Combination of Roll Grinding and High-Pressure Homogenization Can Prepare Stable Bicelles for Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Drug–Lipid Nanoparticle Suspensions
2.3. Freeze-Drying and Reconstitution of NI–Lipid Nanoparticles
2.4. Measurement of Mean Particle Size and Zeta Potential
2.5. Measurement of the Concentration and the Entrapment Efficiency of Drugs in Drug–Lipid Nanoparticle Suspensions
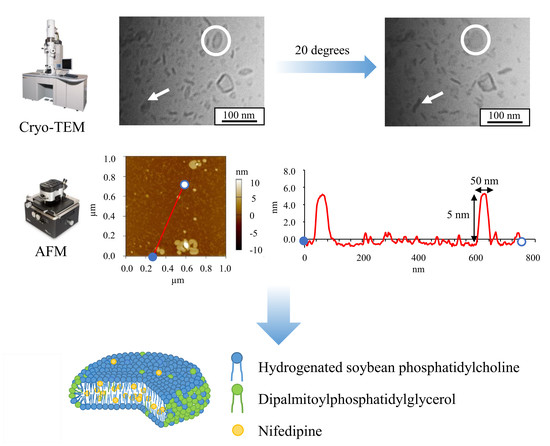
2.6. Structural Analysis of Lipid Nanoparticles
3. Results
3.1. Stractural Feature of Standard NI-LNs
3.1.1. Cryo-TEM Images of Standard NI-LNs
3.1.2. AFM Images of Standard NI-LNs
3.2. Effect of Long-Term Storage and Stabilization on LN Structure
3.3. Effect of Encapsulated Drugs on LN Structure
3.4. Effect of Lipid Composition Ratio on LN Structure
3.5. Effect of Phosphatidylcholine Acyl Chain Length on LN Structure
3.6. Effect of High-Pressure Homogenization on LN Structure
3.7. Effect of Cholesterol on LN Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| APTES | 3-Aminopropyltriethoxysilane |
| Chol | Cholesterol |
| CPP | Critical packing parameter |
| Cryo-TEM | Cryo transmission electron microscopy |
| DDS | Drug delivery system |
| DLS | Dynamic light scattering |
| DHPC | Diheptanoylphosphatidylcholine |
| DMPC | Dimyristoylphosphatidylcholine |
| DPPC | Dipalmitoylphosphatidylcholine |
| DPPG | Dipalmitoylphosphatidylglycerol |
| DSPC | Distearoylphosphatidylcholine |
| EE | Entrapment efficiency |
| HSPC | Hydrogenated soybean phosphatidylcholine |
| LNs | Lipid-based nanoparticles |
| NI | Nifedipine |
| NI-LNs | Nifedipine-encapsulated lipid nanoparticles |
| PC | Phosphatidylcholine |
| PDI | Polydispersity index |
| PEG | Polyethylene glycol |
| PG | Phosphatidylglycerol |
| PTFE | Polytetrafluoroethylene |
| PHT | Phenytoin |
| PHT-LNs | Phenytoin-encapsulated lipid nanoparticles |
| Tc | Phase transition temperature |
References
- Di Maio, S.; Carrier, R.L. Gastrointestinal contents in fasted state and post-lipid ingestion: In vivo measurements and in vitro models for studying oral drug delivery. J. Control. Release 2011, 151, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Saito, N.; Imai, T.; Otagiri, M. Effect of grinding with hydroxypropyl cellulose on the dissolution and particle size of a poorly water-soluble drug. Chem. Pharm. Bull. 1999, 47, 1311–1313. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Okagaki, T.; Narisawa, S.; Koida, Y.; Nakajima, K. Improvement of dissolution characteristics and bioavailability of poorly water-soluble drugs by novel cogrinding method using water-soluble polymer. Int. J. Pharm. 1998, 160, 11–19. