Synthesis, Self-Assembly, and Drug-Release Properties of New Amphipathic Liquid Crystal Polycarbonates
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Measurements
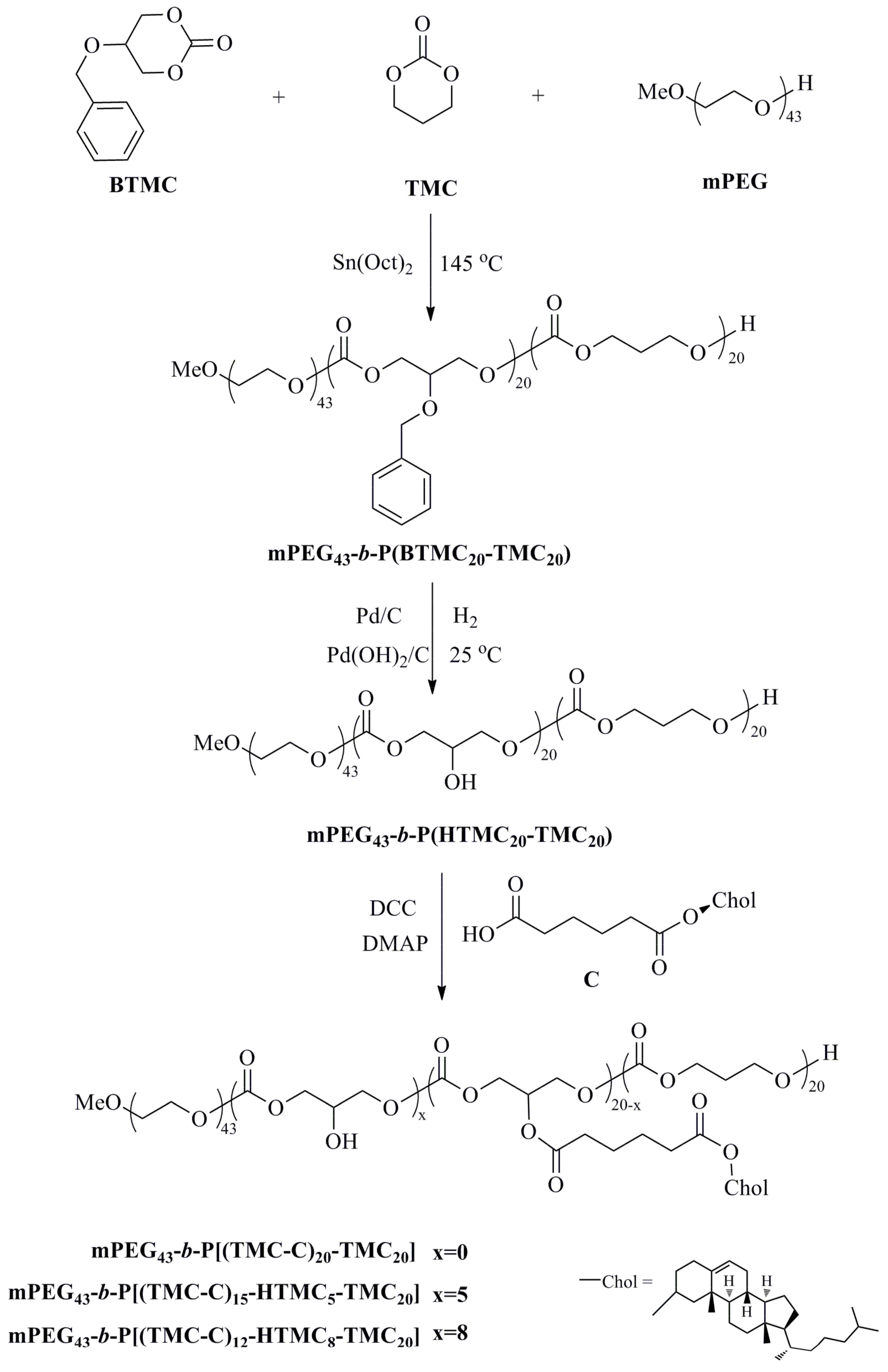
2.3. Synthesis of the Block Copolymers
2.3.1. Synthesis of mPEG43-b-P(BTMC20-TMC20)
2.3.2. Synthesis of mPEG43-b-P(HTMC20-TMC20)
2.3.3. Synthesis of mPEG43-b-P[(TMC-C)20−x-HTMCx-TMC20]
2.4. Nanoprecipitation of Copolymers
2.5. Preparation of DOX-Loaded Micelles
2.6. In Vitro Drug Release
3. Results and Discussion
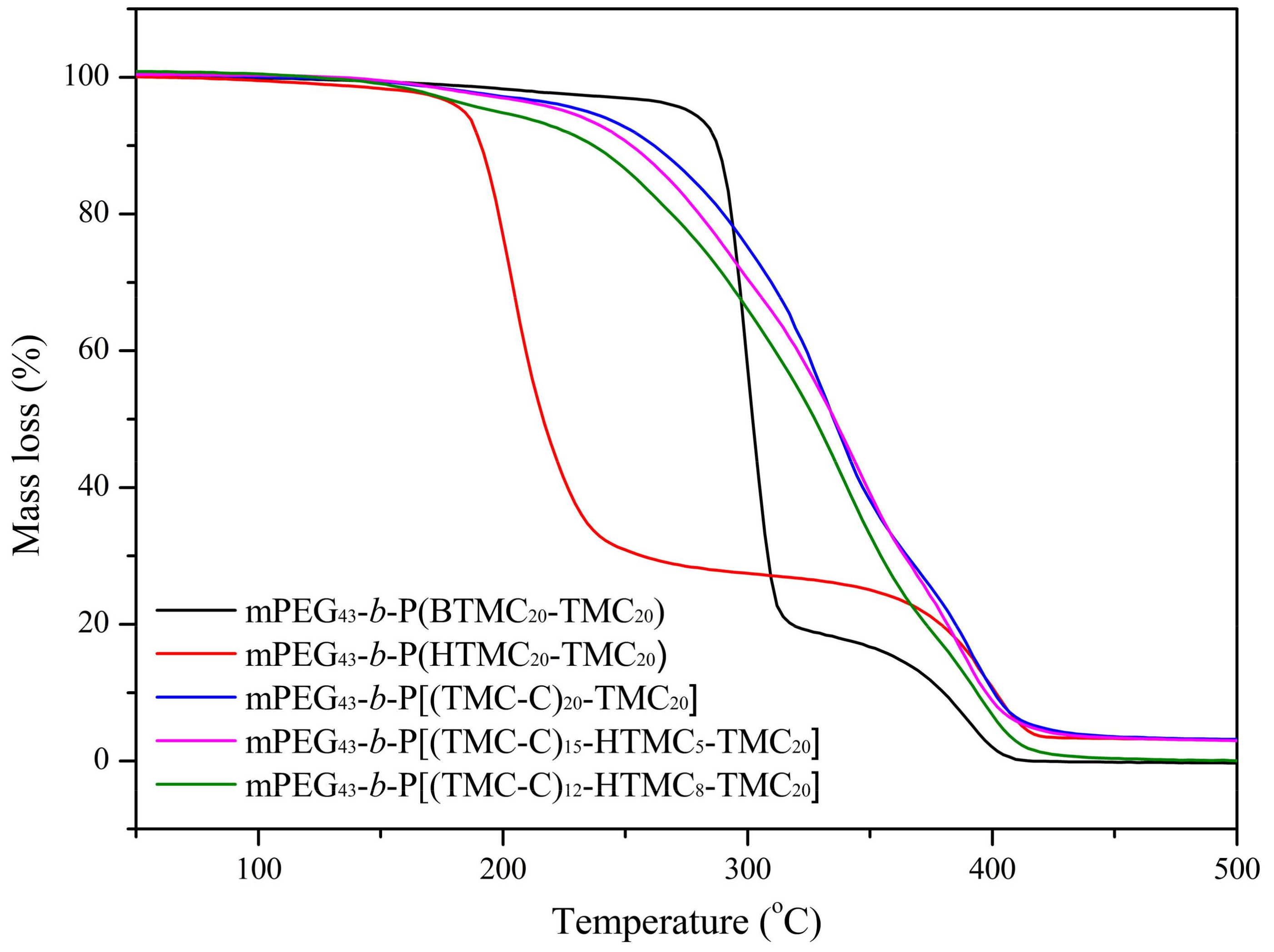
3.1. Thermal Stability
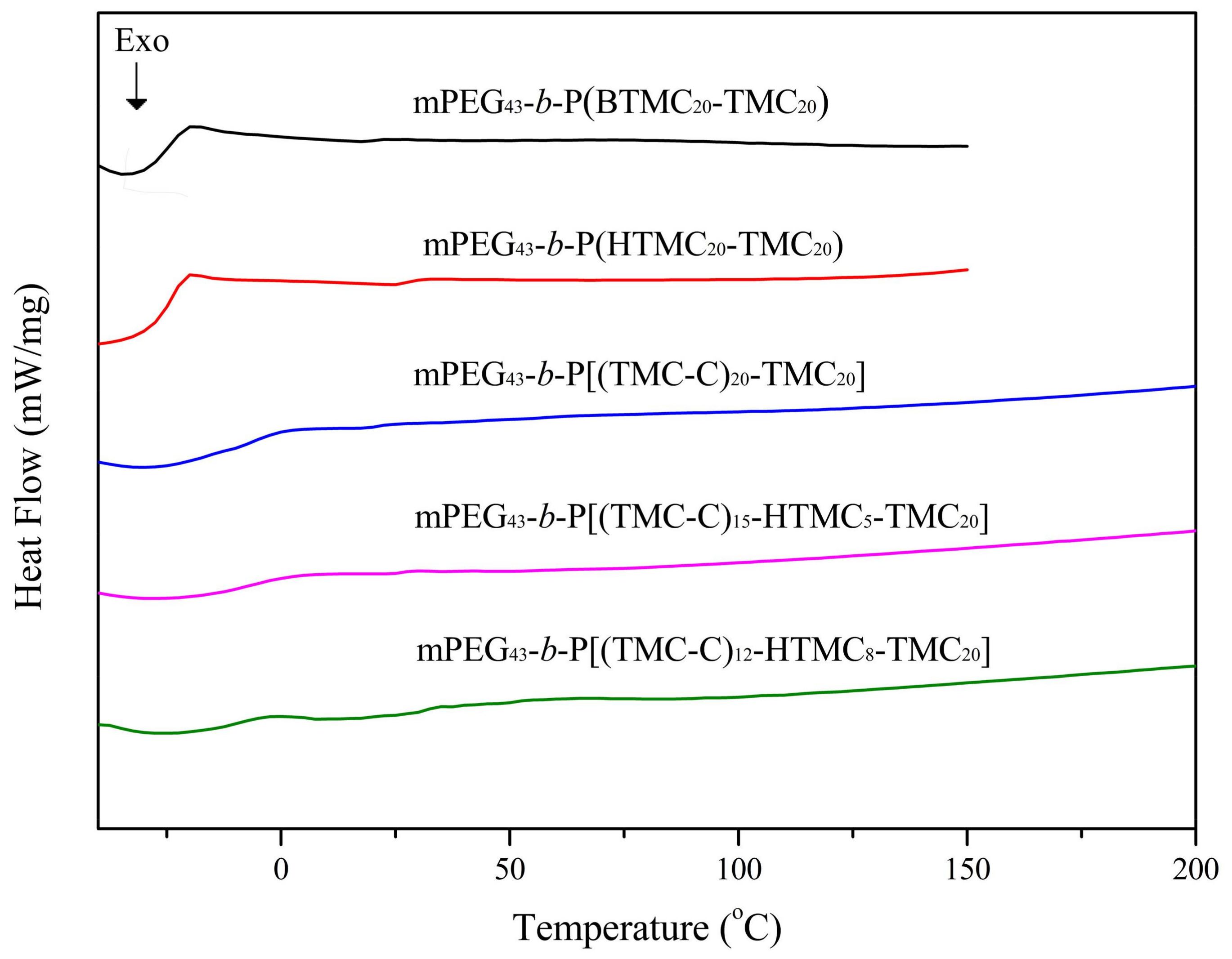
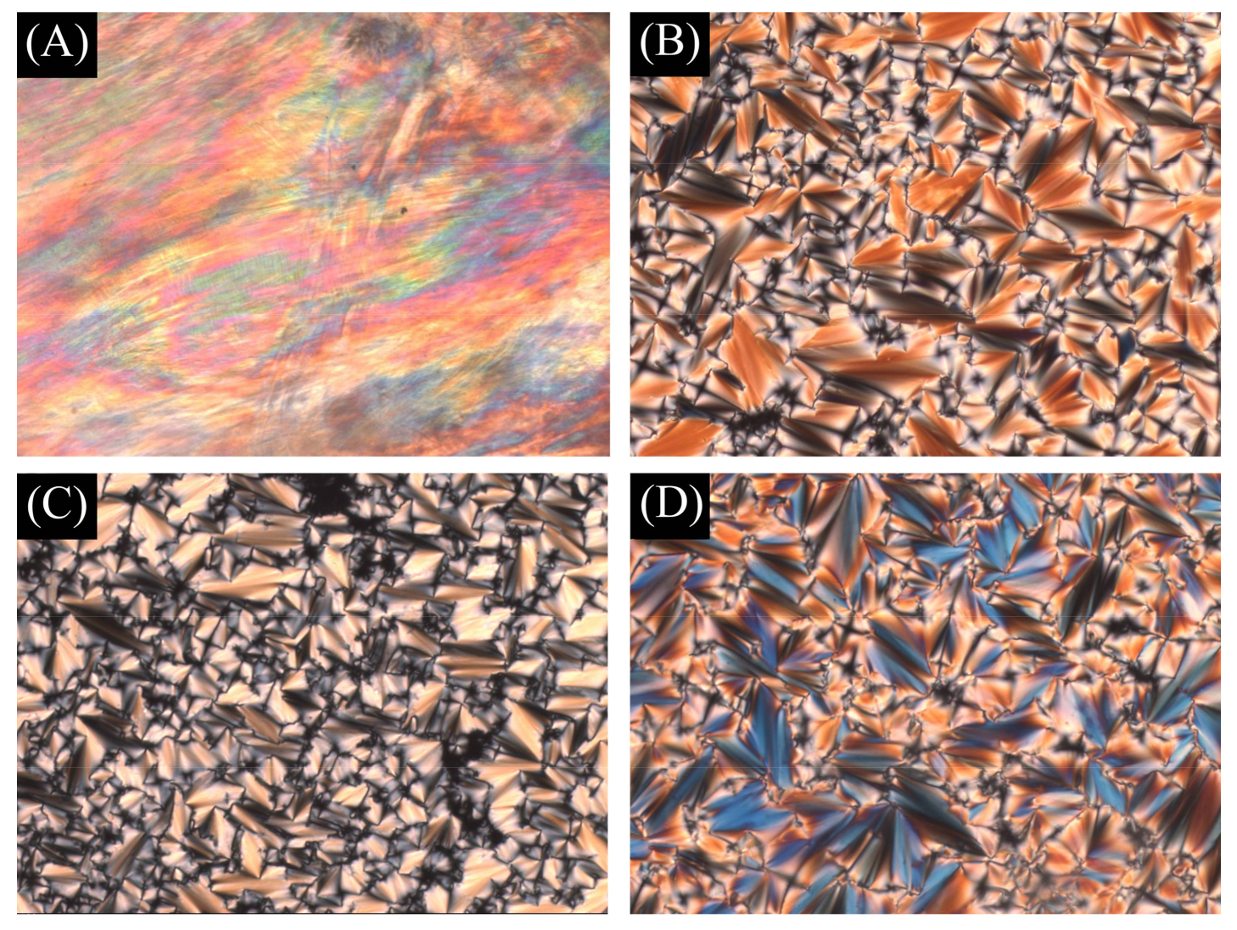
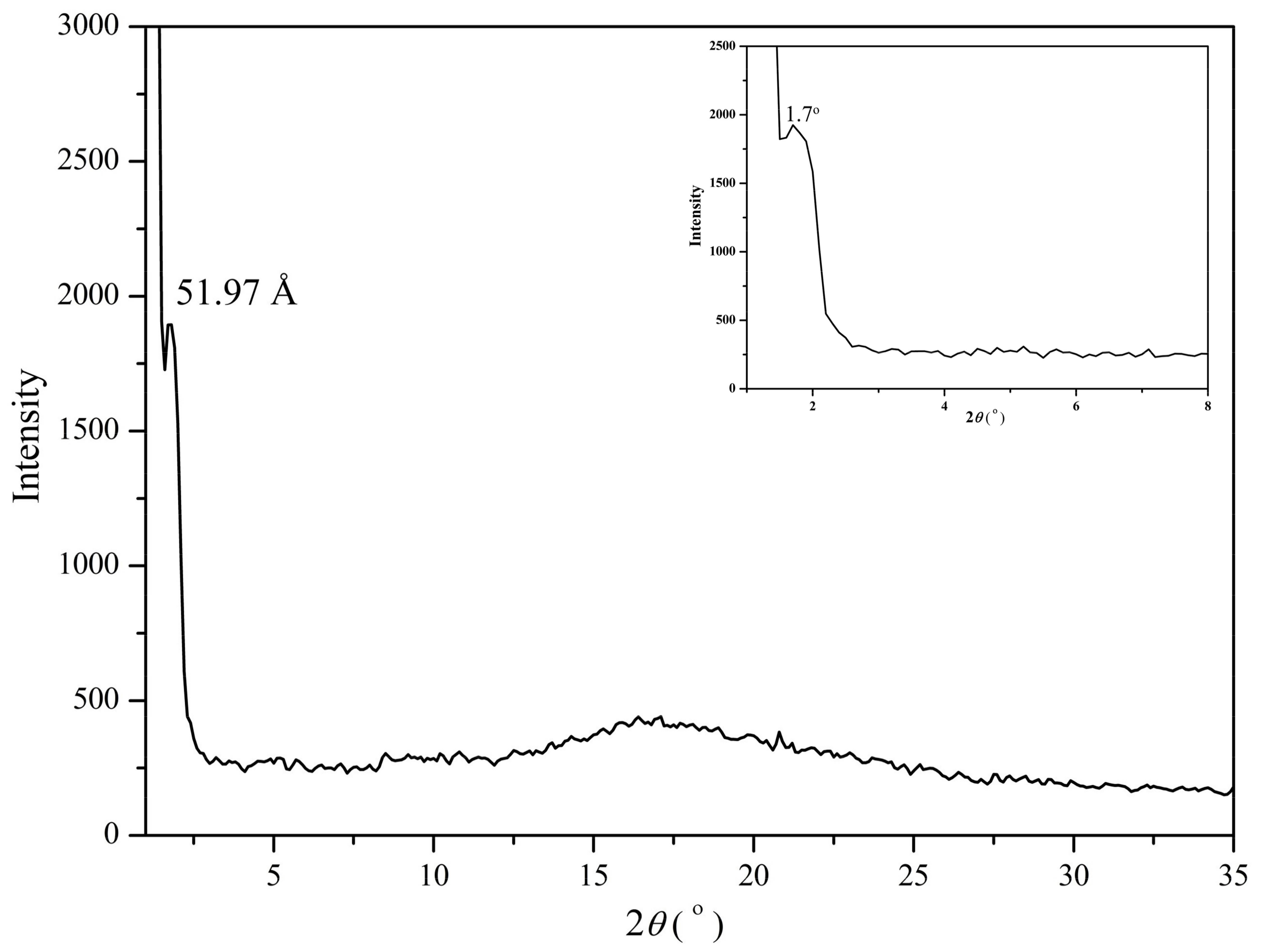
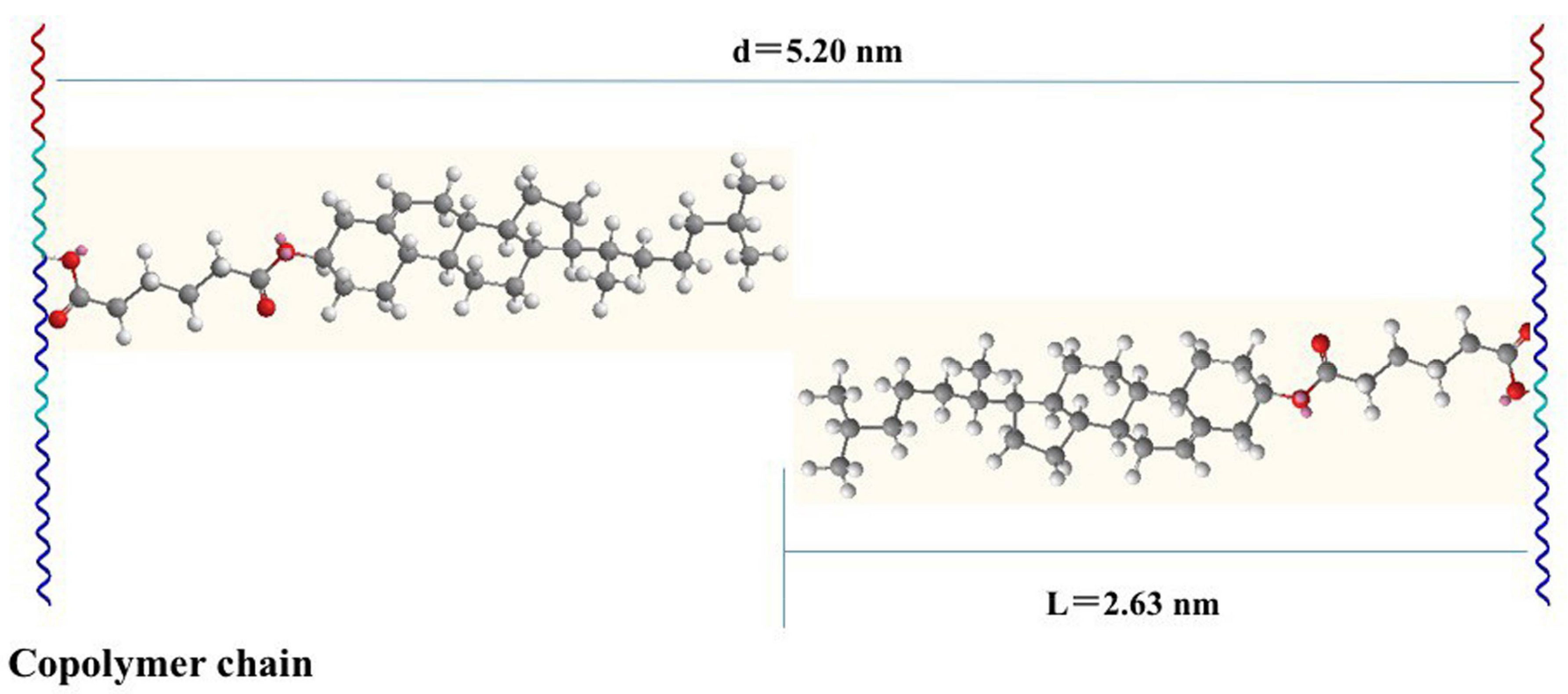
3.2. Liquid Crystal Behavior
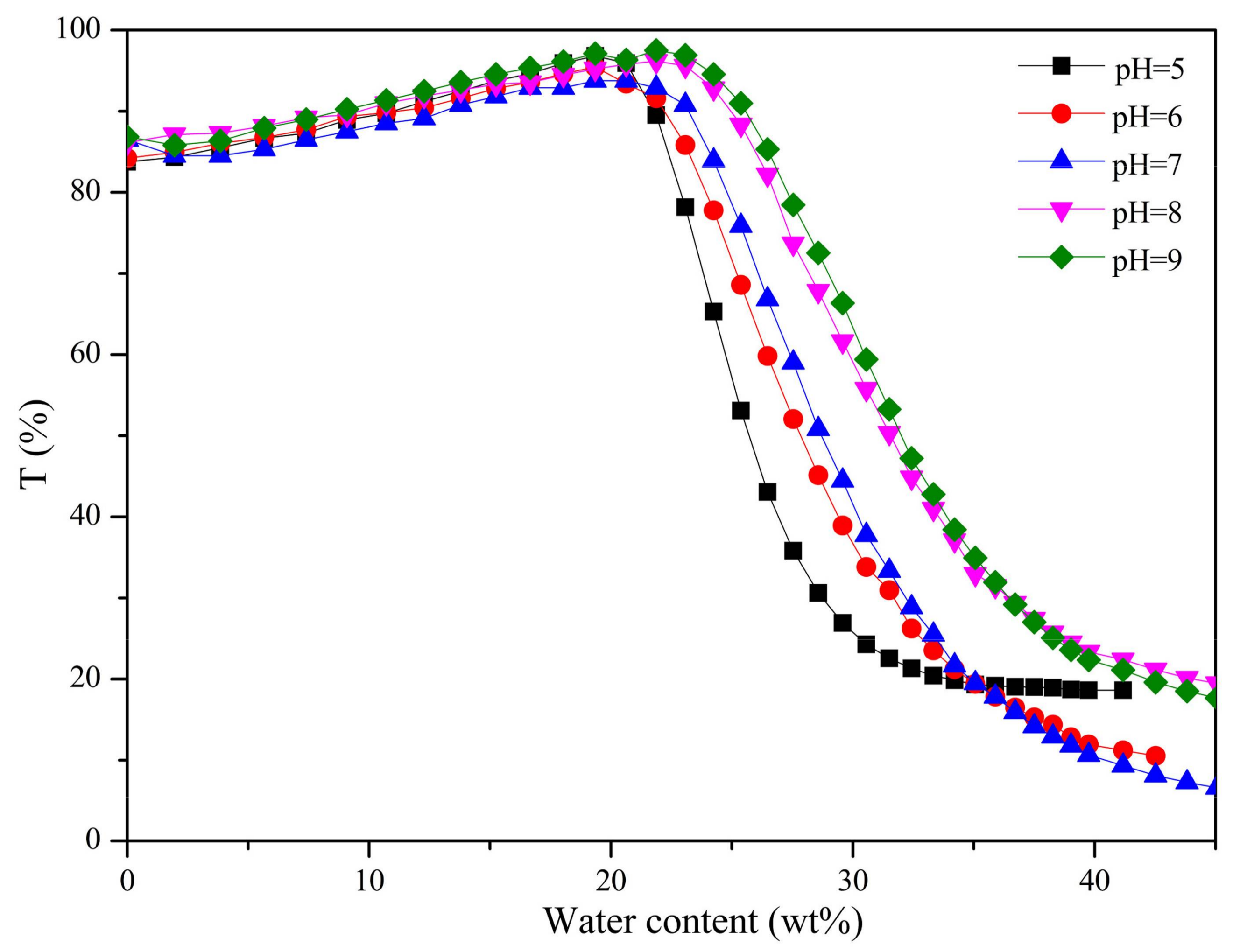
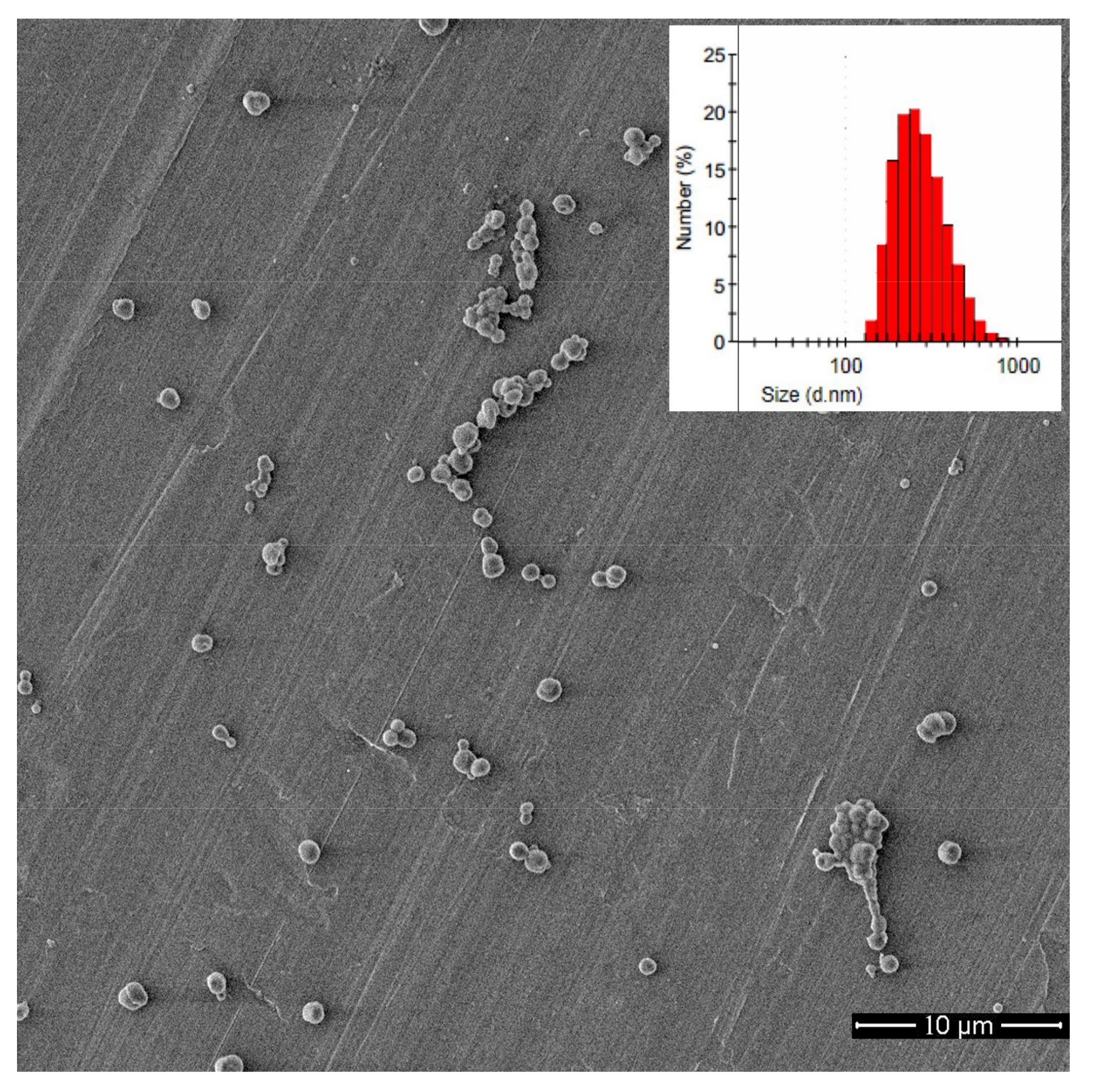
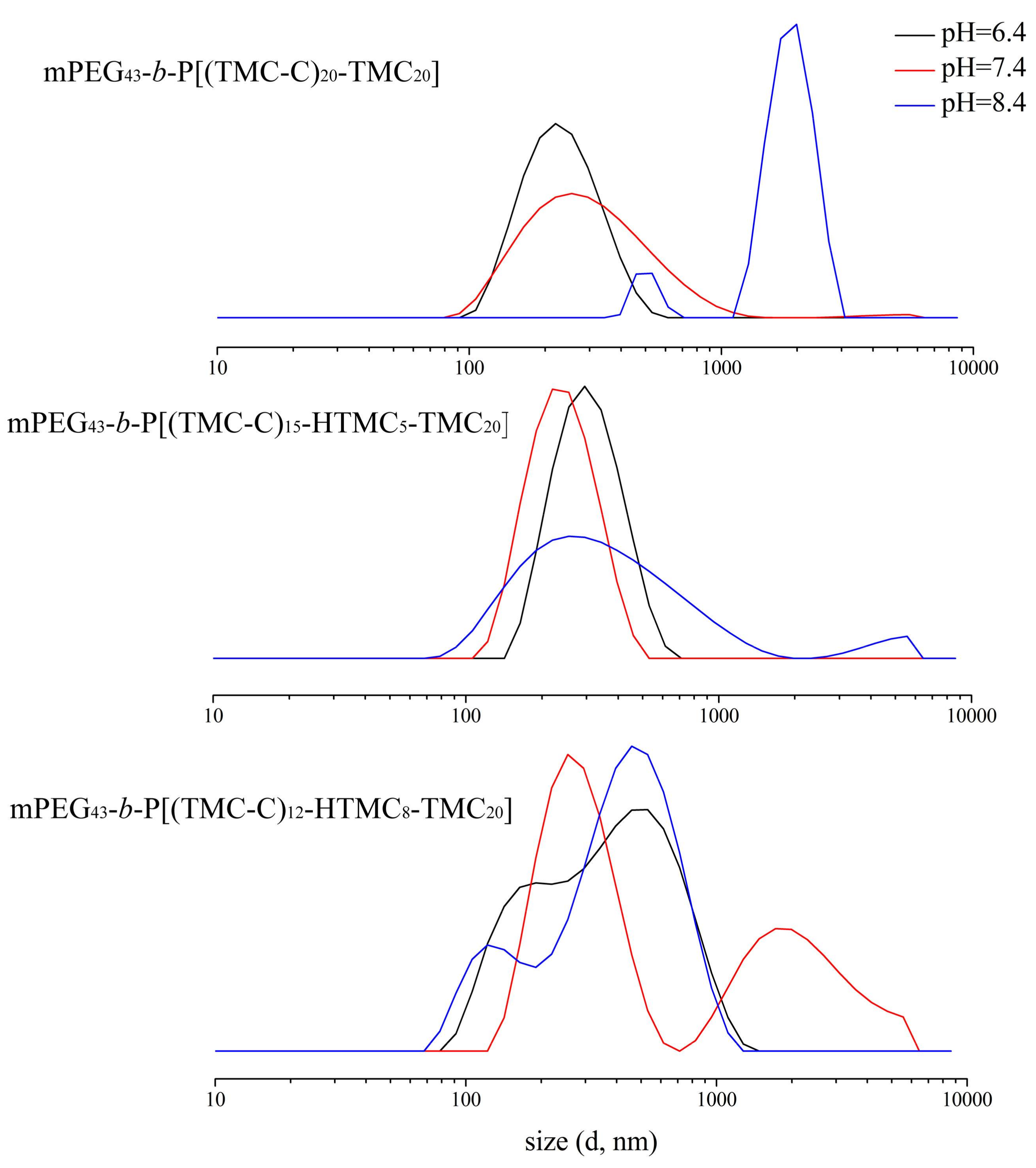
3.3. Self-Assembly Behavior
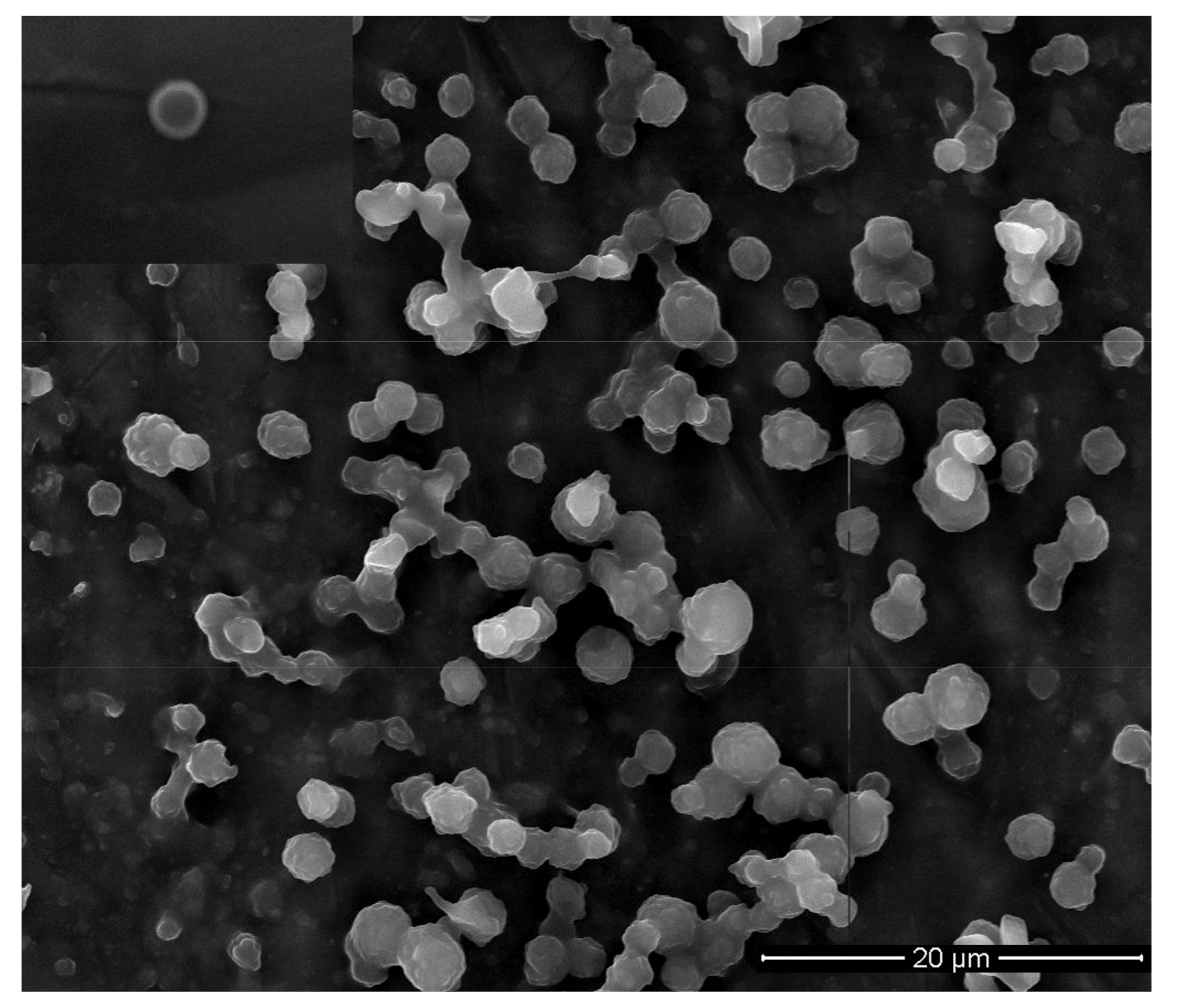
3.4. DOX-Loaded Micelles
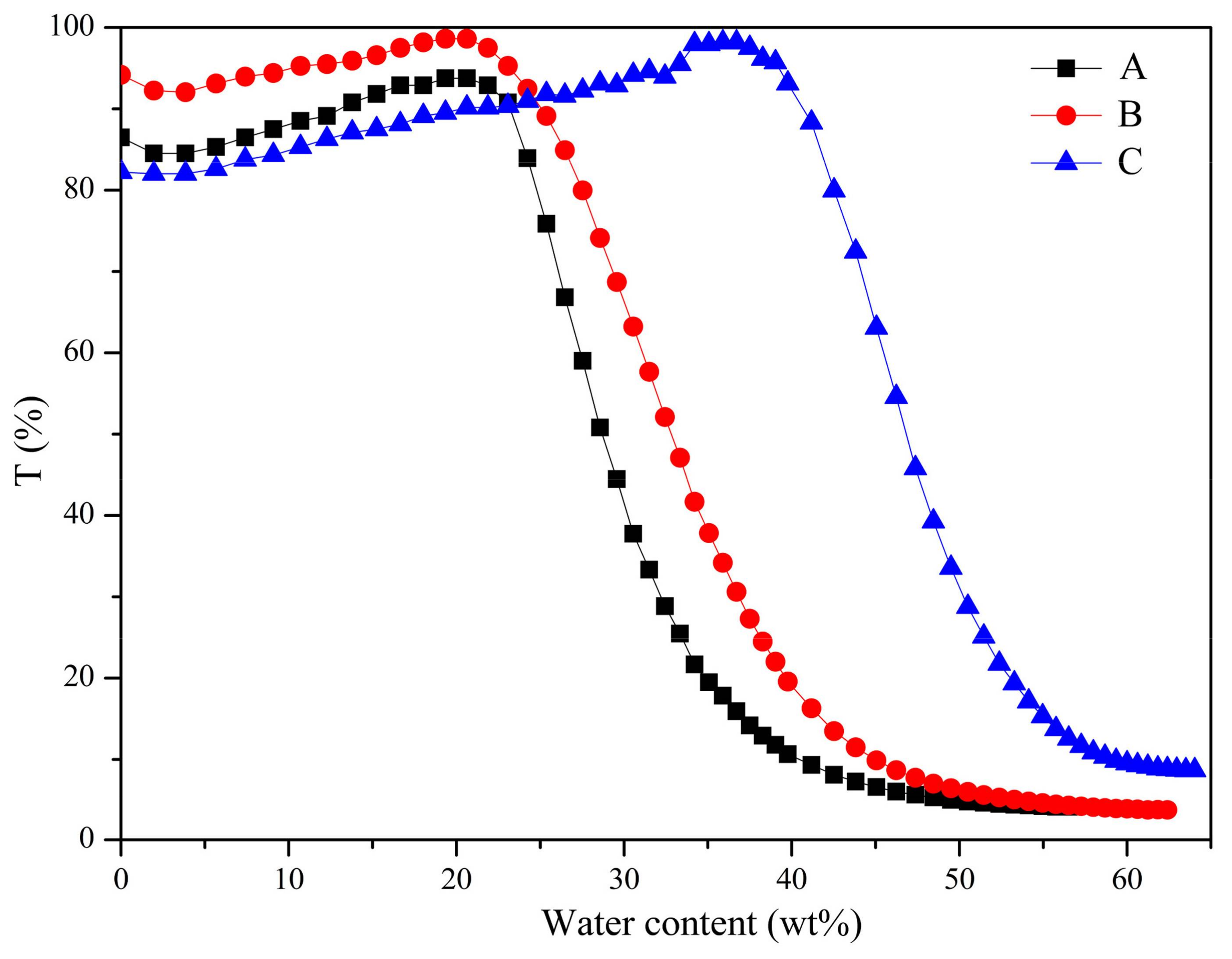
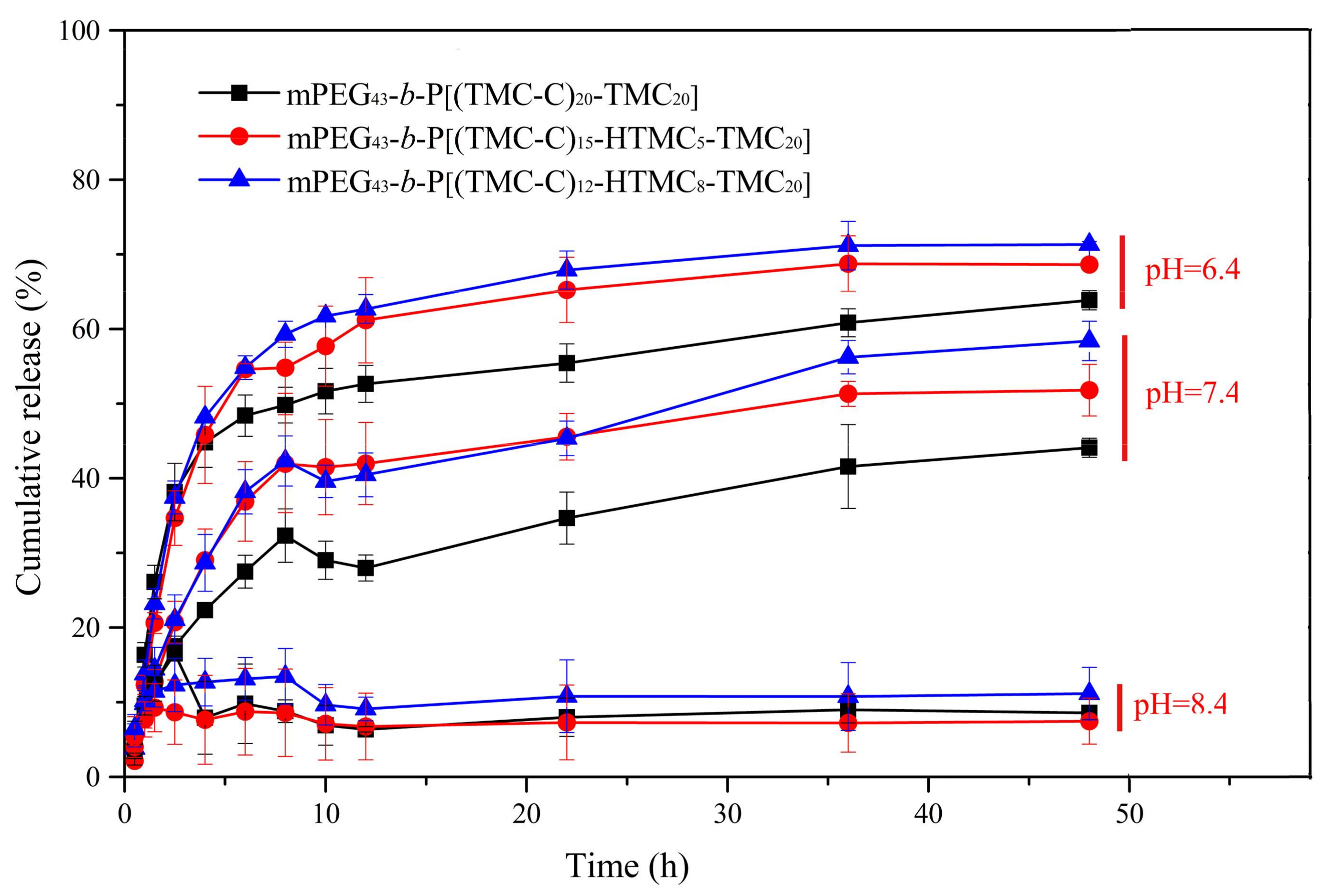
3.5. In Vitro DOX Release from the Micelles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dicastillo, L.C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.L.; Galotto, M.J. Improvement of polylactide properties through cellulose nanocrystals embedded in poly(vinyl alcohol) electrospun nanofibers. Nanomaterials 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Wang, Y.; Chen, Y.J.; Jin, Q.; Ji, J. A biomimic pH-sensitive polymeric prodrug based on polycarbonate for intracellular drug delivery. Polym. Chem. 2014, 5, 854–861. [Google Scholar] [CrossRef]
