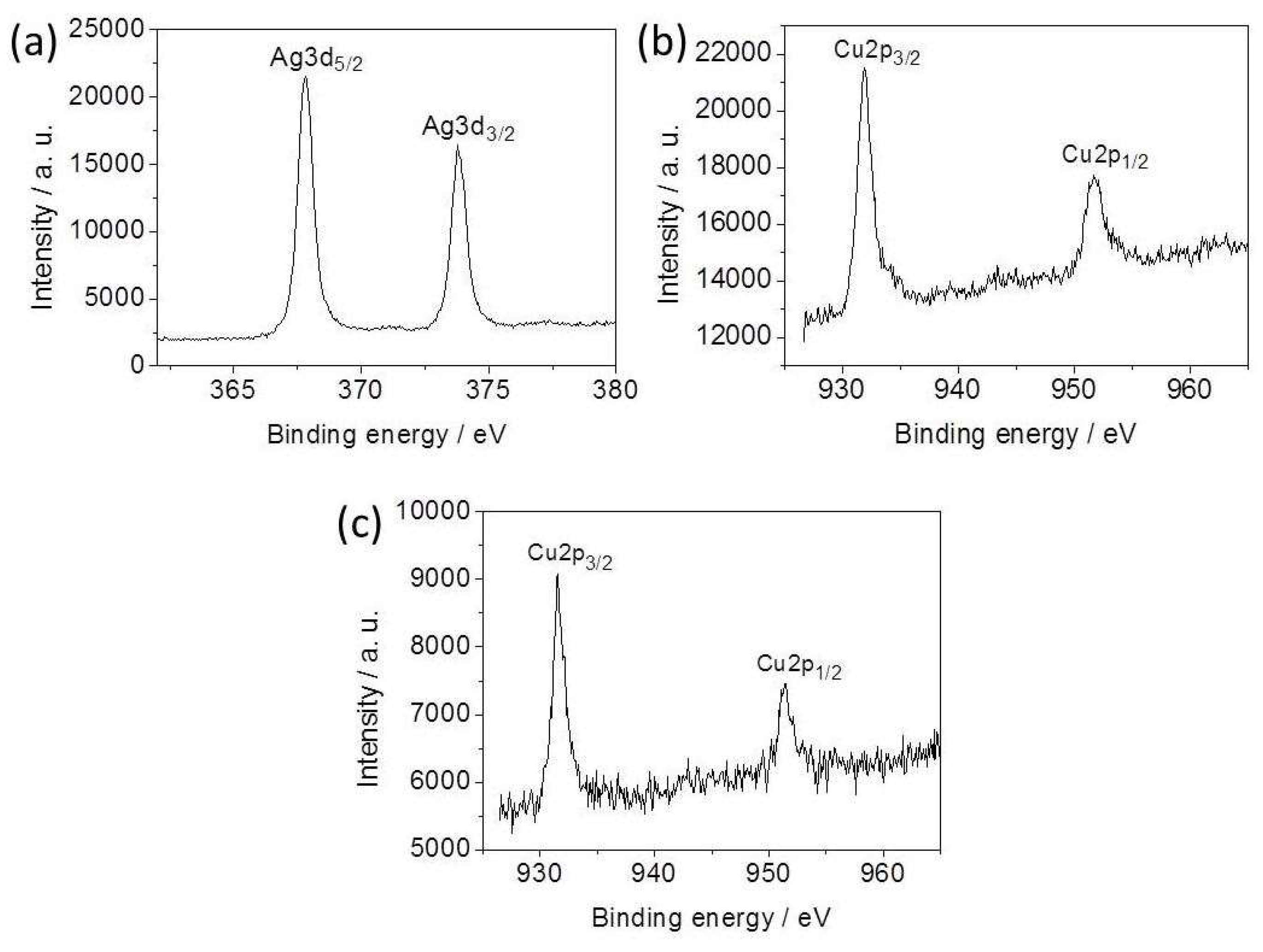
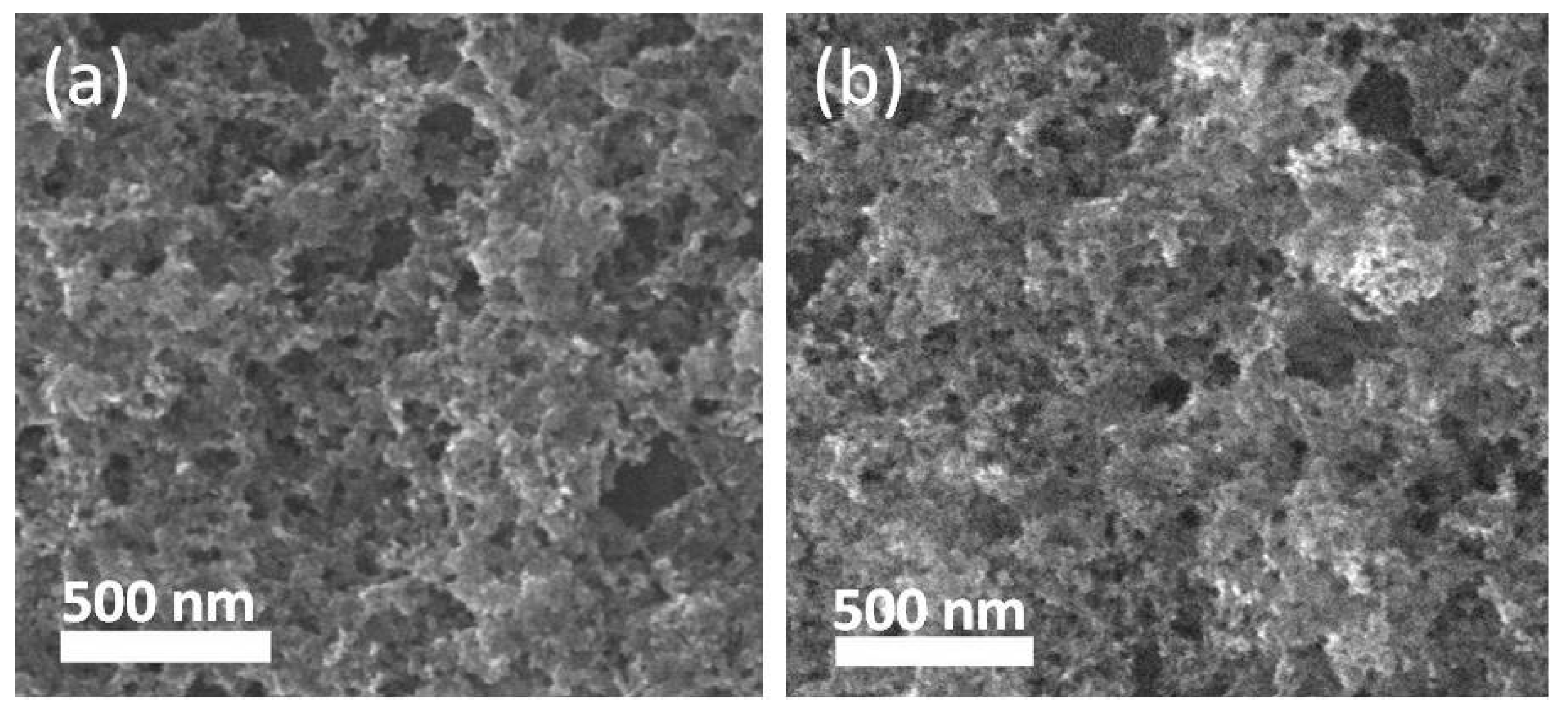
3.1. Morphology and XPS Analysis of the Ag-Cu2O Nanocomposite Films
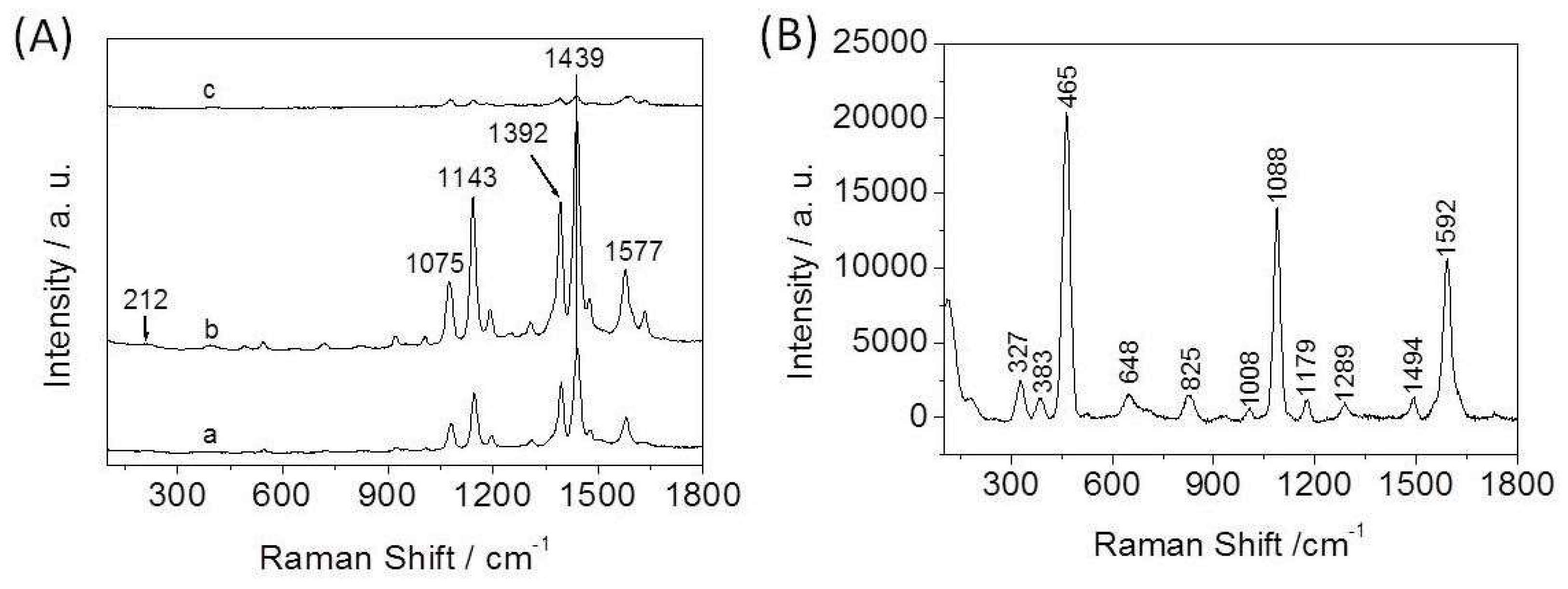
3.2. SERS Spectra of 4-ATP on the Ag-Cu2O Nanocomposite Films
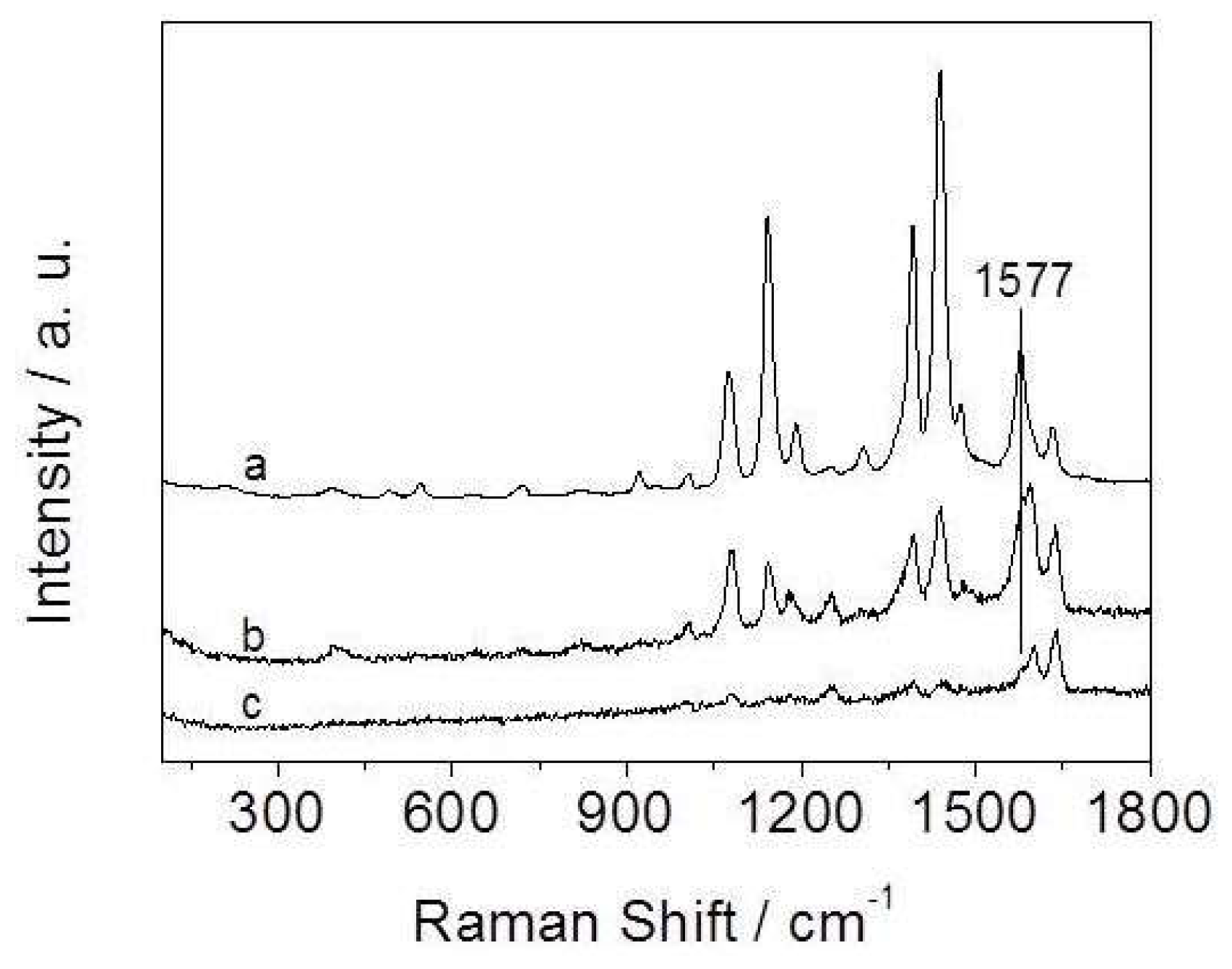
3.2.1. Influence of the Amount of CA in the Reaction System on SERS Activity
3.2.2. Influence of the Molar Ratio of Ag to Cu on SERS Activity
3.3. Electrocatalysis of the Nanocomposite-Films/GCE towards H2O2
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ray, C.; Pal, T. Recent advances of metal-metal oxide nanocomposites and their tailored nanostructures in numerous catalytic applications. J. Mater. Chem. A 2017, 5, 9465–9487. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Jang, Y.H.; Kim, D.H. A study on the mechanism for the interaction of light with noble metal-metal oxide semiconductor nanostructures for various photophysical applications. Chem. Soc. Rev. 2013, 42, 8467–8493. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.W.; Sun, W.; Wei, G.; Su, Z.Q. Cuprous oxide microspheres on graphene nanosheets: An enhanced material for non-enzymatic electrochemical detection of H2O2 and glucose. RSC Adv. 2015, 5, 35338–35345. [Google Scholar] [CrossRef]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Catalysis with TiO2/gold nanocomposites. Effect of metal particle size on the Fermi level equilibration. J. Am. Chem. Soc. 2004, 126, 4943–4950. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.T.; Choi, S.J.; Kim, S.J.; Jang, J.S.; Tuller, H.L.; Kim, I.D. Heterogeneous sensitization of metal-organic framework driven metal@metal oxide complex catalysts on an oxide nanofiber scaffold toward superior gas sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.Y.; Hirata, A.; Fujita, T.; Chen, M.W. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nat. Nanotechnol. 2011, 6, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Jiang, S.P.; Wang, X. Microwave-assisted one-pot synthesis of metal/metal oxide nanoparticles on graphene and their electrochemical applications. Electrochim. Acta 2011, 56, 3338–3344. [Google Scholar] [CrossRef]