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanoparticle Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Zhang, P.; Forsgren, J.; Strømme, M. Stabilisation of amorphous ibuprofen in Upsalite, a mesoporous magnesium carbonate, as an approach to increasing the aqueous solubility of poorly soluble drugs. Int. J. Pharm. 2014, 472, 185–191. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; Redington, W.; O’Reilly, N.J. An investigation into the crystallization tendency/kinetics of amorphous active pharmaceutical ingredients: A case study with dipyridamole and cinnarizine. Eur. J. Pharm. Biopharm. 2016, 104, 59–71. [Google Scholar] [CrossRef]
- Skrdla, P.J.; Floyd, P.D.; Dell’orco, P.C. Practical Estimation of Amorphous Solubility Enhancement Using Thermoanalytical Data: Determination of the Amorphous/Crystalline Solubility Ratio for Pure Indomethacin and Felodipine. J. Pharm. Sci. 2016, 105, 2625–2630. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Preparation and dissolution characteristics of several fast-release solid dispersions of griseofulvin. J. Pharm. Sci. 1969, 58, 1505–1510. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Zhang, J.; Lv, Y.; Liu, X.; Lv, G.; Ma, X. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.S.; Beckford Vera, D.R.; Benhabbour, S.R.; Parrott, M.C. Rapid microwave-assisted synthesis of sub-30 nm lipid nanoparticles. J. Colloid Interface Sci. 2017, 488, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Dokoumetzidis, A.; Macheras, P. A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int. J. Pharm. 2006, 321, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Moreno, J.; Bilgili, E.; Davé, R. Fast dissolution of poorly water soluble drugs from fluidized bed coated nanocomposites: Impact of carrier size. Int. J. Pharm. 2016, 513, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Fabiano, A.; Zambito, Y.; Bernkop-Schnürch, A. About the impact of water movement on the permeation behaviour of nanoparticles in mucus. Int. J. Pharm. 2017, 517, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pandey, R.; Sharma, S.; Khuller, G.K. Chemotherapeutic efficacy of poly (DL-lactide-co-glycolide) nanoparticle encapsulated antitubercular drugs at sub-therapeutic dose against experimental tuberculosis. Int. J. Antimicrob. Agents 2004, 24, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Nishiyama, N.; Kozaki, M.; Nakanishi, T.; Matsuda, Y.; Hirano, M.; Hanada, H.; Hisada, S.; Onodera, H.; Harashima, H.; et al. General considerations regarding the in vitro and in vivo properties of block copolymer micelle products and their evaluation. J. Control. Release 2015, 210, 76–83. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Progress in Polymer Science Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.S.; Yoong, S.L.; Jagusiak, A.; Panczyk, T.; Ho, H.K.; Ang, W.H.; Pastorin, G. Carbon nanotubes for delivery of small molecule drugs. Adv. Drug Deliv. Rev. 2013, 65, 1964–2015. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part II—Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Kamiya, S.; Yamada, M.; Kurita, T.; Miyagishima, A.; Arakawa, M.; Sonobe, T. Preparation and stabilization of nifedipine lipid nanoparticles. Int. J. Pharm. 2008, 354, 242–247. [Google Scholar] [CrossRef]
- Ohshima, H.; Miyagishima, A.; Kurita, T.; Makino, Y.; Iwao, Y.; Sonobe, T.; Itai, S. Freeze-dried nifedipine-lipid nanoparticles with long-term nano-dispersion stability after reconstitution. Int. J. Pharm. 2009, 377, 180–184. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Iwao, Y.; Noguchi, S.; Itai, S. Lipid nanoparticles with no surfactant improve oral absorption rate of poorly water-soluble drug. Int. J. Pharm. 2013, 451, 92–94. [Google Scholar] [CrossRef]
- Shi, L.L.; Cao, Y.; Zhu, X.Y.; Cui, J.H.; Cao, Q.R. Optimization of process variables of zanamivir-loaded solid lipid nanoparticles and the prediction of their cellular transport in Caco-2 cell model. Int. J. Pharm. 2015, 478, 60–69. [Google Scholar] [CrossRef]
- Gonçalves, L.M.D.; Maestrelli, F.; Mannelli, L.C.; Ghelardini, C.; Almeida, A.J.; Mura, P. Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur. J. Pharm. Biopharm. 2016, 102, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: Effect on formulation and characterization parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Shegokar, R.; Rakovsky, E.; Gonzalez-Mira, E.; Lopes, C.M.; Silva, A.M.; Martins-Lopes, P.; Muller, R.H.; Souto, E.B. Cationic solid lipid nanoparticles (cSLN): Structure, stability and DNA binding capacity correlation studies. Int. J. Pharm. 2011, 420, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, J.S.; Kim, C.K. Development of a binary lipid nanoparticles formulation of itraconazole for parenteral administration and controlled release. Int. J. Pharm. 2010, 383, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Crowell, K.J.; Macdonald, P.M. Surface charge response of the phosphatidylcholine head group in bilayered micelles from phosphorus and deuterium nuclear magnetic resonance. Biochim. Biophys. Acta—Biomembr. 1999, 1416, 21–30. [Google Scholar] [CrossRef]
- Diller, A.; Loudet, C.; Aussenac, F.; Raffard, G.; Fournier, S.; Laguerre, M.; Grélard, A.; Opella, S.J.; Marassi, F.M.; Dufourc, E.J. Bicelles: A natural “molecular goniometer” for structural, dynamical and topological studies of molecules in membranes. Biochimie 2009, 91, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Barman, R.K.; Iwao, Y.; Funakoshi, Y.; Ranneh, A.-H.; Noguchi, S.; Wahed, M.I.I.; Itai, S. Development of Highly Stable Nifedipine Solid–Lipid Nanoparticles. Chem. Pharm. Bull. 2014, 62, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Yasuhara, K.; Miki, S.; Nakazono, H.; Ohta, A.; Kikuchi, J. Synthesis of organic–inorganic hybrid bicelles–lipid bilayer nanodiscs encompassed by siloxane surfaces. Chem. Commun. 2011, 47, 4691–4693. [Google Scholar] [CrossRef]
