1. Introduction
Silicon-oxide series of ceramics, such as SiO
2 and Al
2O
3 coatings, are valued for their hardness, high wear resistance, dielectric strength, high corrosion resistance, and chemical stability. They have been extensively used as plasma-resistant materials in plasma etching equipment and in the deposition thin-film processing semiconductor industry [
1,
2]. They are also popular shield materials that protect the interior ceramic parts, chamber windows, cover baffles, rings, and reactor chamber walls of plasma equipment from corrosive fluorocarbon gases (e.g., C
2F
6, CF
4, CHF
3, and C
2F
6) [
3]. However, on the inner walls of the processing chamber, these oxide materials easily interact with fluorine-based plasma, causing significant erosion and particle generation [
4]. As integrated circuits continue to downscale to the nanoscopic level, particle contaminants in their wafers are becoming increasingly worrisome, because they short-current the integration circuit and reduce mass-production yield [
5]. Yttrium oxide (Y
2O
3) promises to replace SiO
2 and Al
2O
3 as the material of plasma-facing inner walls, owing to its much higher chemical stability and reduced erosion rate [
6]
. Nevertheless, as pointed out by mass-production factories, Y
2O
3 inner wall coatings contain pores and crack defects that release particles into the plasma by flake-off [
7]. Yttrium fluoride (YF
3) coatings have recently attracted substantial attention because their high plasma erosion resistance prevents the generation of fluoride particles on the chamber wall surface, reducing particulate contamination [
8]. Moreover, YF
3 reportedly has a high dielectric strength [
9]. Thus, YF
3 coating is a new plasma-facing material. Researchers have fabricated protective coatings in chamber walls using a plasma spray technique. However, as the spray method rapidly forms thick films with porous structures and rough surfaces, it might introduce critical particle impurities during the semiconductor plasma deposition/etching process [
10]. Vacuum coating techniques such as magnetron sputtering can address these problems.
To our knowledge, sputtered YF3 films and their erosion behavior under fluorocarbon plasma etching had not previously been investigated. Herein, we investigate the composition, structure, erosion behavior, and surface morphology changes of YF3 films on sapphire substrate, prepared by magnetron sputtering.
2. Materials and Methods
The sputter material was YF
3 ceramic target (99.99% purity, 2 inch diameter, 3 mm thickness). YF
3 thin films were deposited on c-plane sapphire substrate (single crystal of Al
2O
3) at room temperature by radio frequency magnetron sputtering in a vacuum chamber. The substrates were cleaned before deposition, first in acetone and alcohol, and then by ultrasonic cleansing in de-ionized (DI) water for 30 min. They were blow-dried in nitrogen gas. The sputtering gas was high-purity argon (99.995%), maintained at a constant flow rate (~100 sccm). The sputtering process was performed under a base chamber pressure of approximately 1.5 × 10
−5 torr, with turbo molecular and oil diffusion pumps. The plasma generation was activated by RF power at 13.56 MHz. The target–substrate distance was 15 cm. To ensure uniform film thickness, the substrate holder was rotated at 20 rpm during the deposition process. Substrate heating temperature was varied from 400 to 700 °C in steps of 100 °C. The sputter deposition conditions of the YF
3 films are detailed in
Table 1. The plasma etching behaviors of all samples were performed using an inductively-coupled plasma etcher. The etching gases mixed were CF
4 and O
2. The etching conditions are shown in
Table 2.
The surface morphology, microstructure, and elemental analysis of these coating samples were analyzed by scanning electron microscopy (SEM, S-3000H, Hitachi, Tokyo, Japan) coupled with energy dispersive X-ray diffraction (EDX), atomic force microscopy (AFM, DI-3100, Veeco, New York, NY, USA), and high-resolution transmission electron microcopy (HRTEM, H-600, Hitachi, Tokyo, Japan). The sample compositions were examined by X-ray photoelectron spectroscopy (XPS, PHI 5000 VersaProbe, ULVAC-PHI, Kanagawa, Japan) using a monochromatic Cu Kα X-ray source (λ = 1.541874 Å) at a passing energy of 20 eV with a spot size of 650 μm. After XPS, the sample surface was etched using focused argon-ion sputtering to investigate the chemical compositional depth profile (Thermo Scientific K-Alpha). Finally, the photoelectron spectrum resulting from the core energy levels of yttrium 3D states was deconvoluted by a fitting software program (Thermo Fisher Scientific, Inc., Waltham, MA, USA) to estimate the contributions from bonding with fluorine elements.