- Xiao, F.; Li, Y.Q.; Zan, X.L.; Liao, K.; Xu, R.; Duan, H.W. Growth of metal-metal oxide nanostructures on freestanding graphene paper for flexible biosensors. Adv. Funct. Mater. 2012, 22, 2487–2494. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Maliyekkal, S.M.; Lisha, K.P.; Pradeep, T. Reduced graphene oxide-metal/metal oxide composites: Facile synthesis and application in water purification. J. Hazard. Mater. 2011, 186, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Zhang, Y.; Liu, M.; Wang, Y.; Qu, X.; Liu, Y.; Li, J.; Liu, X.; Yang, J. Design of Cu2O-Au composite microstructures for surface-enhanced Raman scattering study. Coll. Surf. A Physicochem. Eng. Asp. 2016, 507, 96–102. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Chen, D.; Li, C.Y.; Cui, P.L.; Yang, J. Noble metal-based composite nanomaterials fabricated via solution-based approaches. J. Mater. Chem. A 2015, 3, 3182–3223. [Google Scholar] [CrossRef]
- Sang, L.X.; Zhao, Y.X.; Burda, C. TiO2 nanoparticles as functional building blocks. Chem. Rev. 2014, 114, 9283–9318. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.J.; Zhang, P.P.; Ning, J.; Li, J.F.; Su, Z.Q.; Wei, G. A facile fabrication of large-scale reduced graphene oxide-silver nanoparticle hybrid film as a highly active surface-enhanced Raman scattering substrate. J. Mater. Chem. C 2015, 3, 4126–4133. [Google Scholar] [CrossRef]
- Wei, G.; Wang, L.; Liu, Z.G.; Song, Y.H.; Sun, L.L.; Yang, T.; Li, Z. DNA-network-templated self-assembly of silver nanoparticles and their application in surface-enhanced Raman scattering. J. Phys. Chem. B 2005, 109, 23941–23947. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Chen, L.; Schmitz, M.; Bao, F.S.; Zhu, J.H. Hierarchical macrotube/mesopore carbon decorated with mono-dispersed Ag nanoparticles as a highly active catalyst. Green Chem. 2015, 17, 2515–2523. [Google Scholar] [CrossRef]