- Ishii, D.; Ying, T.H.; Mahara, A.; Murakami, S.; Yamaoka, T. In vivo tissue response and degradation behavior of PLLA and stereocomplexed PLA nanofibers. Biomacromolecules 2009, 10, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Li, P.W.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L.X. Manufacturing techniques and surface engineering of polymer based nanoparticles for targeted drug delivery to cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, A.; Miroshnichenko, S.; Kovalskii, A.; Permyakova, E.; Popov, Z.; Dvorakova, E.; Kiryukhantsev-Korneev, P.; Obrosov, A.; Polcak, J.; Zajickova, L.; et al. Immobilization of platelet-rich plasma onto COOH plasma-coated PCL nanofibers boost viability and proliferation of human mesenchymal stem cells. Polymers 2017, 9, 736. [Google Scholar] [CrossRef]
- Liu, X.F.; Xu, X.X.; Li, Q.; Hu, J.S.; Yang, L.Q.; Chen, Q.F.; Lu, Y.F. New side chain cholesterol-functionalised aliphatic polycarbonate copolymer: Synthesis and phase behaviour. Liq. Cryst. 2017, 44, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhuo, R.X.; Liu, L.J.; He, F.; Liu, G. Synthesis and characterization of novel aliphatic polycarbonates. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 70–75. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhuo, R.X.; Huang, S.W.; Liu, L.J.; He, F. Synthesis, characterization and in vitro cytotoxicity of poly[(5-benzyloxy-trimethylene carbonate)-co-(trimethylene carbonate)]. Macromol. Chem. Phys. 2002, 203, 985–990. [Google Scholar] [CrossRef]
- Zeng, F.Q.; Liu, J.B.; Allen, C. Synthesis and characterization of biodegradable poly(ethylene glycol)-block-poly(5-benzyloxy-trimethylene carbonate) copolymers for drug delivery. Biomacromolecules 2004, 5, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, B.A.; Wilmer, C.E.; Kim, J.; Browne, K.P.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. [Google Scholar] [CrossRef]