3. Results and Discussion
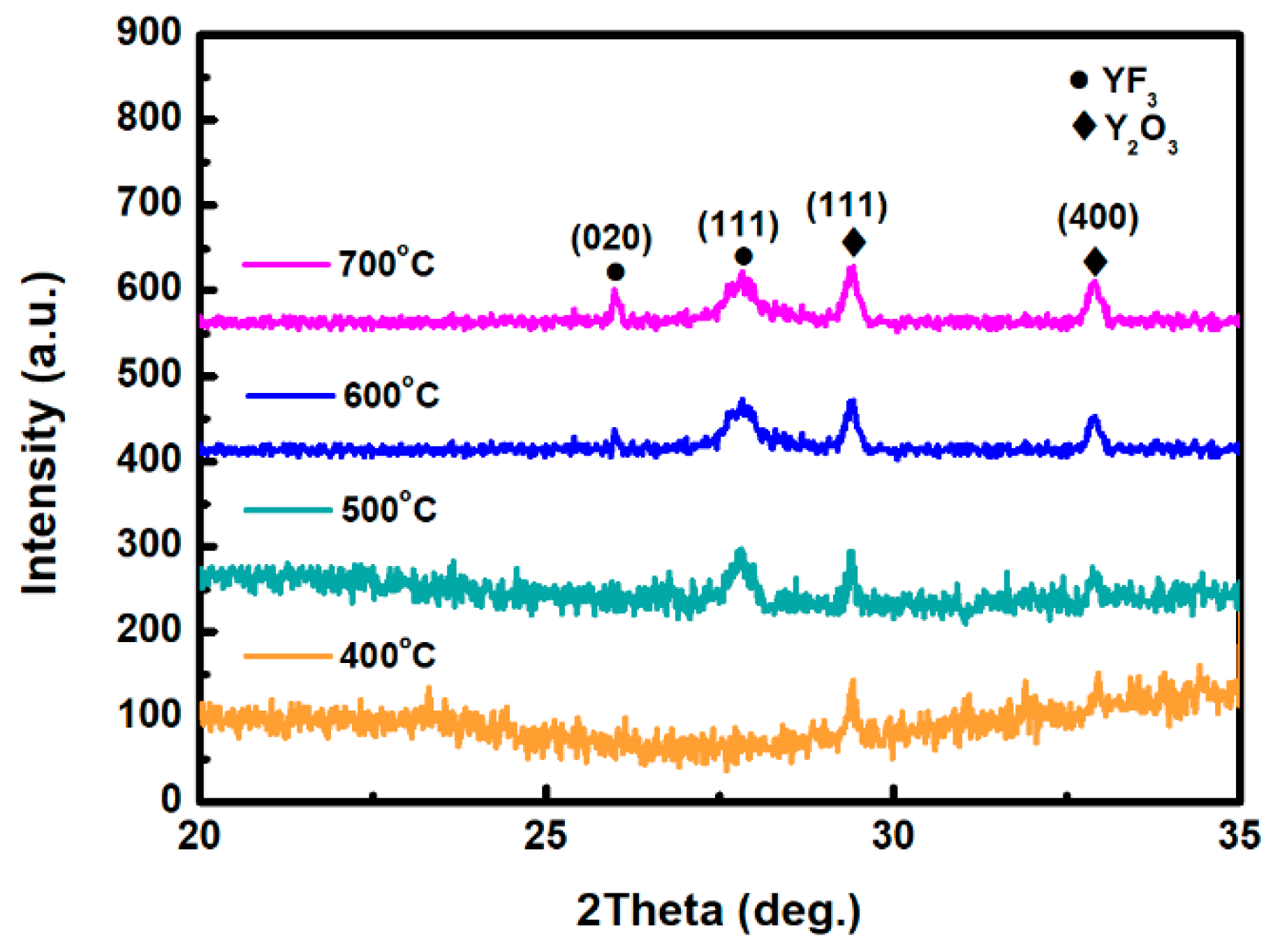
Figure 1 depicts the XRD scan patterns of YF
3 films deposited on Al
2O
3 substrate at 400, 500, 600, and 700 °C. All YF
3 samples were polycrystalline, and their structural orientations depended on the substrate temperature in the deposition process. The (020) plane of the orthorhombic YF
3 phase was formed at substrate temperatures above 500 °C, which is in agreement with the reported data (JCPDS card files No. 74-0911) [
11]. Moreover, the preferential orientation of the orthorhombic YF
3 crystal structure differed in films fabricated at different temperatures. This might be attributed to the high kinetic energy imparted to YF
3 molecules at high substrate temperatures, which enables them to migrate and rearrange on the substrate surface. YF
3 samples fabricated at higher temperatures also showed a weak peak of the cubic Y
2O
3 phase (400) plane (JCPDS card files No. 79-1716), indicating contamination by oxygen atoms during the sputtering process [
9]. As substrate temperature increases, Y
2O
3 formation might be favored by the higher instability of fluoride in the YF
3 crystal lattice. Unstable F ions in the lattice can be gradually replaced by environmental oxygen atoms. Therefore, the number of oxygen atoms in the crystal lattice increases with increasing temperature, forming Y
2O
3 (see
Figure 1). When YF
3 film was deposited at a lower substrate temperature, an amorphous structure was formed. Amorphous film usually has a loose structure, resulting in greater thickness and a higher growth rate. In contrast, with increasing substrate temperature, the YF
