Cytotoxic, Genotoxic, and Polymorphism Effects on Vanilla planifolia Jacks ex Andrews after Long-Term Exposure to Argovit® Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Establishment and Culture Conditions
2.2. Silver Nanoparticles (AgNPs)
2.3. Effect of AgNPs on In Vitro Elongation and Rooting of V. planifolia
2.4. Genotoxic Effect of AgNPs on V. planifolia
2.4.1. Fixation and Staining of Root Tips
2.4.2. Scoring of Slides
2.5. Effect of AgNPs on Somaclonal Variation
2.5.1. DNA Isolation
2.5.2. ISSR-PCR Analysis
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
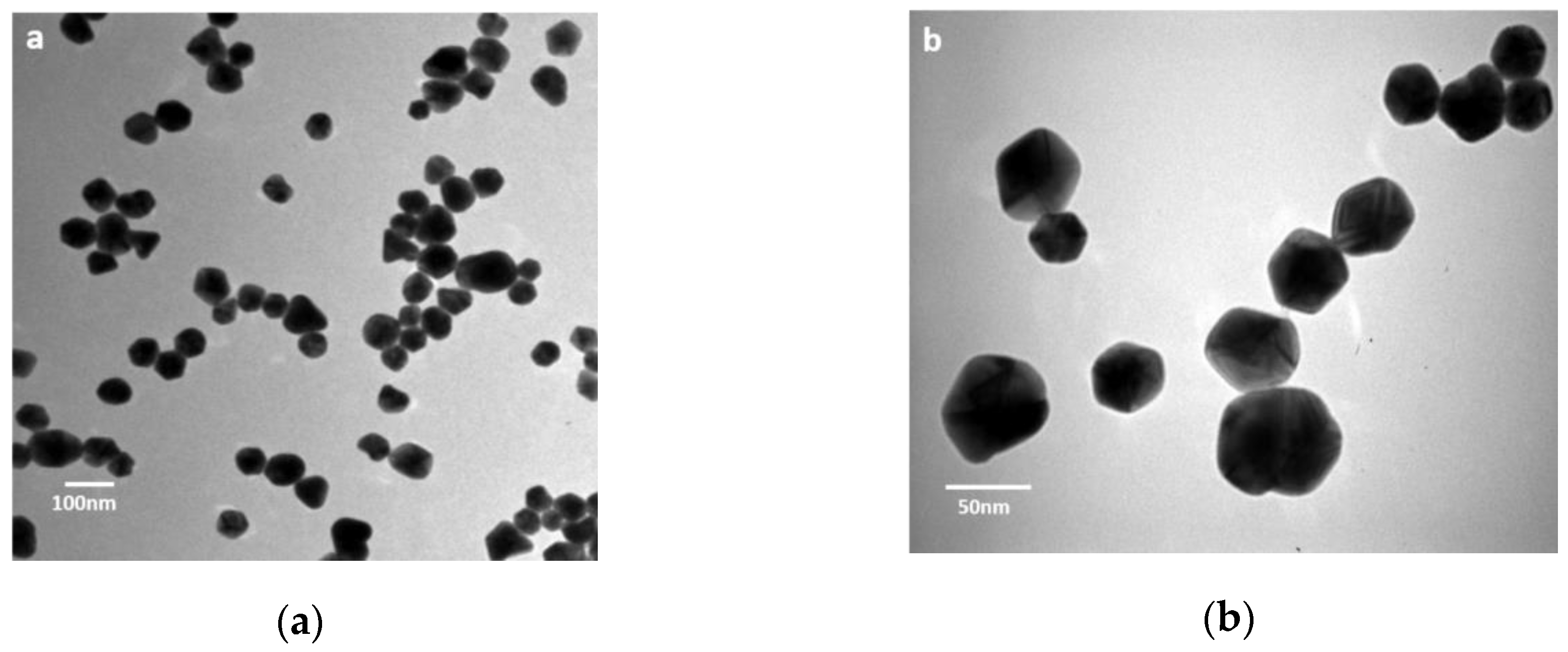
3.1. Characterization of AgNPs
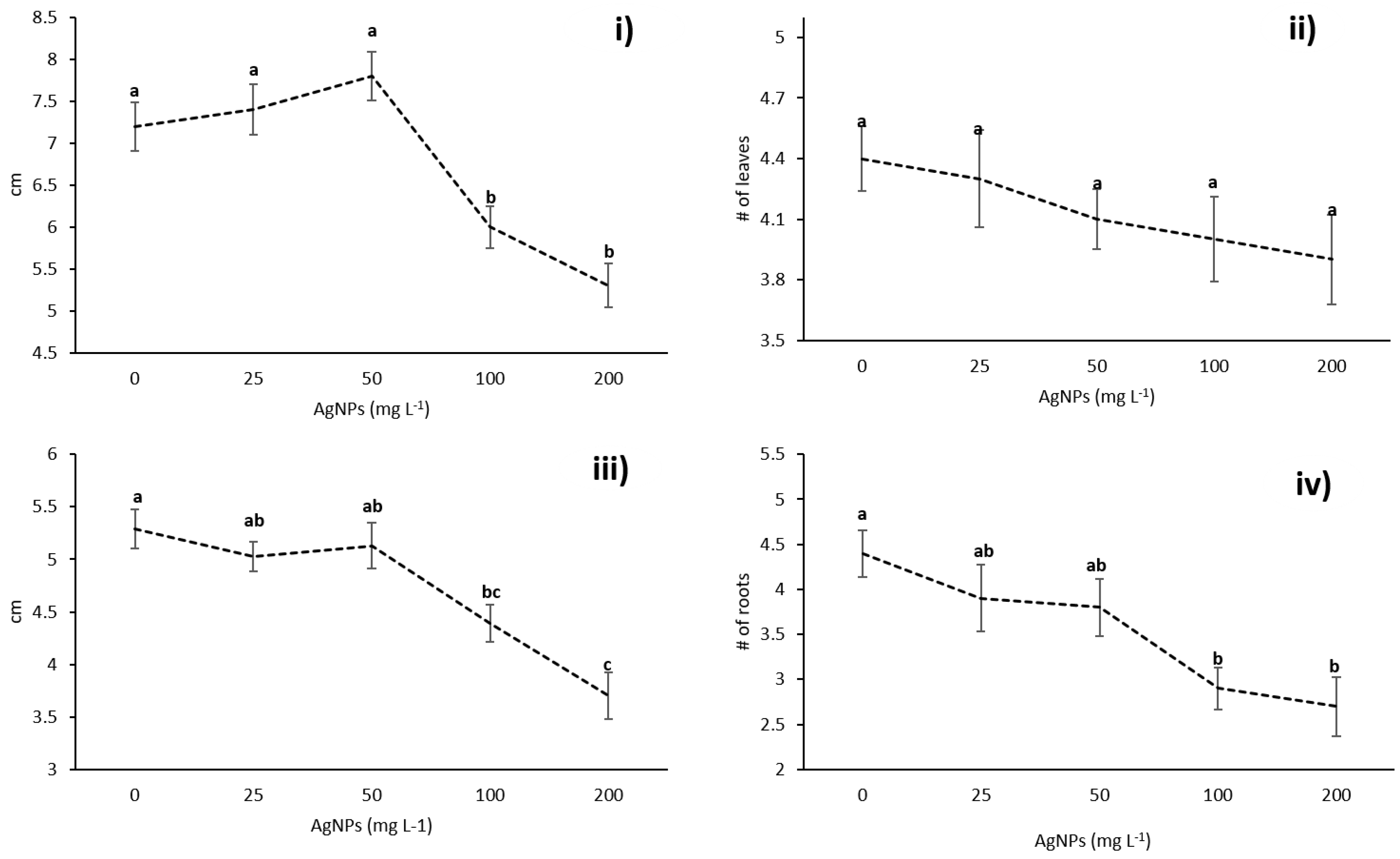
3.2. Effects of AgNPs on Vanilla planifolia Physiological Parameters
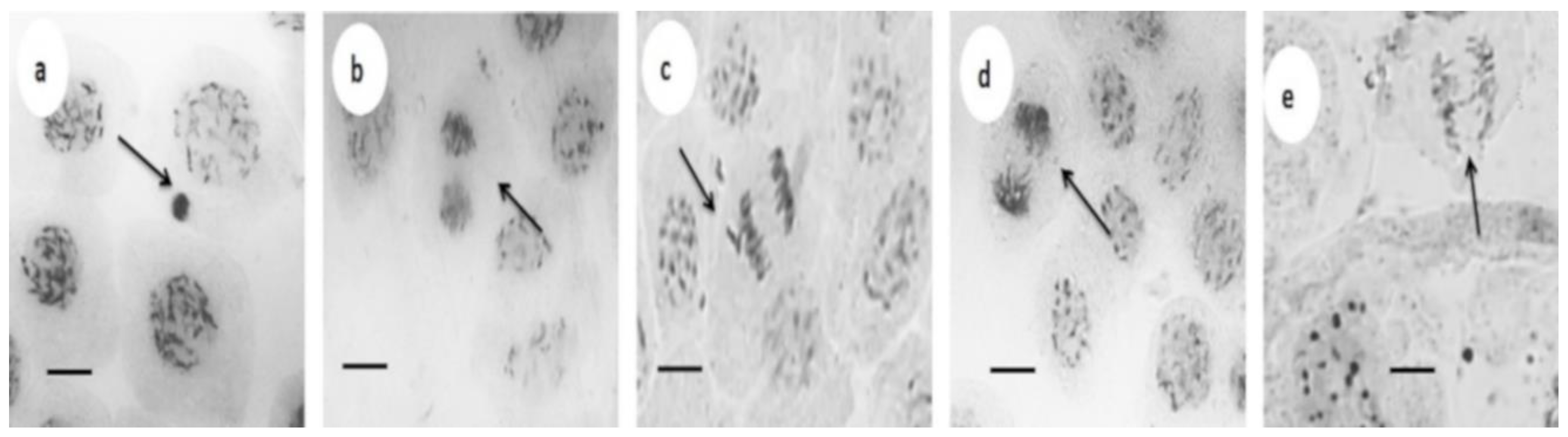
3.3. Cytotoxic and Genotoxic Effects
3.4. Somaclonal Variation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and hormetic effects of silver nanoparticles on in vitro regeneration of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. Plant Cell Tissue Organ Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, M.K.; Salehi, H. Silver Nanoparticles: An Influential Element in Plant Nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Raja muthuramalingam, T.; Shanmugam, C.; Gunasekaran, D.; Duraisamy, N.; Nagappan, R.; Krishnan, K. Bioactive bile salt-capped silver nanoparticles activity against destructive plant pathogenic fungi through in vitro system. RSC Adv. 2015, 5, 71174–71182. [Google Scholar] [CrossRef]
- Villamizar-Gallardo, R.; Cruz, J.F.O.; Ortíz, O.O. Fungicidal effect of silver nanoparticles on toxigenic fungi in cocoa. Pesqui. Agropecu. Bras. 2016, 51, 1929–1936. [Google Scholar] [CrossRef]