- Glazier, R.; Salaita, K. Supported lipid bilayer platforms to probe cell mechanobiology. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 1465–1482. [Google Scholar] [CrossRef]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Hesari, J.; Valizadeh, H.; Faller, R. Molecular dynamics simulations of ternary lipid bilayers containing plant sterol and glucosylceramide. Chem. Phys. Lipids 2017, 203, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Al-Jamal, W.T.; Al-Jamal, K.T.; Kostarelos, K. Doxorubicin-loaded lipid-quantum dot hybrids: Surface topography and release properties. Int. J. Pharm. 2011, 416, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Mahabir, S.; Small, D.; Li, M.; Wan, W.; Kučerka, N.; Littrell, K.; Katsaras, J.; Nieh, M.P. Growth kinetics of lipid-based nanodiscs to unilamellar vesicles—A time-resolved small angle neutron scattering (SANS) study. Biochim. Biophys. Acta—Biomembr. 2013, 1828, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Mariusz, K. Interactions of serum with polyelectrolyte-stabilized liposomes: Cryo-TEM studies. Interactions of serum with polyelectrolyte-stabilized liposomes: Cryo-TEM studies. Colloids Surf. B Biointerfaces 2014, 120, 152–159. [Google Scholar]
- Doroty, C.; Jonathan, C.; Jenna, B.; William, J.M.; Maria, A.O.; Sheng, Q. Disc-shaped polyoxyethylene glycol glycerides gel nanoparticles as novel protein delivery vehicles. Int. J. Pharm. 2015, 496, 1015–1025. [Google Scholar]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and evidences on the lyophilization of polymeric nanoparticles for drug delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.; Mayer, L.D.; Abraham, S.A.; Gallagher, R.C.; Cox, K.A.K.; Tardi, P.G.; Bally, M.B. Improved retention of idarubicin after intravenous injection obtained for cholesterol-free liposomes. Biochim. Biophys. Acta—Biomembr. 2002, 1561, 188–201. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Iwao, Y.; Noguchi, S.; Itai, S. Effect of Alkyl Chain Length and Unsaturation of the Phospholipid on the Physicochemical Properties of Lipid Nanoparticles. Chem. Pharm. Bull. 2015, 63, 731–736. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, R.N.; Gier, J.De; Van Der Neut-Kok, E.C.M. The effect of alterations in fatty acid composition and cholesterol content on the permeability of Mycoplasrno laidlawii B cells and derived liposomes. Biochim. Biophys. Acta—Biomembr. 1973, 298, 500–512. [Google Scholar] [CrossRef]