3 film became crystalline, and its crystal was relatively ordered. This indicates that a denser structure was found in the YF
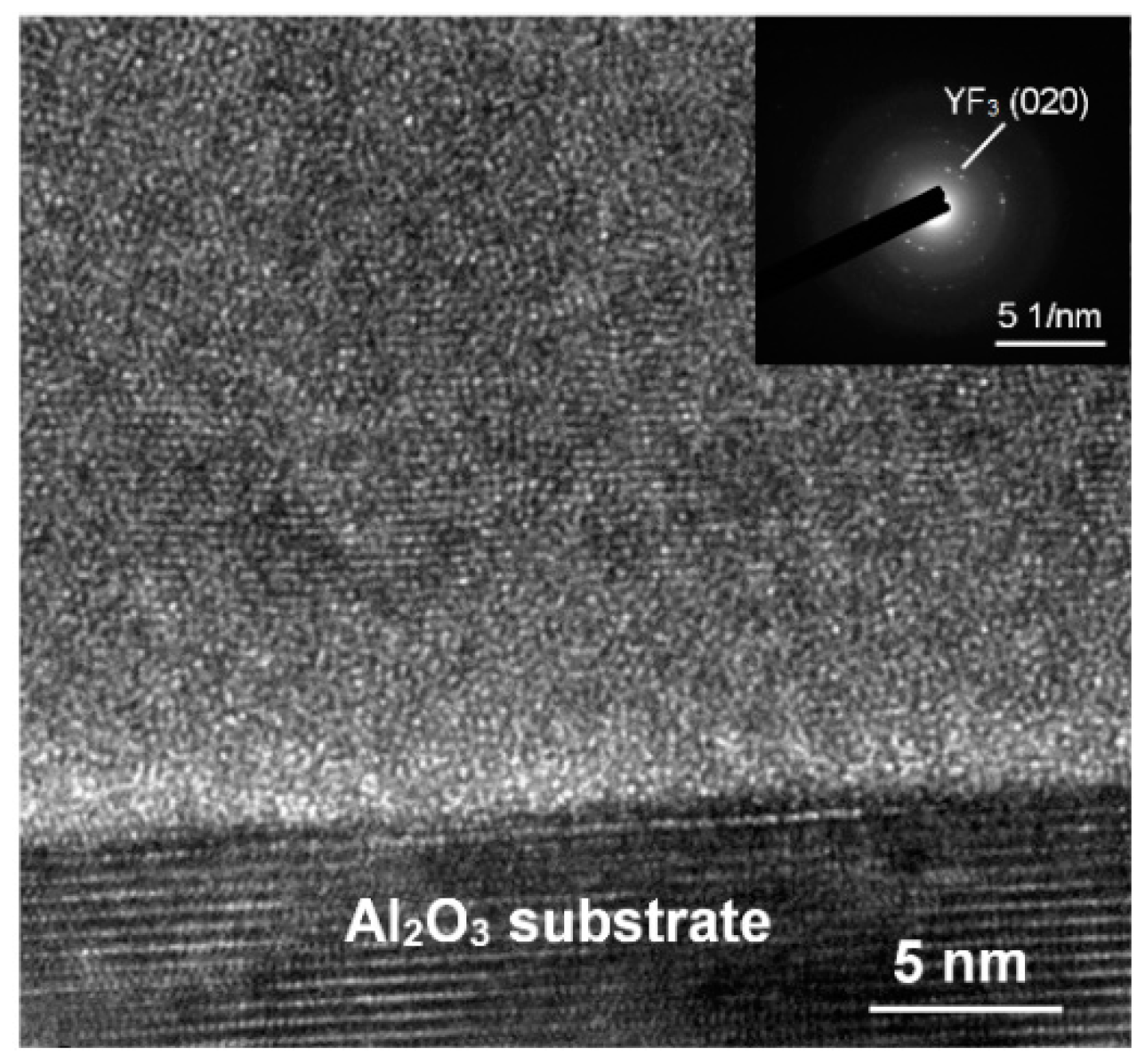
3 film grown at higher substrate temperatures, leading to its smaller thickness and lower growth rate. In TEM analysis, the YF
3 films exhibited nanocrystalline grains with an average size of 5–20 nm (
Figure 2). The regular ring-like electron diffraction pattern of a selected area (
Figure 2, inset) implies a polycrystalline structure of YF
3 film, consistent with the XRD result.
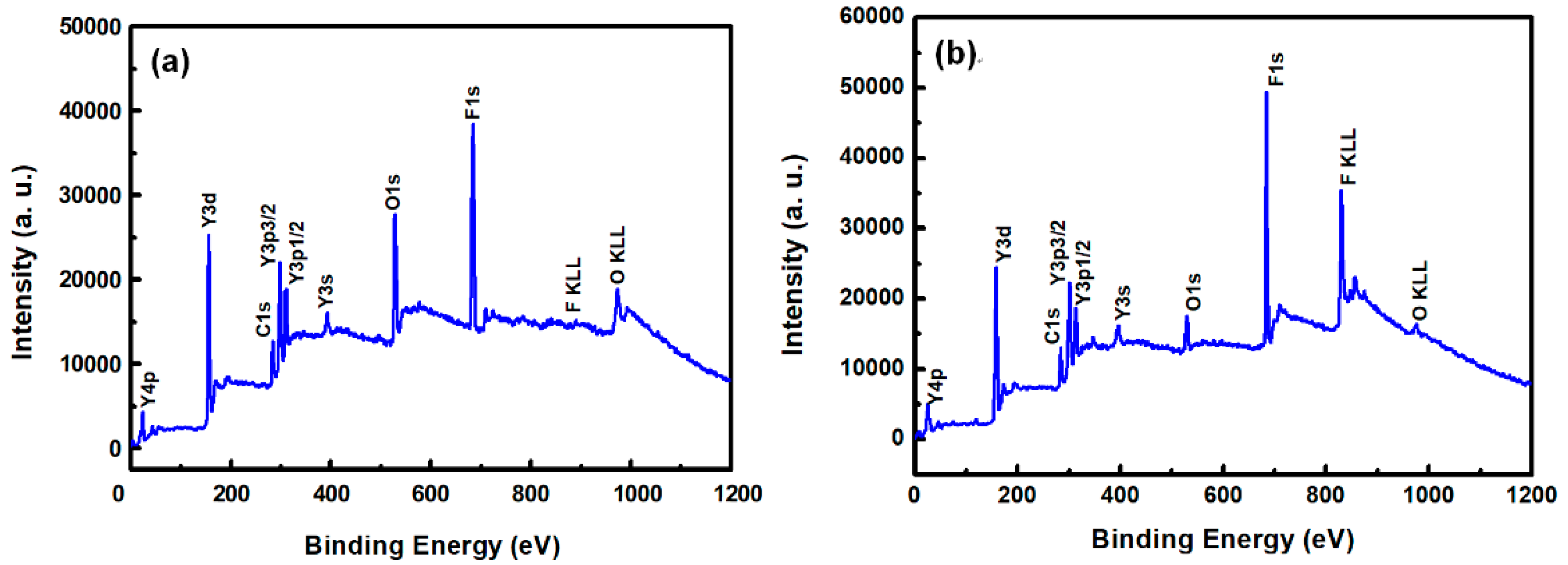
Figure 3a,b show the XPS survey spectra in the 0–1200 eV range of the YF
3 films before and after CF
4/O
2 plasma etching, respectively. To collect the atomic signals, all samples were bombarded by argon ions for 2 min. The XPS peaks in the as-grown YF
3 thin film (
Figure 3a) correspond to Y, F, O, and C elements. The Y and F elements existed in the original YF
3 film, and an O1s peak arose from oxygen contamination during the sputter deposition process. This result is consistent with previous reports [
12]. After exposure to CF
4/O
2 plasma, the spectrum of the YF
3 film exhibited an enhanced F1s peak and an additional C1s peak. The former feature is attributed to the high fluorine content of YF
3, and the latter indicates the formation of a carbon-polymer surface layer under fluorocarbon plasma etching (
Figure 3b).