- Han, L.; Ma, H.W.; Zhu, S.Q.; Liu, P.B.; Shen, H.Y.; Yang, L.C.; Tan, R.; Huang, W.; Li, Y. Effect of topology and composition on liquid crystal order and self-assembly performances driven by asynchronously controlled grafting density. Macromolecules 2017, 50, 8334–8345. [Google Scholar] [CrossRef]
- Zhou, J.S.; Dong, Y.C.; Zhang, Y.Y.; Liu, D.S.; Yang, Z.Q. The assembly of DNA amphiphiles at liquid crystal-aqueous interface. Nanomaterials 2016, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Cano-Sarabia, M.; Angelova, A.; Ventosa, N.; Lesieur, S.; Veciana, J. Cholesterol induced CTAB micelle-to-vesicle phase transitions. J. Colloid Interface Sci. 2010, 350, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zerkoune, L.; Lesieur, S.; Putaux, J.L.; Choisnard, L.; Geze, A.; Wouessidjewe, D.; Angelov, B.; Vebert-Nardin, C.; Doutch, J.; Angelona, A. Mesoporous self-assembled nanoparticles of biotransesterified cyclodextrins and nonlamellar lipids as carriers of water-insoluble substances. Soft Matter 2016, 12, 7539–7550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Bazuin, C.G.; Freiberg, S.; Brisse, F.; Zhu, X.X. Effect of side chain structure on the liquid crystalline properties of polymers bearing cholesterol, dihydrocholesterol and bile acid pendant groups. Polymer 2005, 46, 7266–7272. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Briand, V.A.; Sharma, N.; Ahn, S.K.; Kasi, R.M. Polymers comprising cholesterol: Synthesis, self-assembly, and applications. Materials 2009, 2, 636–660. [Google Scholar] [CrossRef]