- Mabrey, S.; Mateo, P.L.; Sturtevant, J.M.; Mabrey, S.; Mateo, P.L.; Sturtevant, J.M. High-Sensitivity Scanning Calorimetric Study of Mixtures of Cholesterol with Dimyristoyl- and Dipalmitoylphosphatidylcholines. Biochemistry 1978, 17, 2464–2468. [Google Scholar] [CrossRef]
- Prosser, R.S.; Evanics, F.; Kitevski, J.L.; Al-Abdul-Wahid, M.S. Current Applications of Bicelles in NMR Studies of Membrane- Associated Amphiphiles and Proteins. Biochemistry 2006, 45, 8453–8465. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Pearcy, P.; Ramamoorthy, A. Bicelles exhibiting magnetic alignment for a broader range of temperatures: A solid-state NMR study. Langmuir 2014, 30, 1622–1629. [Google Scholar] [CrossRef]
- Isabettini, S.; Liebi, M.; Kohlbrecher, J.; Ishikawa, T.; Windhab, E.J.; Fischer, P.; Walde, P.; Kuster, S. Tailoring bicelle morphology and thermal stability with lanthanide-chelating cholesterol conjugates. Langmuir 2016, 32, 9005–9014. [Google Scholar] [CrossRef] [PubMed]
- Nolandt, O.V.; Walther, T.H.; Grage, S.L.; Ulrich, A.S. Magnetically oriented dodecylphosphocholine bicelles for solid-state NMR structure analysis. Biochim. Biophys. Acta—Biomembr. 2012, 1818, 1142–1147. [Google Scholar] [CrossRef]
- Zetterberg, M.M.; Reijmar, K.; Pränting, M.; Engström, Å.; Andersson, D.I.; Edwards, K. PEG-stabilized lipid disks as carriers for amphiphilic antimicrobial peptides. J. Control. Release 2011, 156, 323–328. [Google Scholar] [CrossRef]
- Meyer, H.W.; Richter, W.; Rettig, W.; Stumpf, M. Bilayer fragments and bilayered micelles (bicelles) of dimyristoylphosphatidylglycerol (DMPG) are induced by storage in distilled water at 4 °C. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 183–185, 495–504. [Google Scholar] [CrossRef]
- Dichello, G.A.; Fukuda, T.; Maekawa, T.; Whitby, R.L.D.; Mikhalovsky, S.V.; Alavijeh, M.; Pannala, A.S.; Sarker, D.K. Preparation of liposomes containing small gold nanoparticles using electrostatic interactions. Eur. J. Pharm. Sci. 2017, 105, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Von White, G.; Chen, Y.; Roder-Hanna, J.; Bothun, G.D.; Kitchens, C.L. Structural and thermal analysis of lipid vesicles encapsulating hydrophobic gold nanoparticles. ACS Nano 2012, 6, 4678–4685. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Suzuki, T.; Yasuda, T.; Oishi, T.; Matsumori, N. Bioorganic & Medicinal Chemistry NMR-based conformational analysis of sphingomyelin in bicelles. Bioorg. Med. Chem. 2012, 20, 270–278. [Google Scholar]
- Ickenstein, L.M.; Arfvidsson, M.C.; Needham, D.; Mayer, L.D.; Edwards, K. Disc formation in cholesterol-free liposomes during phase transition. Biochim. Biophys. Acta—Biomembr. 2003, 1614, 135–138. [Google Scholar] [CrossRef]