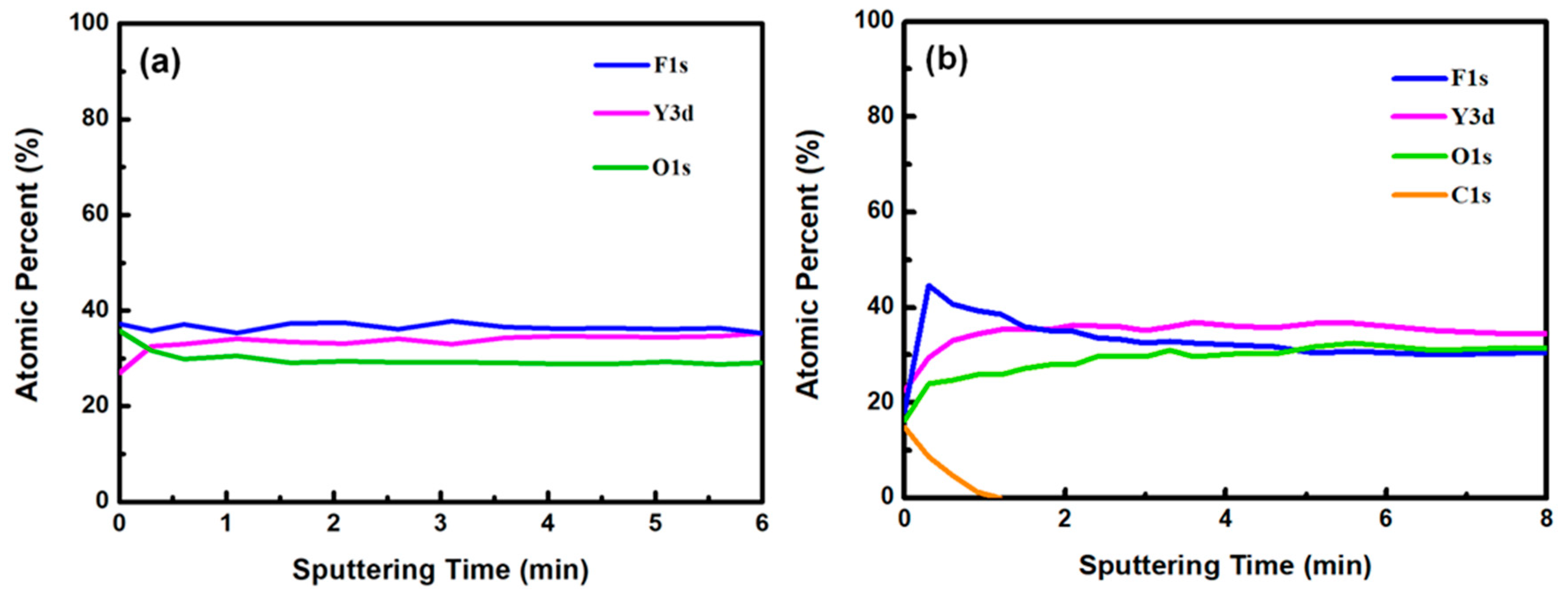
Figure 4a,b plot the compositions of the YF
3 films as functions of sputtering time before and after exposure to the CF
4/O
2 plasma, respectively. Before exposure to the plasma, the maximum F content was 37% and the minimum O content was 32% (
Figure 4a). This result indicates a two-phase mixture of YF
3 and Y
2O
3 in the film, consistent with a previous study that reported a deficiency of fluorine atoms during the sputter deposition process [
13]. The maximum percentage of F atoms in the etched sample was 44.69%, implying a fluorination layer on the YF
3 specimen (
Figure 4b). Similar results were observed in a previous study of surface fluorination by CF
4/O
2 plasma etching [
14]. The carbon content on the YF
3 surface decreased abruptly with sputtering time, indicating a very thin carbon polymer layer on the etched surface. This is because of interactions between the fluorocarbon plasma and Si-based materials, which generate carbon polymer and a SiF
xO
y reaction layer [
15].