- Moradpour, M.; Aziz, M.A. Establishment of in vitro Culture of Rubber (Hevea brasiliensis) from Field-derived Explants: Effective Role of Silver Nanoparticles in Reducing Contamination and Browning. J. Nanomed. Nanotechnol. 2016, 7, 375. [Google Scholar] [CrossRef]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano silver: A novel nanomaterial for removal of bacterial contaminants in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant. 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Mehrian, S.K.; De Lima, R. Nanoparticles cyto and genotoxicity in plants: Mechanisms and abnormalities. Environ. Nanotechnol. Monit. Manag. 2016, 6, 184–193. [Google Scholar] [CrossRef]
- Yanga, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Cabrera, G.L.; Rodriguez, D.M.G. Genotoxicity of soil from farmland irrigated with wastewater using three plant bioassays. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 426, 211–214. [Google Scholar] [CrossRef]
- Wolfe, A.D. ISSR techniques for evolutionary biology. Methods Enzymol. 2005, 395, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, N.; Gill, B.S.; Grover, I.S.; Murin, A.; Osiecka, R.; Sandhu, S.S.; Andersson, H.C. Vicia faba chromosomal aberration assay. Mutat. Res. Regul. Pap. 1994, 310, 231–247. [Google Scholar] [CrossRef]
- Stewart, C.N., Jr.; Via, L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. Biotechniques 1993, 14, 748–751. [Google Scholar] [PubMed]
- Bello-Bello, J.J.; Chavez-Santoscoy, R.A.; Lecona-Guzmán, C.A.; Bogdanchikova, N.; Salinas-Ruíz, J.; Gómez-Merino, F.C.; Pestryakov, A. Hormetic response by silver nanoparticles on in vitro multiplication of sugarcane (Saccharum spp. Cv. Mex 69-290) using a temporary immersion system. Dose-Response 2017, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Shweta; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health 2012, 9, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Manivannan, J.; Sinha, S.; Chakraborty, A.; Mallick, S.K.; Bandyopadhyay, M.; Mukherjee, A. In vitro and in vivo genotoxicity of silver nanoparticles. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 749, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, H.M.; Moustafa, Y. Physiologycal and Cytogenetic Response of Wheat and Barley to Silver Nanopriming Treatment. Int. J. Appl. Biol. Pharm. Technol. 