- Barbosa-Barros, L.; Rodríguez, G.; Cócera, M.; Rubio, L.; López-Iglesias, C.; de la Maza, A.; López, O. Structural Versatility of Bicellar Systems and Their Possibilities as Colloidal Carriers. Pharmaceutics 2011, 3, 636–664. [Google Scholar]
- Rodríguez, G.; Soria, G.; Coll, E.; Rubio, L.; Barbosa-Barros, L.; López-lglesias, C.; Planas, A.M.; Estelrich, J.; De La Maza, A.; López, O. Bicosomes: Bicelles in dilute systems. Biophys. J. 2010, 99, 480–488. [Google Scholar] [CrossRef]
- Vold, R.R.; Prosser, R.S. Magnetically oriented phospholipid bilayered micelles for structural studies of polypeptides. Does the ideal bicelle exist? J. Magn. Reson. Ser. B 1996, 113, 267–271. [Google Scholar] [CrossRef]
- Barbosa-Barros, L.; Rodríguez, G.; Barba, C.; Cócera, M.; Rubio, L.; Estelrich, J.; López-Iglesias, C.; De La Maza, A.; López, O. Bicelles: Lipid nanostructured platforms with potential dermal applications. Small 2012, 8, 807–818. [Google Scholar] [CrossRef]
- Hameed, S.; Bhattarai, P.; Dai, Z. Cerasomes and Bicelles: Hybrid Bilayered Nanostructures With Silica-Like Surface in Cancer Theranostics. Front. Chem. 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
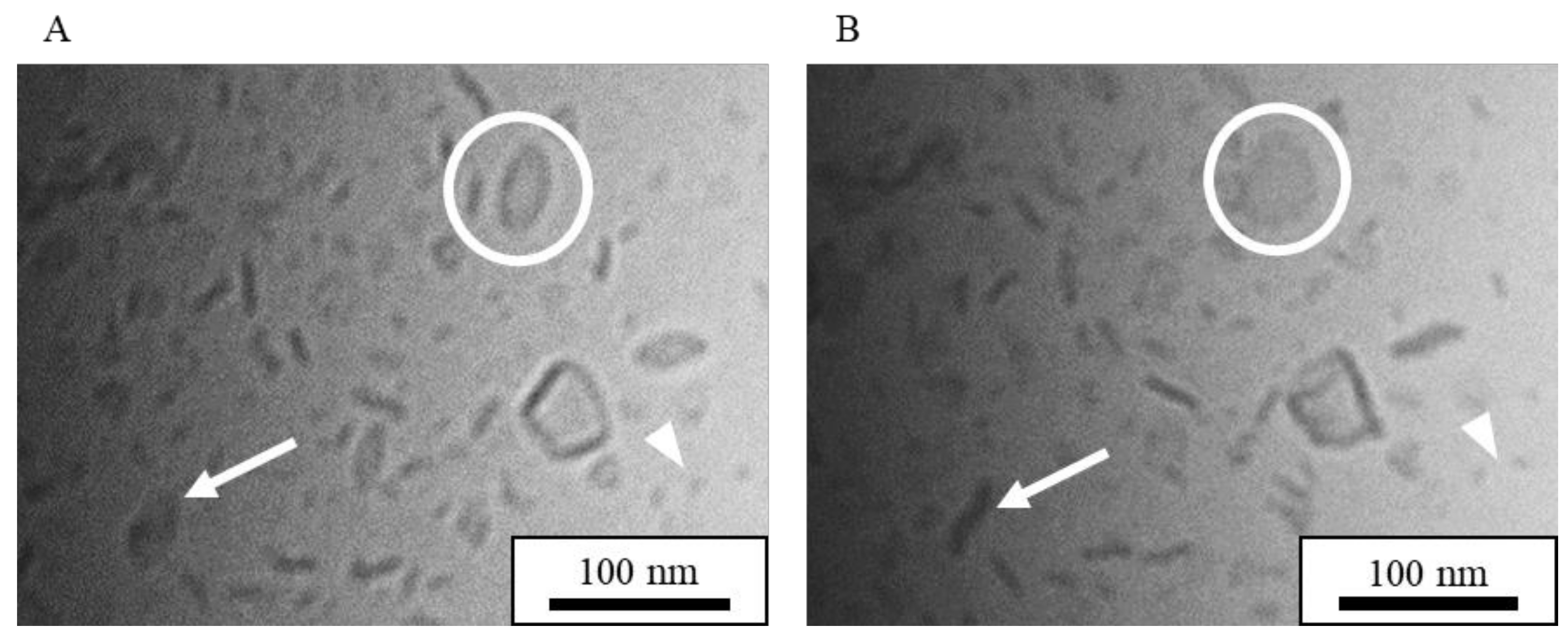
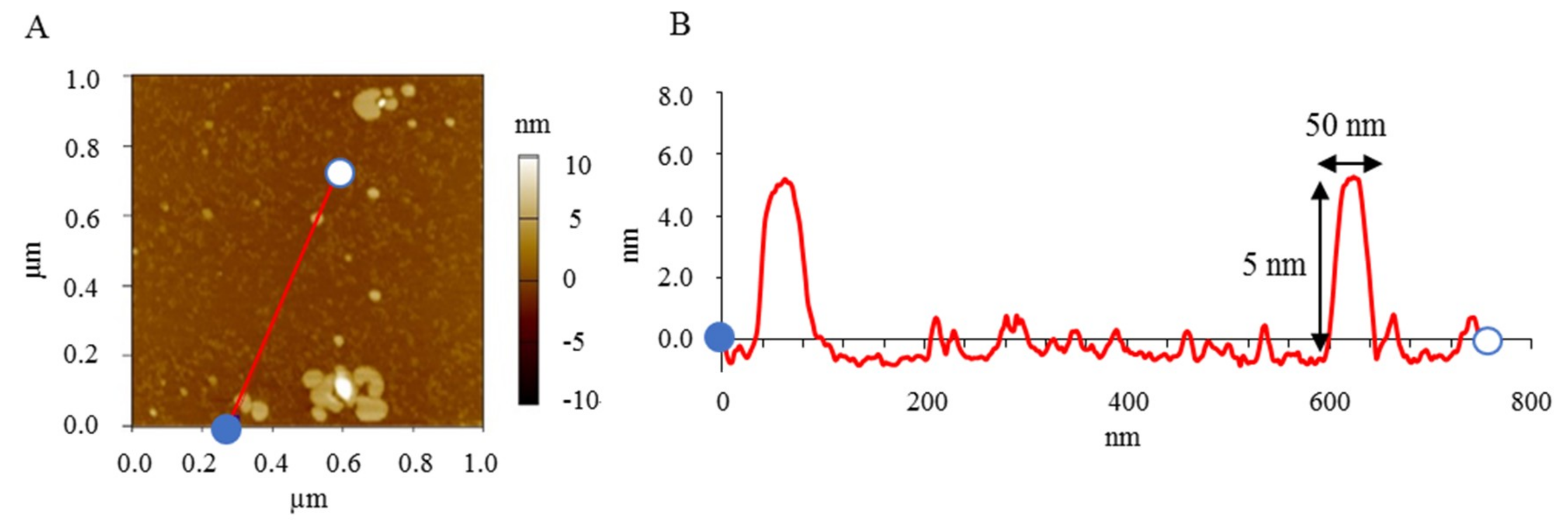
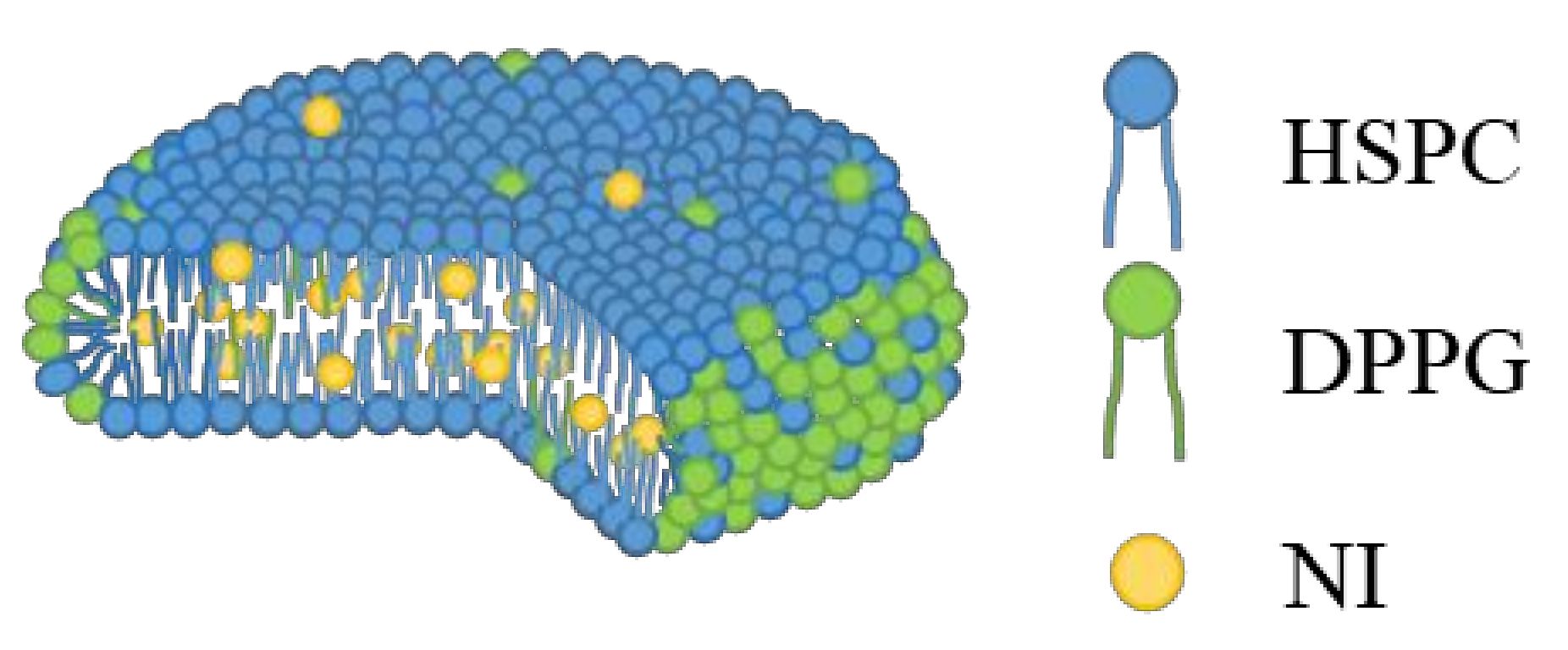
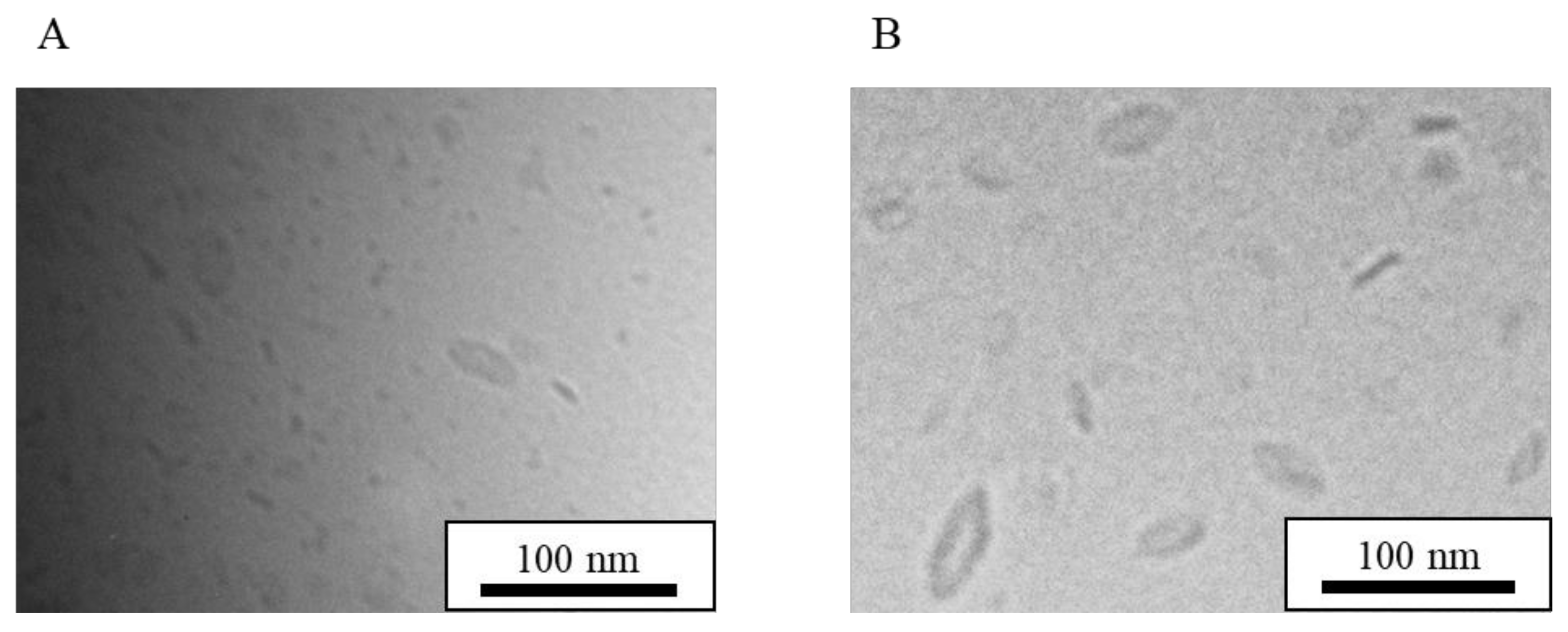
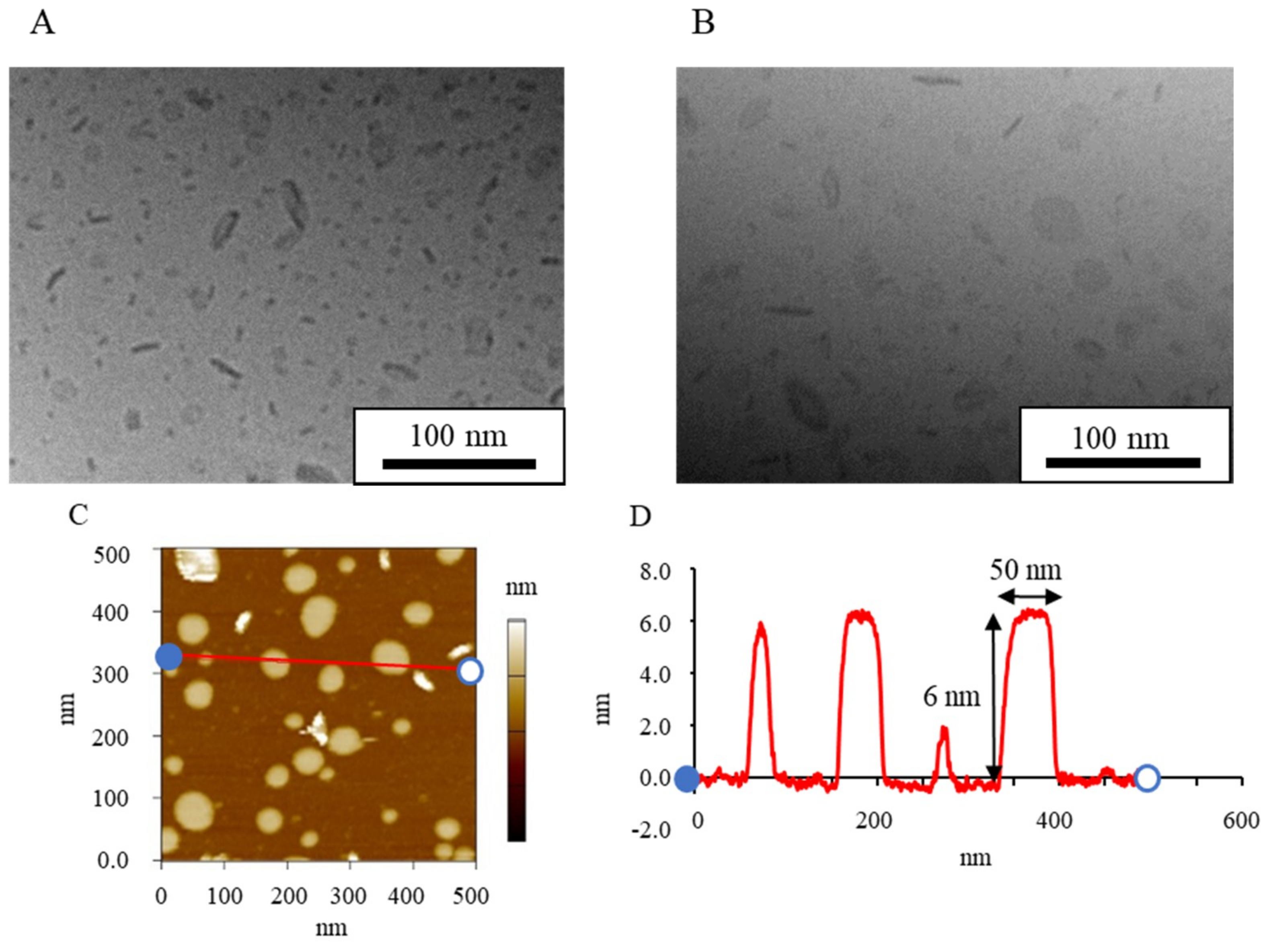
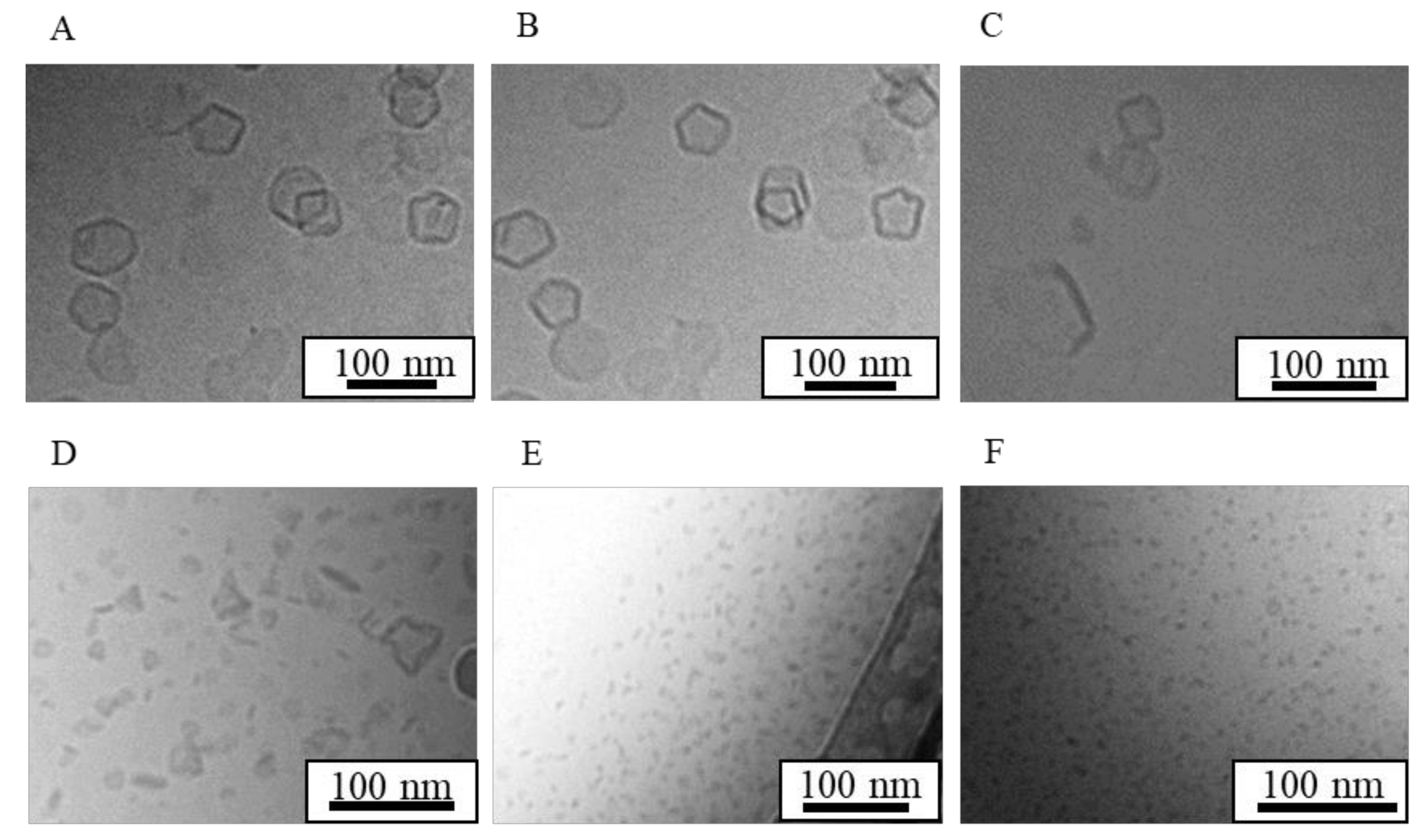
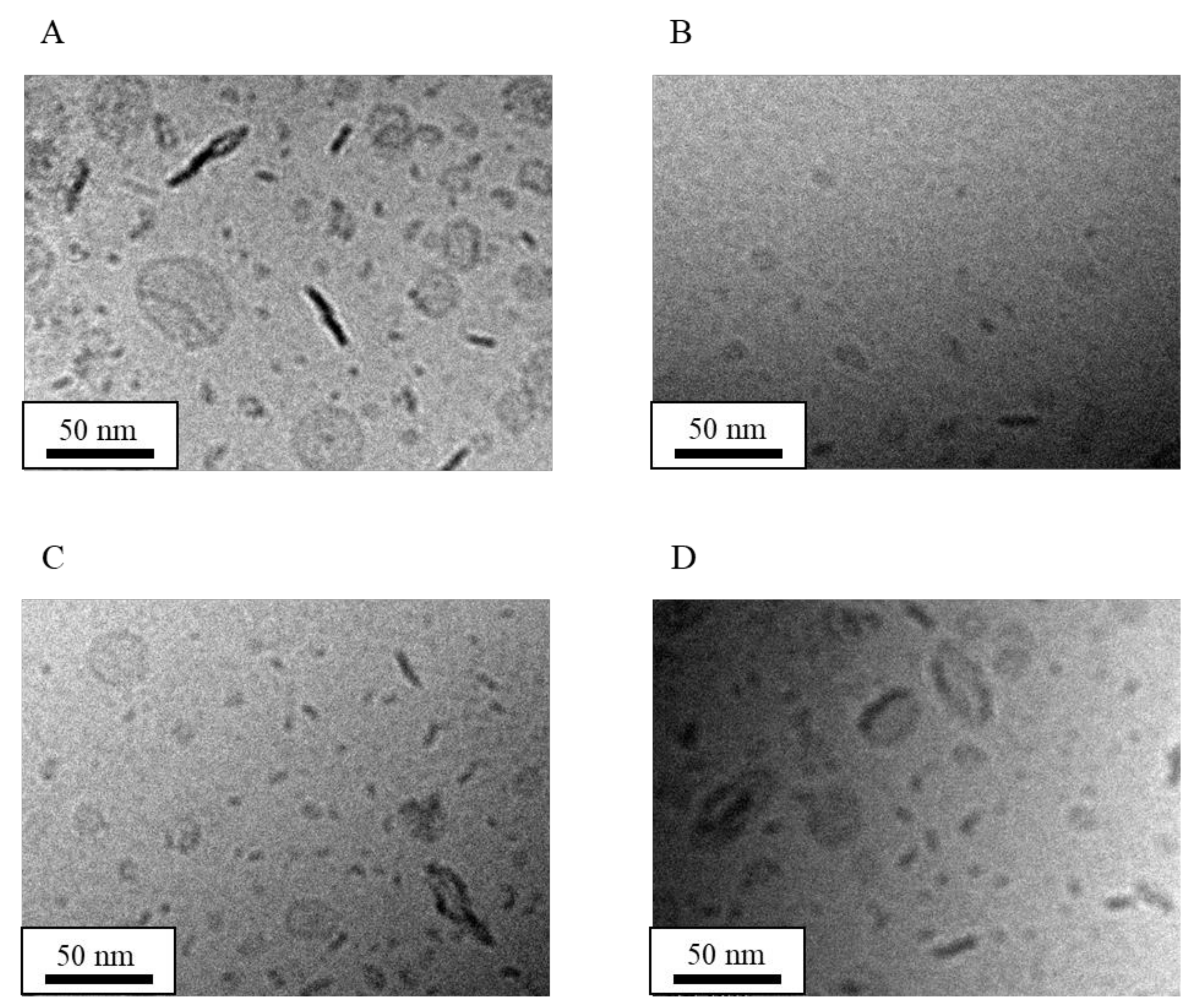
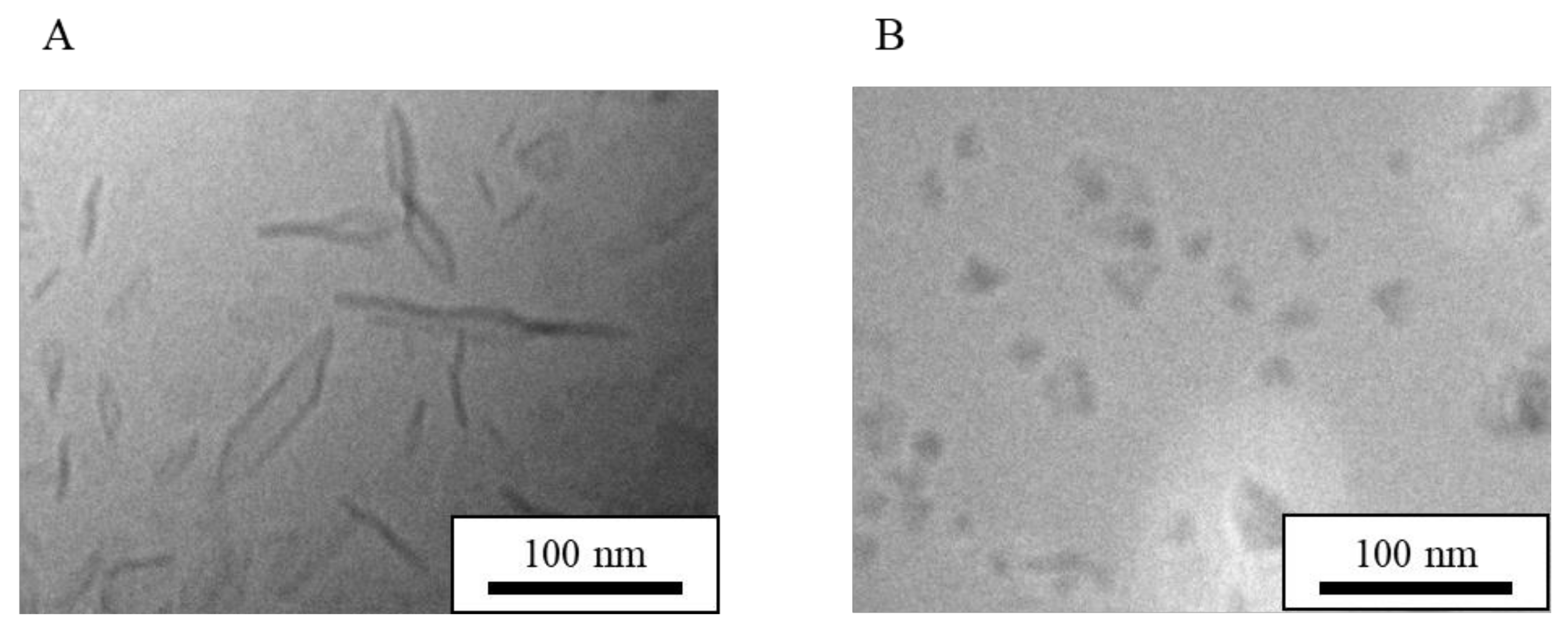
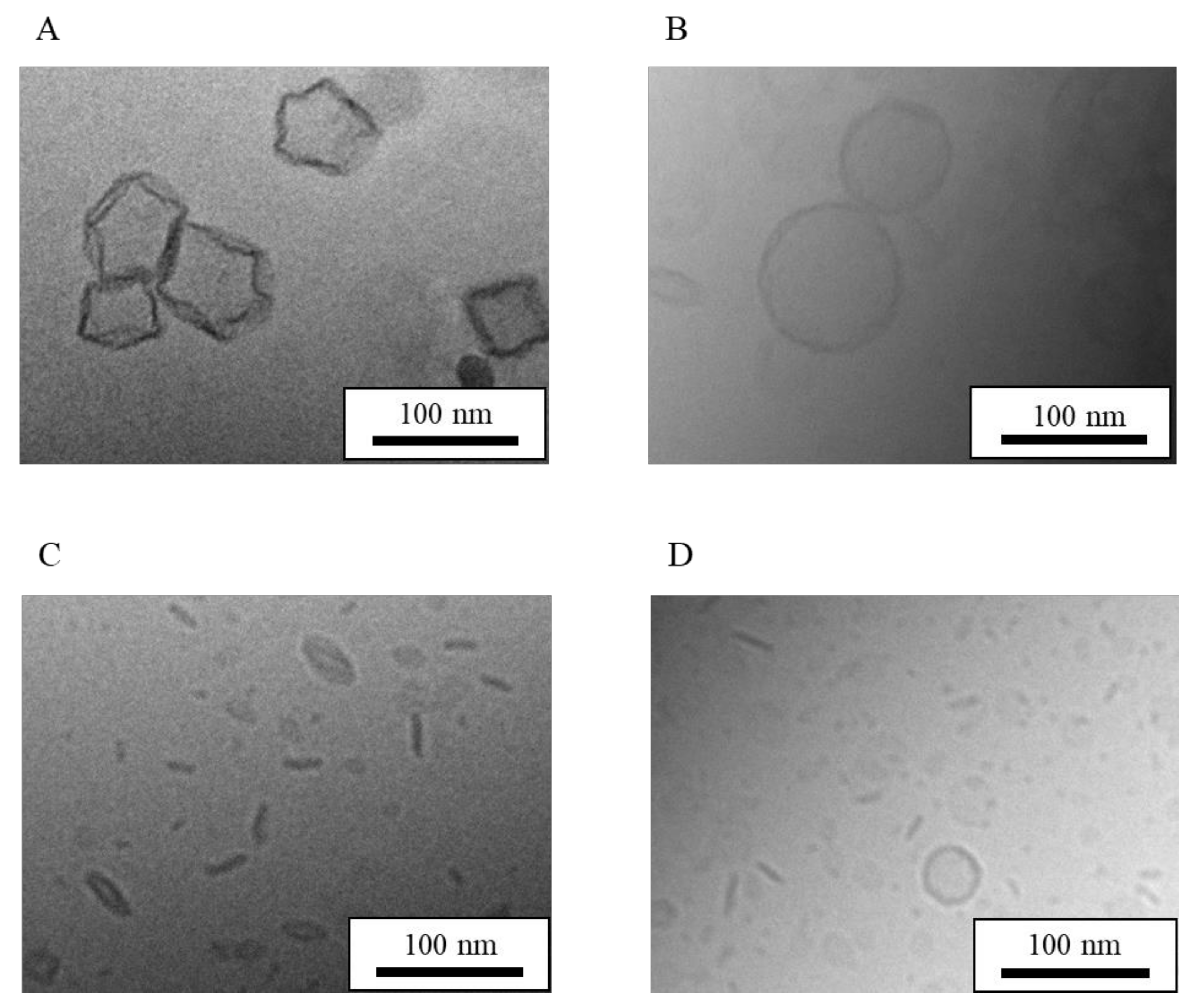
| Formulation | Bicelle (nm) | Micelle (nm) | Mean Particle Size (nm) |
|---|---|---|---|
| Just after preparation | 26.8 ± 8.4 (n = 81) | 8.0 ± 1.7 (n = 19) | 23.2 ± 10.6 (n = 100) |
| Stored for four months at a cold dark place | 23.4 ± 10.9 (n = 59) | 7.6 ± 1.8 (n = 41) | 17.0 ± 11.5 (n = 100) |
| Re-hydrated after freeze-drying with sucrose | 39.7 ± 12.1 (n = 91) | 11.1 ± 1.0 (n = 9) | 37.1 ± 14.2 (n = 100) |
| Formulation | Mean Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Concentration (µg/mL) | EE (%) |
|---|---|---|---|---|---|
| Just after preparation | 47.5 ± 2.9 | 0.307 ± 0.027 | −46.8 ± 9.6 | 67.3 ± 11.3 | 97.1 ± 1.6 |
| Stored for four months at a cold dark place | 46.3 ± 1.0 | 0.310 ± 0.033 | −52.1 ± 2.8 | 67.4 ± 6.2 | 98.1 ± 0.3 |
| Re-hydrated after freeze-drying with sucrose | 63.7 ± 1.2 | 0.351 ± 0.007 | −47.0 ± 4.1 | 30.7 ± 7.6 | 97.8 ± 0.5 |
| Model Drug | Bicelle (nm) | Micelle (nm) | Mean Particle Size (nm) |
|---|---|---|---|
| NI | 26.8 ± 8.4 (n = 81) | 8.0 ± 1.7 (n = 19) | 23.2 ± 10.6 (n = 100) |
| None | 21.1 ± 5.6 (n = 68) | 6.1 ± 2.1 (n = 32) | 16.3 ± 8.7 (n = 100) |
| PHT | 26.5 ± 9.3 (n = 81) | 8.1 ± 1.6 (n = 19) | 23.0 ± 11.1 (n = 100) |