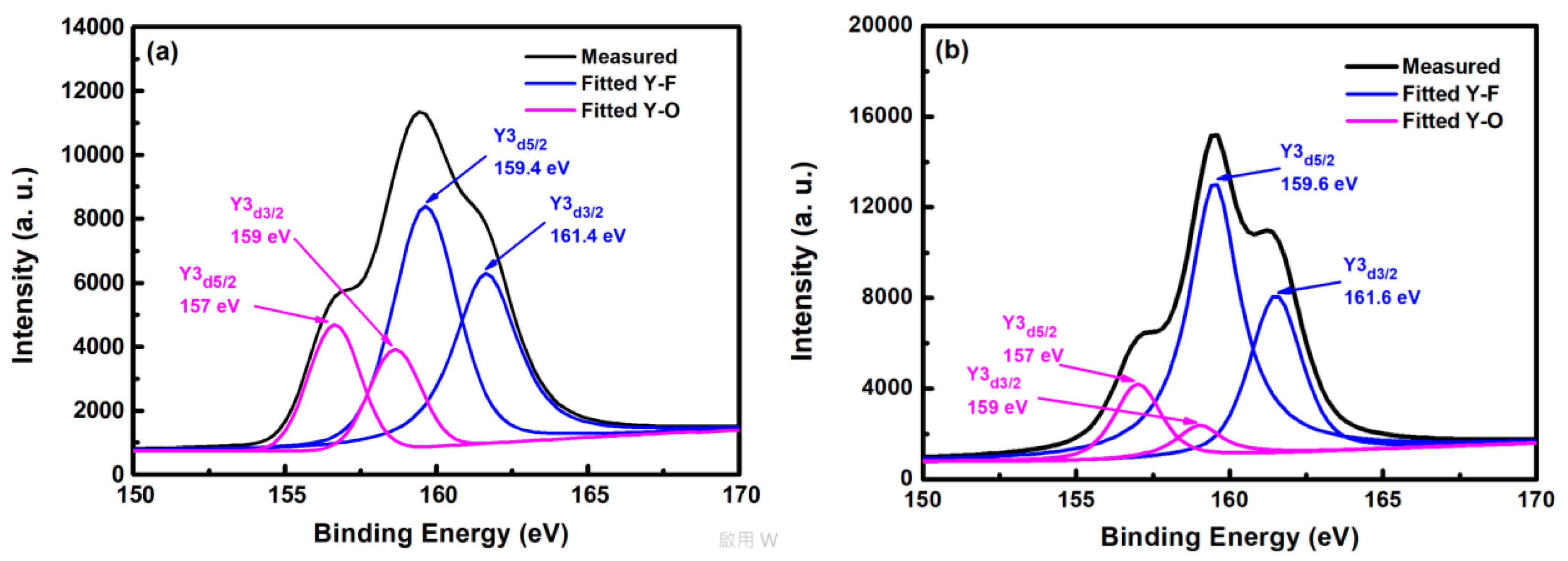
Figure 5 shows the XPS spectra of yttrium atoms of the YF
3 film after CF
4/O
2 plasma etching. In the curve-fitted XPS spectra of the YF
3 film, two peaks represent two bonding sources for Y cations, that is, the Y3d peak splits into a doublet (Y3d
5/2 and Y3d
3/2 electrons) with a binding-energy separation of 2 eV. The two yttrium bonding sources are consistent with the XPS standard [
16]. The Y3d
5/2 and Y3d
3/2 peaks in the spectrum of the as-deposited YF
3 film (
Figure 5a) deconvolute into four peaks. The two peaks located at higher binding energies (159.4 and 161.4 eV) correspond to Y–F bonding in the YF
3 film, whereas those at lower binding energies (157 and 159 eV) are ascribed to Y–O bonding. In the XPS spectra of etched YF
3 film surface, the peaks are more intense than in the un-etched specimen (
Figure 5b). Again, the strong doublet at higher binding energies (159.6 and 161.6 eV) and the weak doublet at lower binding energies (157 and 159 eV) correspond to Y–F and Y–O bonding, respectively. The higher XPS binding energy of the Y–F bonding can be attributed to the higher electronegativity of fluorine atoms (4.0) than oxygen atoms (3.5) [
17]. Consequently, more electrons are transferred to fluorine, decreasing the electron density around the cation and enhancing the binding energy. Meanwhile, the intensity ratio of Y–F to Y–O bonds was estimated as 2.1 on etched YF
3 films, indicating strong chemical interaction between the YF
3 films and the fluorocarbon plasma.