2014, 5, 150–163. [Google Scholar]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of Silver Nanoparticles on Growth of Eukariotic Green Algae. Nano-Micro Lett. 2012, 4, 158–165. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Milošić, A.; Domijan, A.M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, M.K.; Niazi, A.; Salehi, H.; Abolimoghadam, A. Silver nanoparticles affect ACS expression in Tecomella undulata in vitro culture. Plant Cell Tissue Organ Cult. 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Çekiç, F.Ö.; Ekinci, S.; İnal, M.; Ünal, D. Silver nanoparticles induced genotoxicity and oxidative stress in tomato plants. Turkish J. Biol. 2017, 41, 700–707. [Google Scholar] [CrossRef]
- Panda, K.K.; Achary, V.M.M.; Phaomie, G.; Sahu, H.K.; Parinandi, N.L.; Panda, B.B. Polyvinyl polypyrrolidone attenuates genotoxicity of silver nanoparticles synthesized via green route, tested in Lathyrus sativus L. root bioassay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 806, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. Rev. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014, 641759. [Google Scholar] [CrossRef] [PubMed]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.A.T.; Dobránszki, J.; Winarto, B.; Zeng, S. Anthurium in vitro: A review. Sci. Hortic. 2015, 186, 266–298. [Google Scholar] [CrossRef]
- Dobránszki, J.; Teixeira da Silva, J.A. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy-Trojanowska, M.; Bolibok, H. Characteristics and a Comparison of Three Classes of Microsatellite-Based Markers and Their Application in Plants. Cell. Mol. Biol. Lett. 2004, 9, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, M.; Babu, K.N.; Ravindran, P.N.; Peter, K.V.; Plant, A.J.; Res, S. Biotechnology for micropropagation and enhancing variations in Vanilla. Asian J. Plant Sci. Res. 2015, 5, 52–62. [Google Scholar]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia Jacks. Plant Cell Tissue Organ Cult. 2015, 123, 657–664. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales. Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo; Diario Oficial de la Federación: Mexico City, Mexico, 2010; pp. 1–64. [Google Scholar]