| Model Drug | Mean Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Concentration (µg/mL) | EE(%) |
|---|---|---|---|---|---|
| NI | 47.5 ± 2.9 | 0.307 ± 0.027 | −46.8 ± 9.6 | 67.3 ± 11.3 | 97.1 ± 1.6 |
| None | 43.3 ± 0.7 | 0.357 ± 0.086 | −55.6 ± 4.2 | - | - |
| PHT | 46.0 ± 4.4 | 0.267 ± 0.003 | −30.7 ± 6.4 | 30.5 ± 2.8 | 59.4 ± 7.6 |
| HSPC/DPPG (Molar Ratio) | Bicelle (nm) | Micelle (nm) | Liposome (nm) | Mean Particle Size (nm) | |
|---|---|---|---|---|---|
| NI-LNs | (5/0) | - | - | 77.4 ± 31.5 (n = 100) | 77.4 ± 31.5 (n = 100) |
| (5/0.5) | 24.1 ± 9.4 (n = 71) | 9.0 ± 2.1 (n = 22) | 43.9 ± 10.1 (n = 7) | 22.2 ± 12.0 (n = 100) | |
| (5/1) | 26.8 ± 8.4 (n = 81) | 8.0 ± 1.7 (n = 19) | - | 23.2 ± 10.6 (n = 100) | |
| (0/5) | - | 8.6 ± 2.4 (n = 100) | - | 8.6 ± 2.4 (n = 100) | |
| LNs | (5/0) | - | - | 80.5 ± 40.6 (n = 20) | 80.5 ± 40.6 (n = 20) |
| (5/1) | 21.1 ± 5.6 (n = 68) | 6.1 ± 2.1 (n = 32) | - | 16.3 ± 8.7 (n = 100) | |
| (0/5) | - | 10.9 ± 1.7 (n = 100) | - | 10.9 ± 1.7 (n = 100) | |
| HSPC/DPPG (Molar Ratio) | Mean Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Concentration (µg/mL) | EE (%) | |
|---|---|---|---|---|---|---|
| NI-LNs | (5/0) | 93.1 ± 102.4 | 0.443 ± 0.049 | −5.5 ± 9.1 | 2.9 ± 2.5 | 63.4 ± 55.1 |
| (5/0.5) | 50.7 ± 3.1 | 0.294 ± 0.025 | −43.6 ± 7.9 | 58.0 ± 25.8 | 97.0 ± 1.8 | |
| (5/1) | 47.5 ± 2.9 | 0.307 ± 0.027 | −46.8 ± 9.6 | 67.3 ± 11.3 | 97.1 ± 1.6 | |
| (0/5) | 54.5 ± 28.4 | 0.793 ± 0.210 | −49.9 ± 3.7 | 98.0 ± 24.2 | 99.3 ± 0.1 | |
| LNs | (5/0) | 217.7 ± 135.1 | 0.238 ± 0.026 | −13.5 ± 0.4 | - | - |
| (5/1) | 48.7 ± 9.4 | 0.357 ± 0.086 | −55.6 ± 4.2 | - | - | |
| (0/5) | 92.4 ± 10.3 | 0.540 ± 0.029 | −55.4 ± 5.4 | - | - | |
| Formulation | Bicelle (nm) | Micelle (nm) | Mean Particle Size (nm) |
|---|---|---|---|
| HSPC/DPPG (5/1) | 26.8 ± 8.4 (n = 81) | 8.0 ± 1.7 (n = 19) | 23.2 ± 10.6 (n = 100) |
| DMPC/DPPG (5/1) | 15.6 ± 3.3 (n = 40) | 6.7 ± 1.0 (n = 15) | 13.2 ± 4.9 (n = 55) |
| DPPC/DPPG (5/1) | 25.7 ± 11.3 (n = 82) | 7.2 ± 1.4 (n = 18) | 22.4 ± 12.5 (n = 100) |
| DSPC/DPPG (5/1) | 26.4 ± 8.8 (n = 77) | 6.1 ± 0.6 (n = 23) | 21.7 ± 11.5 (n = 100) |
| Formulation | Mean Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Concentration (µg/mL) | EE (%) |
|---|---|---|---|---|---|
| HSPC/DPPG (5/1) | 47.5 ± 2.9 | 0.307 ± 0.027 | −46.8 ± 9.6 | 67.3 ± 11.3 | 97.1 ± 1.6 |
| DMPC/DPPG (5/1) | 34.4 ± 17.2 | 0.668 ± 0.194 | −35.1 ± 10.0 | 61.0 ± 19.0 | 98.9 ± 0.2 |
| DPPC/DPPG (5/1) | 50.4 ± 11.1 | 0.361 ± 0.086 | −53.1 ± 1.8 | 61.5 ± 19.6 | 97.9 ± 1.9 |
| DSPC/DPPG (5/1) | 62.5 ± 5.9 | 0.285 ± 0.041 | −47.6 ± 3.7 | 59.1 ± 17.9 | 97.7 ± 2.3 |
| Conditions | Bicelle (nm) | Micelle (nm) | Mean Particle Size (nm) |
|---|---|---|---|