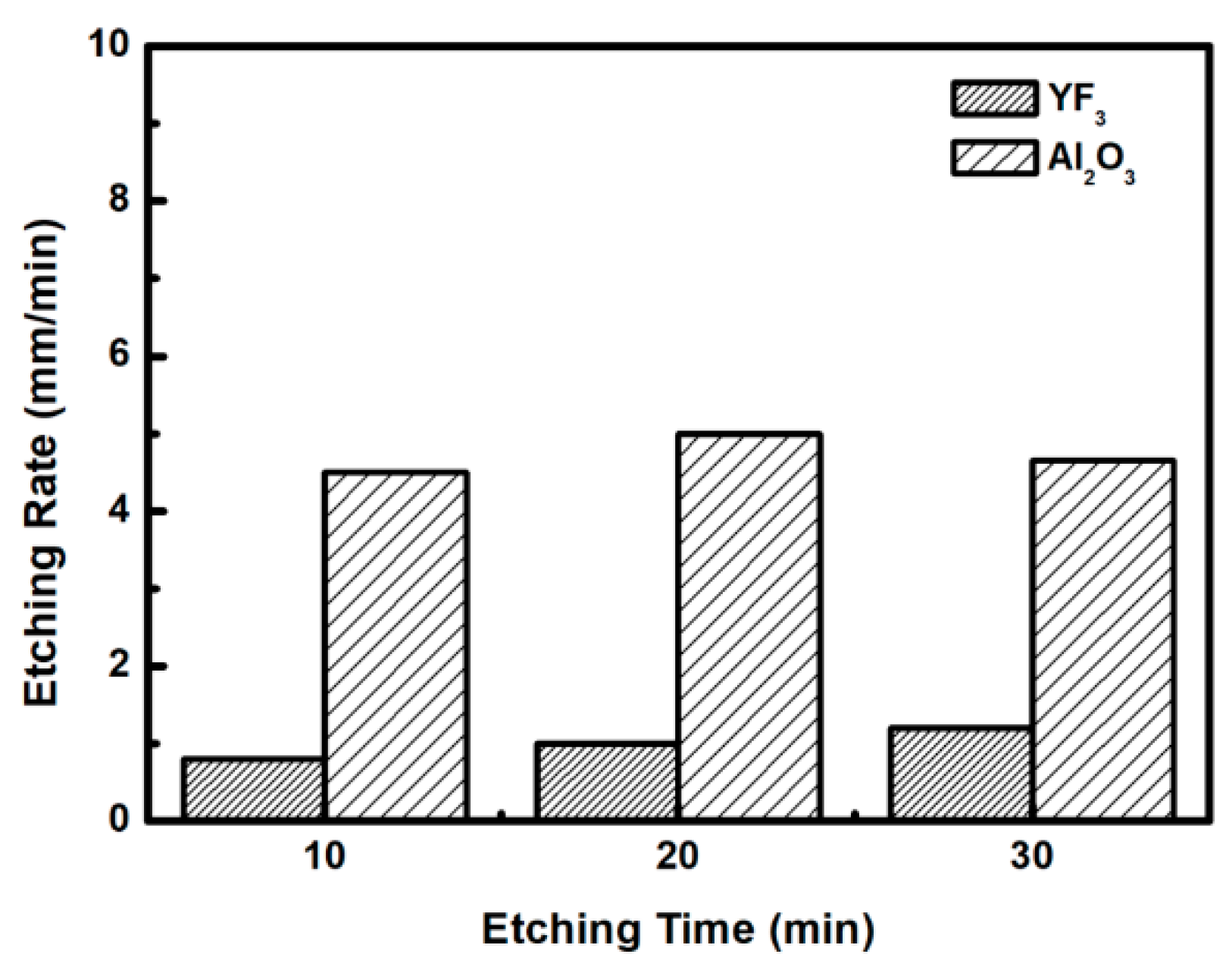
To evaluate the plasma erosion resistance of as-deposited YF
3 films, the etching rate of the YF
3-coated Al
2O
3 substrate was measured after exposure to the CF
4/O
2 plasma for different etching times. The etching rate of bare Al
2O
3 crystal was used as the reference.
Figure 6 shows that the etching depths of both samples linearly increased with increasing etching time. After 30 min, the etching depth reached 120 mm in the sapphire, but only 38 mm in YF
3. The erosion resistance of the YF
3 film to CF
4/O
2 plasma etching was more than three times higher than that of sapphire crystal. This is attributed to the chemical stability of YF
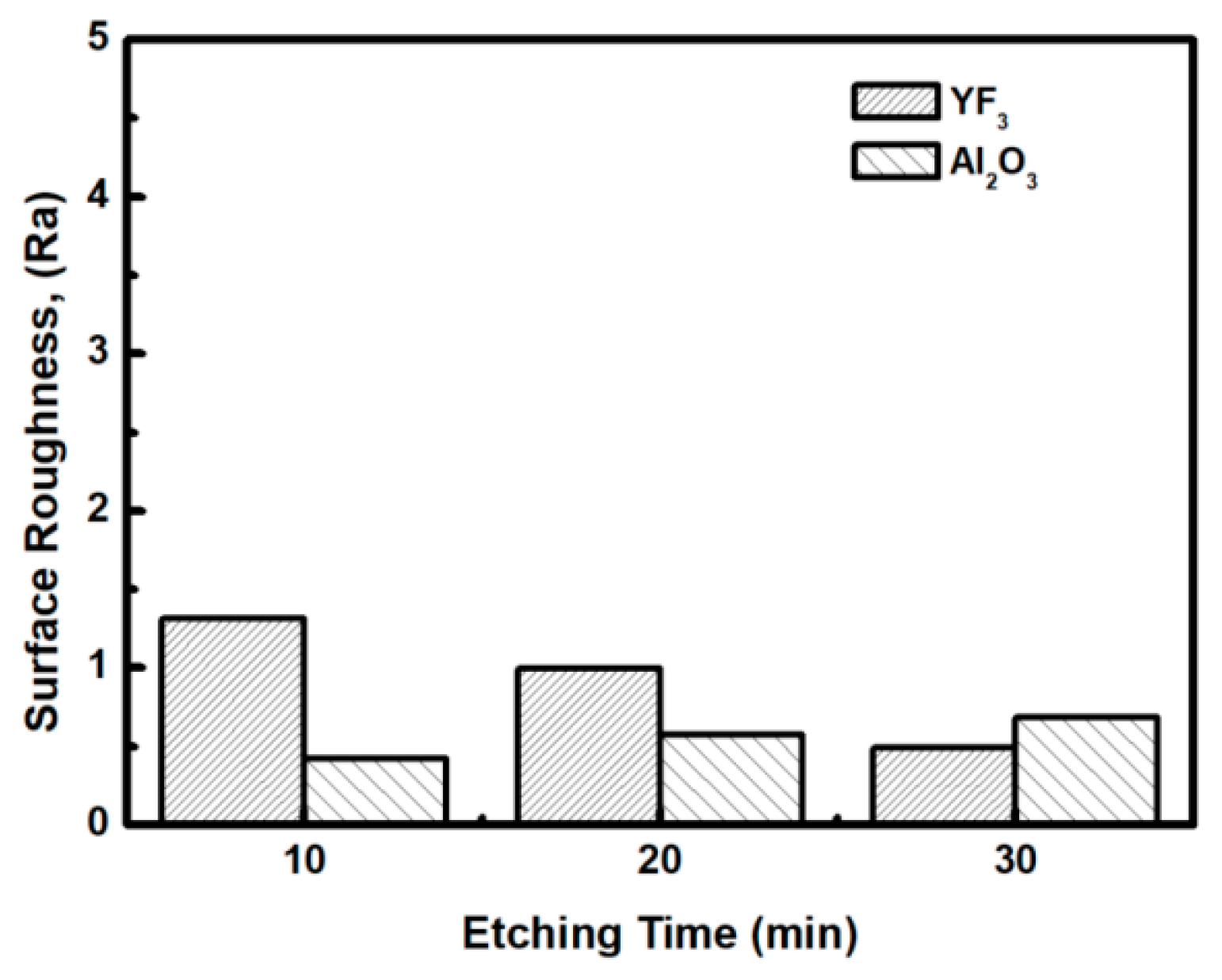
3 in a chemical environment dominated by fluorocarbon plasma. The surface morphologies and roughness of the YF