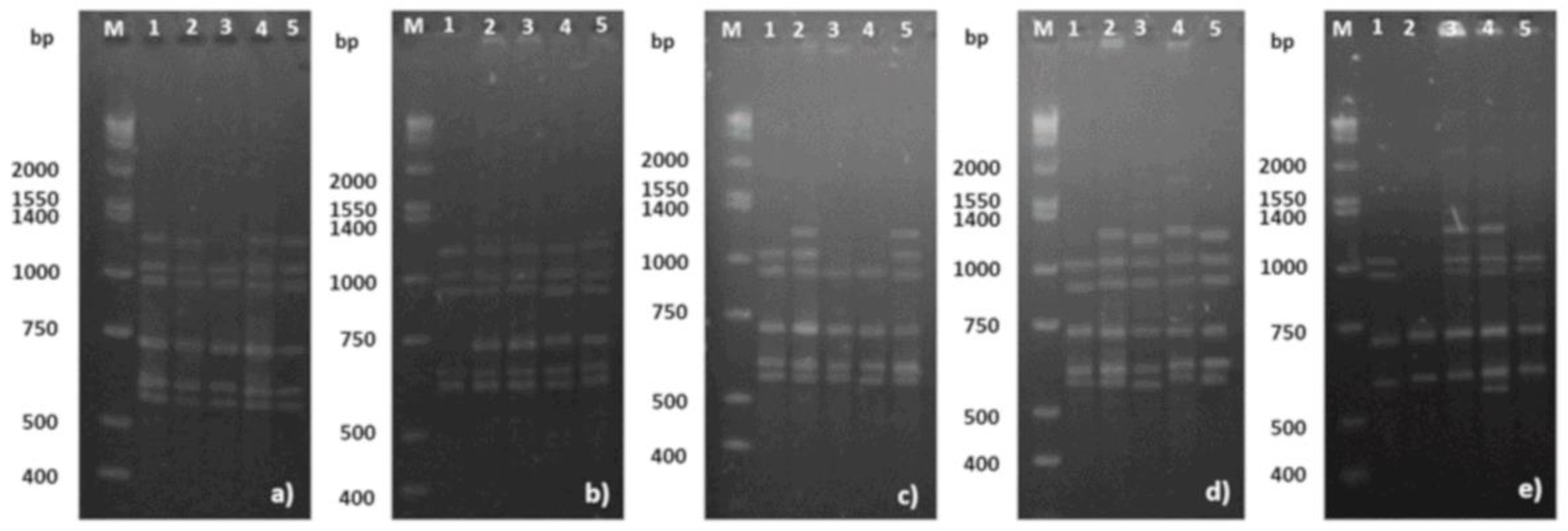
| Primer | Sequence (5′–3′) | °Tm (°C) 2 | No. of Bands | Range (bp) 3 | Polymorphism (%) |
|---|---|---|---|---|---|
| UBC 809 | AGAGAGAGAGAGAGAGG | 45 | 10 | 300–2000 | 30 |
| T 06 | AGAGAGAGAGAGAGAGGT | 50 | 9 | 300–1550 | 66.66 |
| UBC 840 | GAGAGAGAGAGAGAGAYT 1 | 50 | 8 | 200–1400 | 25 |
| UBC 836 | AGAGAGAGAGAGAGAGYA 1 | 50 | 7 | 200–1550 | 42.86 |
| UBC 812 | GAGAGAGAGAGAGAGAA | 50 | 7 | 200–1400 | 28.57 |
| UBC 825 | ACACACACACACACACT | 51 | 6 | 500–3000 | 83.33 |
| UBC 808 | AGAGAGAGAGAGAGAGC | 52 | 10 | 200–750 | 60 |
| T 05 | CGTTGTGTGTGTGTGTGT | 54 | 8 | 300–2000 | 25 |
| C 07 | GAGAGAGAGAGAGAGAC | 56 | 7 | 300–1400 | 71.43 |
| Properties | Mean |
|---|---|
| Metallic silver content | 12 mg/L |
| Form Factor (Spheroid) | 0.82 |
| Roundness | 0.88 |
| Size interval of metallic silver particles by TEM (nm) | 1–80 |
| Average diameter by TEM (nm) | 38 ± 15 |
| Zeta potential (mV) | −15 |
| Surface plasmon resonance (nm) | 420 |
| AgNPs (mg L−1) 1 | Cells in Division | Mitotic Index (%) | Aberration (%) 2 | Total Aberration (%) | |||
|---|---|---|---|---|---|---|---|
| CB | CF | BC | MN | ||||
| 0 | 2582 ± 92 * | 88.21 ± 0.48 * | 0.03 | 0 | 0 | 0 | 0.03 ± 0.00 * |
| 25 (1.5) | 2348 ± 75 * | 83.18 ± 1.16 * | 0.10 | 0.08 | 0 | 0 | 0.18 ± 0.06 * |
| 50 (3.0) | 2338 ± 87 * | 82.15 ± 2.40 * | 0.30 | 0.50 | 0.17 | 0 | 0.97 ± 0.03 ** |
| 100 (6.0) | 1786 ± 99 ** | 60.35 ± 0.90 ** | 1.5 | 1.0 | 0.16 | 1.5 | 4.16 ± 0.17 *** |
| 200 (12.0) | 1018 ± 72 *** | 33.53 ± 1.91 *** | 1.5 | 1.4 | 1.0 | 3.0 | 6.90 ± 0.22 **** |
| Plant | AgNPs Source and Physicochemical Properties | Active Component Concentration (Metallic Silver Content) | Exposure Time and (AgNPs) Used | DNA Damage or Genotoxic Effect | Ref. |