- Ahn, S.K.; Le, L.T.N.; Kasi, R.M. Synthesis and characterization of side-chain liquid crystalline polymers bearing cholesterol mesogen. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2690–2701. [Google Scholar] [CrossRef]
- Nagahama, K.; Ueda, Y.; Ouchi, T.; Ohya, Y. Exhibition of soft and tenacious characteristics based on liquid crystal formation by introduction of cholesterol groups on biodegradable lactide copolymer. Biomacromolecules 2007, 8, 3938–3943. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, Z.B.; Jiang, G.Q.; Liu, J.J.; Chen, X.F.; Li, Y.W.; Zhou, N.C.; Zhu, X.L. Self-assembly of amphiphilic macrocycles containing polymeric liquid crystal grafts in solution. Polym. Chem. 2016, 7, 2785–2789. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, D.P.; Hocine, S.; Pilone, A.; Trepout, S.; Marco, S.; Thomas, C.; Guo, J.; Li, M.H. Transition from smectic nanofibers to smectic vesicles in the self-assemblies of PEG-b-liquid crystal polycarbonates. Polym. Chem. 2017, 8, 4776–4780. [Google Scholar] [CrossRef]
- Venkataraman, S.; Lee, A.L.; Maune, H.T.; Hedrick, J.L.; Prabhu, V.M.; Yang, Y.Y. Formation of disk-and stacked-disk-like self-assembled morphologies from cholesterol-functionalized amphiphilic polycarbonate diblock copolymers. Macromolecules 2013, 46, 4839–4846. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, T.; Sheng, R.L.; Li, H.; Sun, J.J.; Cao, A.M. Amphiphilic diblock terpolymer PMAgala-b-P(MAA-co-MAChol)s with attached galactose and cholesterol grafts and Their intracellular pH-responsive doxorubicin delivery. Biomacromolecules 2015, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zou, T.; Cheng, S.X.; Zhuo, R.X. Synthesis and characterization of biodegradable cholesteryl end-capped polycarbonates. Biomacromolecules 2005, 6, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Cheng, S.X.; Zhuo, R.X. Synthesis and enzymatic degradation of end-functionalized biodegradable polyesters. Colloid Polym. Sci. 2005, 283, 1091–1099. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.R.; Jiang, X.S.; Jiang, X.S.; Cheng, S.X.; Zhuo, R.X. Studies on functionalization of poly (ε-caprolactone) by a cholesteryl moiety. J. Biomater. Sci. Polym. Ed. 2005, 16, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Li, F.; Cheng, S.X.; Zhuo, R.X. Synthesis and characterization of end-capped biodegradable oligo/poly(trimethylene carbonate)s. J. Biomater. Sci. Polym. Ed. 2006, 17, 1093–1106. [Google Scholar] [CrossRef]
- Chen, G.X.; Yang, L.Q.; Liu, X.F.; Xie, Y.J.; Guo, Z.H.; Li, M.; Guo, J.; Hu, J.S. Main-chain biodegradable liquid crystal derived from cholesteryl derivative end-capped poly(trimethylene carbonate): Synthesis and characterisation. Liq. Cryst. 2017, 44, 1050–1058. [Google Scholar] [CrossRef]
- Xu, X.X.; Liu, X.F.; Li, Q.; Hu, J.S.; Chen, Q.F.; Yang, L.Q.; Lu, Y.H. New amphiphilic polycarbonates with side functionalized cholesteryl groups as biomesogenic units: Synthesis, structure and liquid crystal behavior. RSC Adv. 2017, 7, 14176–14185. [Google Scholar] [CrossRef]
- Qi, Y.P.; Miao, Z.M.; Cheng, S.X.; Zhang, X.Z. Fabrication and drug release properties of poly(5-benzyloxy-trimethylene-co-glycolide) microspheres. J. Appl. Polym. Sci. 2010, 115, 3451–3455. [Google Scholar] [CrossRef]
- Han, J.Y.; Zhao, D.D.; Li, D.; Wang, X.H.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.F.; Li, Y.W.; Zou, A.H. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Garamus, V.M.; Drechsler, M.; Angelova, A. Structural analysis of nanoparticulate carriers for encapsulation of macromolecular drugs. J. Mol. Liq. 2017, 235, 83–89. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Mutafchieva, R.; Lesieur, S.; Couvreur, P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc. Chem. Res. 2010, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Angelov, B.; Angelova, A.; Filippov, S.K.; Drechsler, M.; Stepanek, P.; Lesieur, S. Multicompartment lipid cubic nanoparticles with high protein upload: Millisecond dynamics of formation. ACS Nano 2014, 8, 5216–5226. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Fajolles, C.; Hocquelet, C.; Djedaini-Pilard, F.; Lesieur, S.; Bonnet, V.; Perly, B.; Lebas, G.; Mauclaire, L. Physico-chemical investigation of asymmetrical peptidolipidyl-cyclodextrins. J. Colloid Interface Sci. 2008, 322, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Angelova, A.; Angelov, B.; Drechsler, M.; Garamus, V.M.; Willumeit-Romer, R.; Zou, A.H. Sterically stabilized spongosomes for multidrug delivery of anticancer nanomedicines. J. Mater. Chem. B 2015, 3, 7734–7744. [Google Scholar] [CrossRef]
- Zerkoune, L.; Angelova, A.; Lesieur, S. Nano-assemblies of modified cyclodextrins and their complexes with guest molecules: Incorporation in nanostructured membranes and amphiphile nanoarchitectonics design. Nanomaterials 2014, 4, 741–765. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Liu, X.F.; Hu, J.S.; Yang, L.Q.; Chen, Z.P. Synthesis and self-assembled behavior of ph-responsive chiral liquid crystal amphiphilic copolymers based on diosgenyl-functionalized aliphatic polycarbonate. Nanomaterials 2017, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals Chemistry and Physics; Chapter 3; Taylor & Francis: London, UK, 1997. [Google Scholar]