3 films etched for different lengths of time were measured by AFM measurements, with a scanning area of 5 m
2. The surface roughness results are shown in
Figure 7. The etching time of the CF
4/O
2 plasma did not remarkably influence the surface roughness of the YF
3 film.
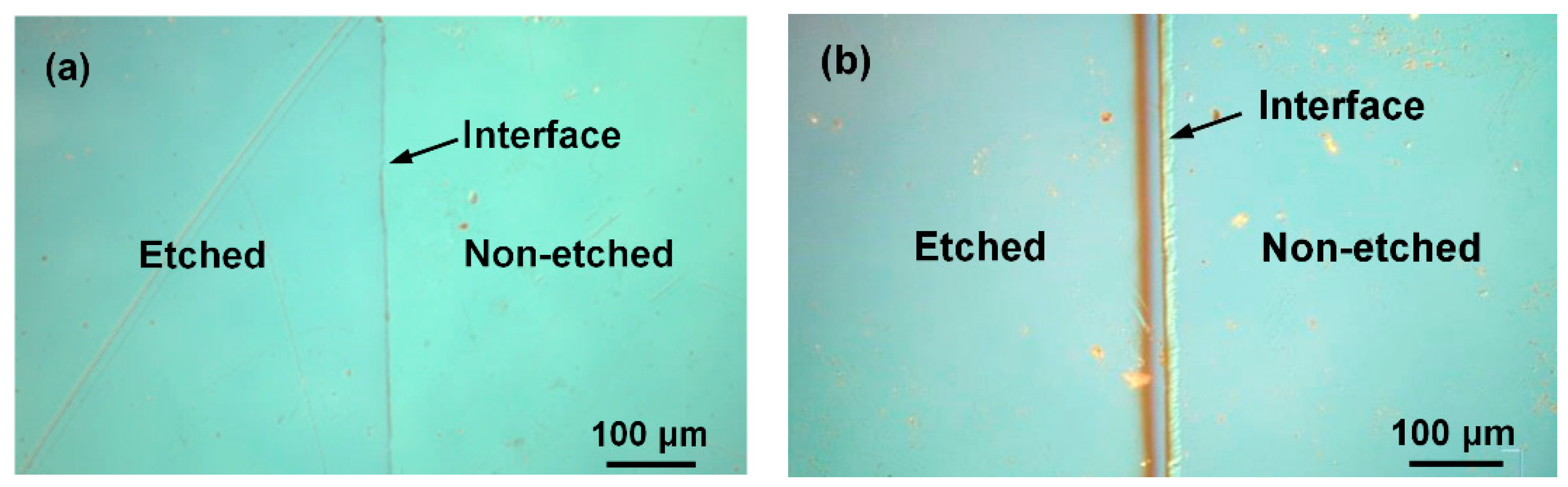
Figure 8a,b show the surface microstructures of YF
3 and Al
2O
3 samples, respectively, after the etching experiment. The micrographs were acquired by optical microscopy (OM). The right and left images in each panel show the un-etched and etched surfaces, respectively. The step height of the Al
2O
3 sample changed after CF
4/O
2 plasma etching. These images confirm the hardness and stronger CF
4/O
2 corrosion resistance of the YF
3 films compared to conventional Al
2O
3 substrate.