|---|---|---|---|---|---|
| Vanilla planifolia | Commercial Vector-Vita PVP-AgNPs Size: 35 ± 15 nm, coating agent: PVP; ζ potential: −15 mV; hydrodynamic diameter: 70 nm | Metallic silver content quantified by ICP-OES 1.5, 3, 6, and 12 mg/L of metallic silver 13.9, 27.8, 55.6, and 111.25 µM of metallic silver | 42 days (1008 h) 25, 50, 100, and 200 mg/L of AgNPs | A dose-dependent increase in the frequency of cells with CA. 1.5 and 3 MN were observed in 3000 counted cells for the concentrations 100 and 200 mg/L, respectively | This work |
| Allium cepa | Commercial Sigma-Aldrich size: <100 nm, purity: 99.5% trace metal basis, coating agent: NR | NR | 4 h 25, 50, 75, and 100 mg/L | CA and cell disintegration. | [20] |
| Vicia faba | Commercial Ocean Nanotech LLC, size: 60 nm; purity: 99.5% trace metal basis, coating agent: NR Characterization made by the authors Size: 63 ± 41 nm, ζ potential: −33.2 mV | NR | 4 h of exposure and 24 h of recovery 12.5, 25, 50, and 100 mg/L | Dose-dependence increase in the frequency of cells with CA and MN. MN frequency with 100 mg/L of AgNPs is triplicated compared with control (control 5.86 ± 0.66; AgNPs 100 mg/L: 18.4 ± 0.75). | [21] |
| Nicotiana tabacum | Commercial Sigma-Aldrich Size: <100 nm, purity: 99.5% trace metal basis, coating agent: NR Characterization made by the authors size: TEM 70–130 nm, av. ~125 nm; SEM: 90–180 nm, av. 120 nm; ζ potential: −4.86 mV | NR | 24 h 25, 50, and 75 mg/L | No damage was observed in nuclei isolated from shoots. Nuclei isolated from roots exposed to 50 and 75 µg/mL shown DNA damage determined by comet assay. Dose-dependence for DNA damage. | [22] |
| Triticum durum Desf. cv. Beni Sweif 1 | Synthesis, spherical, size: ~20 nm; coating agent: NR | NR | Soaked by 24 h in AgNPs solution and germinated by a period of 72 and 120 h, respectively. No concentrations reported | Time-dependent increase in the CA and MN frequency | [23] |