- Liu, J.H.; Hung, H.J.; Yang, P.C.; Tien, K.H. Thermal Recordable Novel Cholesteric Liquid Crystalline Polyacrylates Containing Various Chiral Moieties. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6214–6228. [Google Scholar] [CrossRef]
- Favier, A.; Charreyre, M.T. Experimental requirements for an efficient control of free-radical polymerizations via the reversible addition-fragmentation chain transfer (RAFT) process. Macromol. Rapid Commun. 2006, 27, 653–692. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 7–16. [Google Scholar]
| Copolymer | m (–OH) | n (–OH) | m (–COOH) | n (–COOH) |
|---|---|---|---|---|
| g | mol | g | mol | |
| mPEG43-b-P[(TMC-C)20-TMC20] | 5.00 | 0.016 | 8.28 | 0.016 |
| mPEG43-b-P[(TMC-C)15-HTMC5-TMC20] | 5.00 | 0.016 | 6.21 | 0.012 |
| mPEG43-b-P[(TMC-C)12-HTMC8-TMC20] | 5.00 | 0.016 | 3.58 | 0.008 |
| Copolymer | Tda (°C) | Weight Loss (%) | ||||
|---|---|---|---|---|---|---|
| 200 °C | 250 °C | 300 °C | 350 °C | 400 °C | ||
| mPEG43-b-P(BTMC20-TMC20) | 276.5 | 2.04 | 3.40 | 42.83 | 83.68 | 98.35 |
| mPEG43-b-P(HTMC20-TMC20) | 184.2 | 23.19 | 69.22 | 72.63 | 75.04 | 89.32 |
| mPEG43-b-P[(TMC-C)20-TMC20] | 234.4 | 3.31 | 7.79 | 25.31 | 62.36 | 90.09 |
| mPEG43-b-P[(TMC-C)15-HTMC5-TMC20] | 225.8 | 3.45 | 9.74 | 29.86 | 61.31 | 91.62 |
| mPEG43-b-P[(TMC-C)12-HTMC8-TMC20] | 197.2 | 6.15 | 14.33 | 34.86 | 67.91 | 94.21 |
| Copolymer | Phase Transition Temperature (°C) |
|---|---|
| mPEG43-b-P(BTMC20-TMC20) | g −25.0 I |
| mPEG43-b-P(HTMC20-TMC20) | g −24.1 I |
| mPEG43-b-P[(TMC-C)20-TMC20] | g −4.6 SmA 180.0 I |
| mPEG43-b-P[(TMC-C)15-HTMC5-TMC20] | g −7.4 SmA 169.8 I |
| mPEG43-b-P[(TMC-C)12-HTMC8-TMC20] | g −11.2 SmA 142.6 I |
| Copolymer | pH | Z-Average, d/nm | PDI |
|---|---|---|---|
| P1 | 5 | 346.3 | 0.136 |