AlF
3 is a typical fluoride of Al
2O
3 materials [
4]. The boiling temperature of YF
3 (2230 °C) is higher than the sublimation temperature of AlF
3 (1275 °C). Hence, YF
3 is more stable and more difficult to vaporize than AlF
3. Therefore, its fluorinated layer can be removed by a physical sputtering process. The sputtering yields of the films are inversely proportional to sublimation enthalpies of their compounds [
18]. The sublimation enthalpy of YF
3 (481 ± 21 kJ/mol) is higher than that of AlF
3 (301 ± 4 kJ/mol). Moreover, the enthalpy of formation of the metal–oxygen bond is lower in YF
3 (−392 kJ mol
−1) than in Al
2O
3 (−279 kJ mol
−1). Therefore, YF
3 is chemically more stable than Al
2O
3 [
19]. In particular, YF
3 is extremely stable under the fluorocarbon plasma etching process.
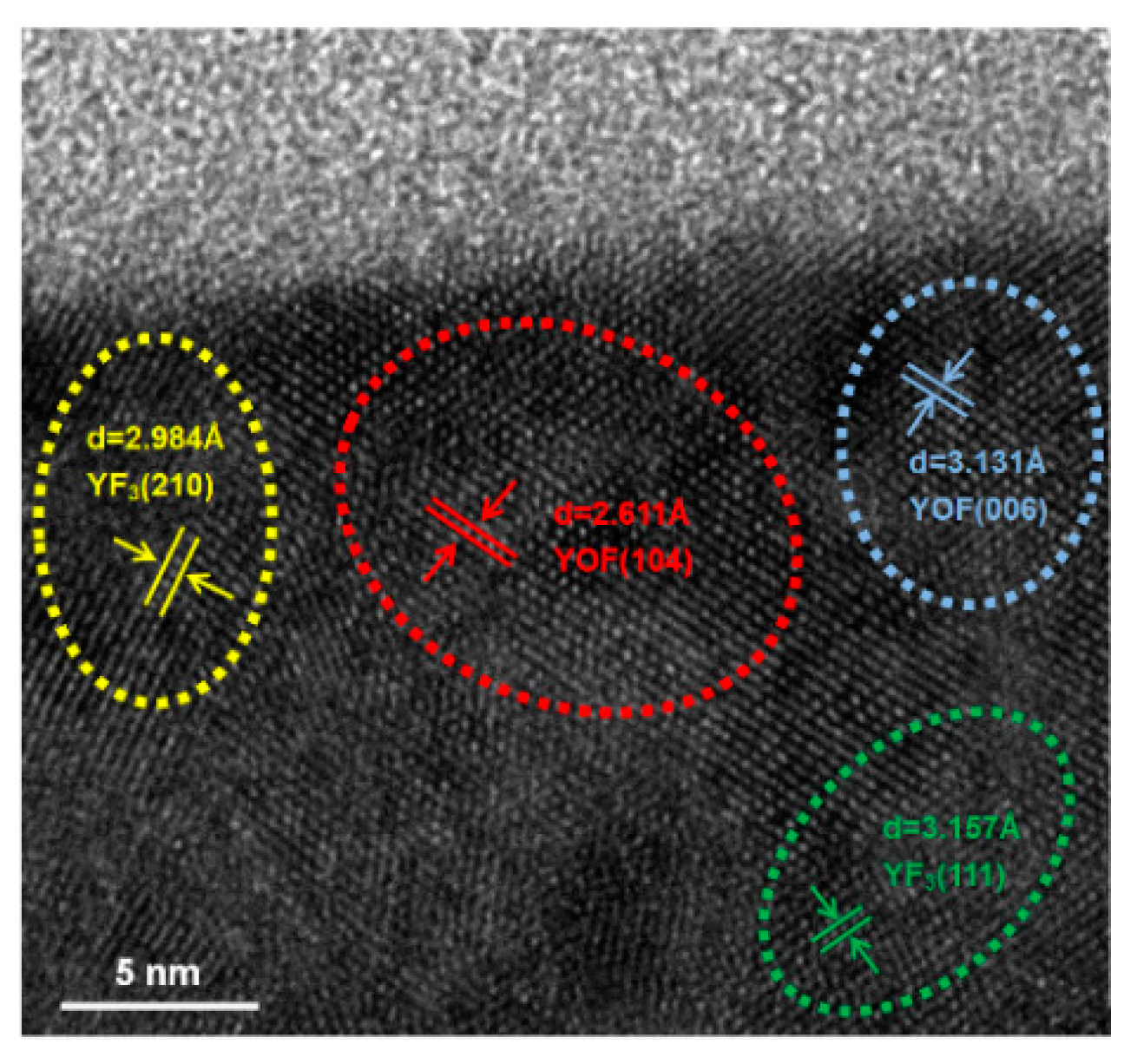
Figure 9 shows a cross-sectional HRTEM image of the YF
3 film after CF
4/O
2 plasma exposure. Continuous and nearly-complete lattice fringes were observed near the surface, indicating that the YF
3 ceramic crystalline lattice was not distorted by the fluorine plasma irradiation. Furthermore, the d-spacings of the near surface of film were 3.131 Å (blue dotted circle) and 2.621 Å (red dotted circle), very close to the d-spacings of the altered layers of yttrium oxyflouride (YOF): 3.147 Å (006) and 2.698 Å (104) [
11]. The thin YOF layer formed on the YF
3 surface plays a protective role, suppressing the particle generation during CF
4/O
2 plasma etching process. The reaction and formation of an altered YOF layer has been reported previously [
14]. Therefore, YF
3 film is a very attractive plasma-corrosion-resistant material that produces fewer contamination particles during the semiconductor fabrication process.