- Kandula, S.; Jeevanandam, P. Synthesis of Cu2O@Ag polyhedral core-shell nanoparticles by a thermal decomposition approach for catalytic applications. Eur. J. Inorg. Chem. 2016, 2016, 1548–1557. [Google Scholar] [CrossRef]
- Sun, S.D. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 2015, 7, 10850–10882. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Amini, M.; Anbari, A.P. Preparation and characterization of TiO2-nanotube/Ti plates loaded Cu2O nanoparticles as a novel heterogeneous catalyst for the azide-alkyne cycloaddition. Catal. Commun. 2016, 76, 72–75. [Google Scholar] [CrossRef]
- Wang, G.; van den Berg, R.; Donega, C.D.; de Jong, K.P.; de Jongh, P.E. Silica-supported Cu2O nanoparticles with tunable size for sustainable hydrogen generation. Appl. Catal. B Environ. 2016, 192, 199–207. [Google Scholar] [CrossRef]
- Xu, Z.H.; Ye, S.J.; Fan, Z.; Ren, F.H.; Gao, C.J.; Li, Q.B.; Li, G.Q.; Zhang, G.L. Preparation of Cu2O nanowire-blended polysulfone ultrafiltration membrane with improved stability and antimicrobial activity. J. Nanopart. Res. 2015, 17, 409. [Google Scholar] [CrossRef]
- Qi, C.C.; Zheng, J.B. Novel nonenzymatic hydrogen peroxide sensor based on Ag/Cu2O nanocomposites. Electroanalysis 2016, 28, 477–483. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.; Zhao, Y.; Kou, Q.; Wang, Y.; Liu, Y.; Zhang, Y.; Yang, J.; Jung, Y.M. Enhanced catalyst activity by decorating of Au on Ag@Cu2O nanoshell. Appl. Surf. Sci. 2018, 435, 72–78. [Google Scholar] [CrossRef]
- Yang, S.Y.; Zhang, S.S.; Wang, H.J.; Yu, H.; Fang, Y.P.; Peng, F. Controlled preparation of Ag-Cu2O nanocorncobs and their enhanced photocatalytic activity under visible light. Mater. Res. Bull. 2015, 70, 296–302. [Google Scholar] [CrossRef]