| P1 | 6 | 307.4 | 0.123 |
| P1 | 7 | 296.4 | 0.146 |
| P1 | 8 | 295.1 | 0.149 |
| P1 | 9 | 301.7 | 0.179 |
| P2 | 7 | 313.4 | 0.132 |
| P3 | 7 | 352.9 | 0.067 |
| Copolymer | P1 | P2 | P3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH = 6.4 | pH = 7.4 | pH = 8.4 | pH = 6.4 | pH = 7.4 | pH = 8.4 | pH = 6.4 | pH = 7.4 | pH = 8.4 | |
| Size (nm) | 219.0 | 256.6 | 304.5 | 286.3 | 225.2 | 298.2 | 319.6 | 398.6 | 305.6 |
| PDI | 0.112 | 0.224 | 0.506 | 0.083 | 0.071 | 0.312 | 0.322 | 0.428 | 0.253 |
| DL (%) | 0.544 | 0.541 | 0.226 | 0.484 | 0.572 | 0.343 | 0.447 | 0.508 | 0.360 |
| EE (%) | 33.21 | 31.82 | 13.20 | 32.92 | 32.62 | 20.74 | 28.92 | 27.62 | 21.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Liu, X.; Hu, Z.; Hou, Z.; Guo, Z.; Chen, Z.; Hu, J.; Yang, L. Synthesis, Self-Assembly, and Drug-Release Properties of New Amphipathic Liquid Crystal Polycarbonates. Nanomaterials 2018, 8, 195. https://doi.org/10.3390/nano8040195
Xie Y, Liu X, Hu Z, Hou Z, Guo Z, Chen Z, Hu J, Yang L. Synthesis, Self-Assembly, and Drug-Release Properties of New Amphipathic Liquid Crystal Polycarbonates. Nanomaterials. 2018; 8(4):195. https://doi.org/10.3390/nano8040195
Chicago/Turabian StyleXie, Yujiao, Xiaofeng Liu, Zhuang Hu, Zhipeng Hou, Zhihao Guo, Zhangpei Chen, Jianshe Hu, and Liqun Yang. 2018. "Synthesis, Self-Assembly, and Drug-Release Properties of New Amphipathic Liquid Crystal Polycarbonates" Nanomaterials 8, no. 4: 195. https://doi.org/10.3390/nano8040195
APA StyleXie, Y., Liu, X., Hu, Z., Hou, Z., Guo, Z., Chen, Z., Hu, J., & Yang, L. (2018). Synthesis, Self-Assembly, and Drug-Release Properties of New Amphipathic Liquid Crystal Polycarbonates. Nanomaterials, 8(4), 195. https://doi.org/10.3390/nano8040195