| 175 MPa/100 pass | 26.8 ± 8.4 (n = 81) | 8.0 ± 1.7 (n = 19) | 23.2 ± 10.6 (n = 100) |
| 100 MPa/100 pass | 47.0 ± 21.5 (n = 84) | 9.4 ± 1.3 (n = 16) | 41.0 ± 24.0 (n = 100) |
| 175 MPa/150 pass | 23.1 ± 6.0 (n = 49) | 10.4 ± 1.9 (n = 11) | 20.7 ± 7.3 (n = 60) |
| Conditions | Mean Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Concentration (µg/mL) | EE (%) |
|---|---|---|---|---|---|
| 175 MPa/100 pass | 47.5 ± 2.9 | 0.307 ± 0.027 | −46.8 ± 9.6 | 67.3 ± 11.3 | 97.1 ± 1.6 |
| 100 MPa/100 pass | 67.9 ± 8.5 | 0.251 ± 0.004 | −41.2 ± 19.7 | 24.6 ± 6.7 | 98.7 ± 0.6 |
| 175 MPa/150 pass | 39.1 ± 14.4 | 0.264 ± 0.004 | −28.1 ± 31.1 | 37.0 ± 3.3 | 99.2 ± 0.4 |
| Formulation | Bicelle (nm) | Micelle (nm) | Liposome (nm) | Mean Particle Size (nm) |
|---|---|---|---|---|
| HSPC/DPPG/Chol (5/0/0) | - | - | 77.4 ± 31.5 (n = 100) | 77.4 ± 31.5 (n = 100) |
| HSPC/DPPG/Chol (5/0/2) | - | - | 46.3 ± 9.6 (n = 100) | 46.3 ± 9.6 (n = 100) |
| HSPC/DPPG/Chol (5/1/0) | 26.8 ± 8.4 (n = 81) | 8.0 ± 1.7 (n = 19) | - | 23.2 ± 10.6 (n = 100) |
| HSPC/DPPG/Chol (5/1/2) | 22.8 ± 8.2 (n = 60) | 7.6 ± 1.0 (n = 32) | 34.7 ± 8.9 (n = 8) | 25.8 ± 8.8 (n = 100) |
| HSPC/DPPG/Chol | Mean Particle Size (nm) | PDI | Zeta Potential (mV) | Drug Concentration (µg/mL) | EE (%) |
|---|---|---|---|---|---|
| (5/0/0) | 93.1 ± 102.4 | 0.443 ± 0.049 | −5.5 ± 9.1 | 2.9 ± 2.5 | 63.4 ± 55.1 |
| (5/0/2) | 69.2 ± 6.2 | 0.368 ± 0.004 | 44.0 ± 1.6 | 12.6 ± 1.0 | 98.8 ± 0.6 |
| (5/1/0) | 47.5 ± 2.9 | 0.307 ± 0.027 | −46.8 ± 9.6 | 67.3 ± 11.3 | 97.1 ± 1.6 |
| (5/1/2) | 80.0 ± 21.3 | 0.468 ± 0.004 | −55.2 ± 1.1 | 109.2 ± 17.1 | 98.2 ± 1.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, S.; Higashi, K.; Moribe, K.; Kimura, S.-i.; Itai, S.; Kondo, H.; Iwao, Y. Combination of Roll Grinding and High-Pressure Homogenization Can Prepare Stable Bicelles for Drug Delivery. Nanomaterials 2018, 8, 998. https://doi.org/10.3390/nano8120998
Matsuo S, Higashi K, Moribe K, Kimura S-i, Itai S, Kondo H, Iwao Y. Combination of Roll Grinding and High-Pressure Homogenization Can Prepare Stable Bicelles for Drug Delivery. Nanomaterials. 2018; 8(12):998. https://doi.org/10.3390/nano8120998
Chicago/Turabian StyleMatsuo, Seira, Kenjirou Higashi, Kunikazu Moribe, Shin-ichiro Kimura, Shigeru Itai, Hiromu Kondo, and Yasunori Iwao. 2018. "Combination of Roll Grinding and High-Pressure Homogenization Can Prepare Stable Bicelles for Drug Delivery" Nanomaterials 8, no. 12: 998. https://doi.org/10.3390/nano8120998
APA StyleMatsuo, S., Higashi, K., Moribe, K., Kimura, S.-i., Itai, S., Kondo, H., & Iwao, Y. (2018). Combination of Roll Grinding and High-Pressure Homogenization Can Prepare Stable Bicelles for Drug Delivery. Nanomaterials, 8(12), 998. https://doi.org/10.3390/nano8120998