- Xu, Z.H.; Ye, S.J.; Zhang, G.L.; Li, W.B.; Gao, C.J.; Shen, C.; Meng, Q. Antimicrobial polysulfone blended ultrafiltration membranes prepared with Ag/Cu2O hybrid nanowires. J. Membr. Sci. 2016, 509, 83–93. [Google Scholar] [CrossRef]
- Ren, S.T.; Zhao, G.L.; Wang, Y.Y.; Wang, B.Y.; Wang, Q. Enhanced photocatalytic performance of sandwiched ZnO@Ag@Cu2O nanorod films: The distinct role of Ag NPs in the visible light and UV region. Nanotechnology 2015, 26, 125403. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Sun, L.; Yan, Y.; Zhu, Z.F. One-step in-situ fabrication of silver-modified Cu2O crystals with enhanced visible photocatalytic activity. Micro Nano Lett. 2016, 11, 363–365. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.Y.; Song, X.Y.; Yin, Z.L.; Bu, Y.X. Construction of reduced graphene oxide-supported Ag-Cu2O composites with hierarchical structures for enhanced photocatalytic activities and recyclability. J. Mater. Chem. A 2015, 3, 5923–5933. [Google Scholar] [CrossRef]
- Pan, Q.; Wang, M.; Wang, H.; Zhao, J.; Yin, G. Novel method to deposit metal particles on transition metal oxide films and its application in lithium-ion batteries. Electrochim. Acta 2008, 54, 197–202. [Google Scholar] [CrossRef]
- Wei, S.; Shi, J.; Ren, H.; Li, J.; Shao, Z. Fabrication of Ag/Cu2O composite films with a facile method and their photocatalytic activity. J. Mol. Catal. A 2013, 378, 109–114. [Google Scholar] [CrossRef]
- Fu, S.Y.; Hsu, Y.K.; Chen, M.H.; Chuang, C.J.; Chen, Y.C.; Lin, Y.G. Silver-decorated hierarchical cuprous oxide micro/nanospheres as highly effective surfaceenhanced Raman scattering substrates. Opt. Express 2014, 22, 14617–14624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Sun, Y.J.; Che, G.B.; Li, Z. Self-assembled silver nanoparticle films at an air-liquid interface and their applications in SERS and electrochemistry. Appl. Surf. Sci. 2011, 257, 7150–7155. [Google Scholar] [CrossRef]