| Hordeum vulgare L. cv. Giza 130 | Synthesis, spherical, size: ~20 nm; coating agent: NR | NR | Soaked by 24 h in AgNPs solution and germinated by a period of 72 and 120 h, respectively. No concentrations reported | Time-dependent increase in the CA and MN frequency | [23] |
| Pithophora oedogonia (Mont.) Wittrock/Chara vulgaris Linn. | Synthesis; size: 10–15 nm, coating agent: NR | NR | 5 and 10 days 0.9 and 1.5 mM | CA observed with 0.9 mM after exposure of 5 days. Longer exposure (10 days) or higher concentrations enhance the magnitude of CA. | [24] |
| Triticum aestivum L. | Green synthesis: Rhodophyta extraction + AgNO3 Chemical synthesis: NaOH + AgNO3 + PEG No characterization data, coating agent: NR | NR | 8, 16, and 24 h 10, 20, 40, and 50 mg/L | Both AgNPs showed concentration- and time-dependent increase in the frequency of cells with CA and MN. | [25] |
| Triticum aestivum L. cv. Blasco | Commercial nanoComposix Size: 10 nm, coating agent: PVP Characterization made by the authors Size: 13.2 nm | NR | Soaked by 4 h in 32 mL of 1 and 10 mg/L PVP-AgNPs solution, respectively. | No differences between the genetic polymorphism of roots treated with AgNPs and control samples by AFLP. | [14] |
| Allium cepa | Synthesis AgNPs-citrate, size: 61.2 ± 33.9 nm; TEM: rod-like; ζ potential: −39.8 ± 3.4 mV AgNPs-PVP, size: 9.4 ± 1.3 nm; TEM: spherical; ζ potential: −4.8 ± 0.6 mV AgNPs-CTAB, size: 5.6 ± 2.1 nm; TEM: spherical; ζ potential: 42.5 ± 2.7 mV | Metallic silver content quantified by ICP-MS for each sample 25, 50, 75, and 100 µM | 72 h 25, 50, 75, and 100 µM | No DNA damage was observed with any of the AgNPs-citrate concentrations employed. Increase in tail DNA was recorded after exposure to AgNPs-PVP at 100 μM concentration. AgNPs-CTAB produces DNA damage only with 50 μM concentration. | [26] |
| Tecomella undulata (Roxb.) Seem. | NR | NR | 16 days (384 h) 30, 60, and 120 mg/L | More than 30 mg/L of AgNPs decreases ACS expression levels | [27] |