- Li, S.K.; Shen, Y.H.; Xie, A.J.; Yu, X.R.; Qiu, L.G.; Zhang, L.; Zhang, Q.F. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Wang, L.; Ni, P.J.; Wei, G.; Wang, J.; Li, Z. Collagen nanofiber-templated silver nanowires on graphene nanosheets for a nonenzymatic amperometric biosensor of hydrogen peroxide. Chem. Lett. 2014, 43, 544–546. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, F.; Deng, X.; Xue, X.; Wang, L.; Sun, Y.; Feng, J.; Zhang, Y.; Wang, Y.; Jung, Y.M. SERS study of surface plasmon resonance induced carrier movement in Au@Cu2O core-shell nanoparticles. Spectrochim. Acta Part A 2018, 189, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, H.H.; Zhao, Y.; Zhang, Y.J.; Wang, Y.X.; Liu, Y.; Zhang, X.L.; Jiang, Y.H.; Hua, Z.; Yang, J.H. Plasmonic-induced SERS enhancement of shell-dependent Ag@Cu2O core-shell nanoparticles. RSC Adv. 2017, 7, 16553–16560. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wang, L.; Sun, L.L.; Guo, C.L.; Yang, T.; Liu, Z.L.; Xu, F.G.; Li, Z. Fabrication, characterization, and application in surface-enhanced Raman spectrum of assembled type-I collagen-silver nanoparticle multilayered films. J. Chem. Phys. 2008, 128, 074704. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.G.; Wang, T.; Wang, L.; Dong, S.J. Surface-enhanced Raman scattering of 4-aminothiophenol self-assembled monolayers in sandwich structure with nanoparticle shape dependence: Off-surface plasmon resonance condition. J. Phys. Chem. C 2007, 111, 6962–6969. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wei, G.; Song, Y.H.; Wang, L.; Sun, L.L.; Guo, C.L.; Yang, T.; Li, Z. Type I collagen-templated assembly of silver nanoparticles and their application in surface-enhanced Raman scattering. Nanotechnology 2008, 19, 115604. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hu, X.G.; Dong, S.J. Surfactantless synthesis of multiple shapes of gold nanostructures and their shape-dependent SERS spectroscopy. J. Phys. Chem. B 2006, 110, 16930–16936. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.B.; Ruan, W.D.; Jiang, X.; Zhao, B.; Xu, W.Q.; Lombardi, J.R. Contribution of ZnO to charge-transfer induced surface-enhanced Raman scattering in Au/ZnO/pATP assembly. J. Phys. Chem. C 2009, 113, 117–120. [Google Scholar] [CrossRef]
- Zhou, Q.; Fan, Q.; Zhuang, Y.; Li, Y.; Zhao, G.; Zheng, J.W. Effect of substrate on surface-enhanced Raman scattering of molecules adsorbed on immobilized silver nanoparticles. J. Phys. Chem. B 2006, 110, 12029–12033. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Han, X.X.; Zhao, B. Charge-Transfer-Induced Enhancement of Raman Scattering Based on Semiconductors. In Recent Developments in Plasmon-Supported Raman Spectroscopy; Kneipp, K., Ozaki, Y., Tian, Z., Eds.; World Scientific Publishing Europe Ltd.: Hackensack, NJ, USA, 2017; pp. 451–482. [Google Scholar]
- Leopold, N.; Lendl, B. A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Lu, L.H.; Eychmuller, A.; Kobayashi, A.; Hirano, Y.; Yoshida, K.; Kikkawa, Y.; Tawa, K.; Ozaki, Y. Designed fabrication of ordered porous Au/Ag nanostructured films for surface-enhanced Raman scattering substrates. Langmuir 2006, 22, 2605–2609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Hu, H.L.; Jing, S.Y.; Wang, Y.X.; Sun, Z.H.; Zhao, B.; Zhao, C.; Lombardi, J.R. Enhanced Raman scattering as a probe for 4-mercaptopyridine surface-modified copper oxide nanocrystals. Anal. Sci. 2007, 23, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, X.W.; Fan, Q.; Zhang, X.X.; Zheng, J.W. Charge transfer between metal nanoparticles interconnected with a functionalized molecule probed by surface-enhanced Raman spectroscopy. Angew. Chem. Int. Ed. 2006, 118, 4074–4077. [Google Scholar] [CrossRef]
- Hsieh, S.C.; Lin, P.Y.; Chu, L.Y. Improved performance of solution-phase surface-enhanced Raman scattering at Ag/CuO anocomposite surfaces. J. Phys. Chem. C 2014, 118, 12500–12505. [Google Scholar] [CrossRef]