| Solanum lycopersicum L. | Commercial Sigma-Aldrich (Catalog number 576832) Nanopowder, size: <100 nm, PVP as dispersant, purity: 99.5% trace metal basis | NR | 14 days (336 h) 10, 20, 40, and 80 mg/L | GTS decreases as AgNPs concentration increases. | [28] |
| Lathyrus sativus L. | Synthesis. All have shown spherical shape AgNPsI: AgNO3 + extract. 14 ± 5.4 nm AgNPsII: AgNO3 + TSC+ extract. 19.2 ± 6.6 nm AgNPsIII: AgNO3 + TSC + PVPV + extract. 18.8 ± 6.6 nm AgNPsIV: AgNO3 + TSC + PVP + extract. 44.6 ± 13.2 nm | NR | Exposure for 3 h and recovery time of 4, 8, 12, and 24 h 1, 5, 10, 20, and 40 mg/L | Authors report that all AgNPs induce genotoxic effects from the concentration of 1 mg/L, with exception of AgNPsIV which induced genotoxicity only at the higher concentration of 40 mg/L. | [29] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello-Bello, J.J.; Spinoso-Castillo, J.L.; Arano-Avalos, S.; Martínez-Estrada, E.; Arellano-García, M.E.; Pestryakov, A.; Toledano-Magaña, Y.; García-Ramos, J.C.; Bogdanchikova, N. Cytotoxic, Genotoxic, and Polymorphism Effects on Vanilla planifolia Jacks ex Andrews after Long-Term Exposure to Argovit® Silver Nanoparticles. Nanomaterials 2018, 8, 754. https://doi.org/10.3390/nano8100754
Bello-Bello JJ, Spinoso-Castillo JL, Arano-Avalos S, Martínez-Estrada E, Arellano-García ME, Pestryakov A, Toledano-Magaña Y, García-Ramos JC, Bogdanchikova N. Cytotoxic, Genotoxic, and Polymorphism Effects on Vanilla planifolia Jacks ex Andrews after Long-Term Exposure to Argovit® Silver Nanoparticles. Nanomaterials. 2018; 8(10):754. https://doi.org/10.3390/nano8100754
Chicago/Turabian StyleBello-Bello, Jericó Jabín, José Luis Spinoso-Castillo, Samantha Arano-Avalos, Eduardo Martínez-Estrada, María Evarista Arellano-García, Alexey Pestryakov, Yanis Toledano-Magaña, Juan Carlos García-Ramos, and Nina Bogdanchikova. 2018. "Cytotoxic, Genotoxic, and Polymorphism Effects on Vanilla planifolia Jacks ex Andrews after Long-Term Exposure to Argovit® Silver Nanoparticles" Nanomaterials 8, no. 10: 754. https://doi.org/10.3390/nano8100754