- Rai, P.; Khan, R.; Raj, S.; Majhi, S.M.; Park, K.K.; Yu, Y.T.; Lee, I.H.; Sekhar, P.K. Au@Cu2O core-shell nanoparticles as chemiresistors for gas sensor applications: Effect of potential barrier modulation on the sensing performance. Nanoscale 2014, 6, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.B.; Jiang, X.; Ruan, W.D.; Yang, J.X.; Zhao, B.; Xu, W.Q.; Lombardi, J.R. Charge-transfer-induced surface-enhanced Raman scattering on Ag-TiO2 nanocomposites. J. Phys. Chem. C 2009, 113, 16226–16231. [Google Scholar] [CrossRef]
- Yang, L.H.; Lv, J.; Sui, Y.M.; Fu, W.Y.; Zhou, X.M.; Ma, J.W.; Su, S.; Zhang, W.J.; Lv, P.; Wu, D.; et al. Fabrication of Cu2O/Ag composite nanoframes as surface-enhanced Raman scattering substrates in a successive one-pot procedure. CrystEngComm 2014, 16, 2298–2304. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Qi, H.; Chen, L.; Sun, Y.; Li, Z. Self-Assembled Ag-Cu2O Nanocomposite Films at Air-Liquid Interfaces for Surface-Enhanced Raman Scattering and Electrochemical Detection of H2O2. Nanomaterials 2018, 8, 332. https://doi.org/10.3390/nano8050332
Wang L, Qi H, Chen L, Sun Y, Li Z. Self-Assembled Ag-Cu2O Nanocomposite Films at Air-Liquid Interfaces for Surface-Enhanced Raman Scattering and Electrochemical Detection of H2O2. Nanomaterials. 2018; 8(5):332. https://doi.org/10.3390/nano8050332
Chicago/Turabian StyleWang, Li, Huan Qi, Lei Chen, Yantao Sun, and Zhuang Li. 2018. "Self-Assembled Ag-Cu2O Nanocomposite Films at Air-Liquid Interfaces for Surface-Enhanced Raman Scattering and Electrochemical Detection of H2O2" Nanomaterials 8, no. 5: 332. https://doi.org/10.3390/nano8050332
APA StyleWang, L., Qi, H., Chen, L., Sun, Y., & Li, Z. (2018). Self-Assembled Ag-Cu2O Nanocomposite Films at Air-Liquid Interfaces for Surface-Enhanced Raman Scattering and Electrochemical Detection of H2O2. Nanomaterials, 8(5), 332. https://doi.org/10.3390/nano8050332




