Direct Growth of Bismuth Film as Anode for Aqueous Rechargeable Batteries in LiOH, NaOH and KOH Electrolytes
Abstract
:1. Introduction
2. Results and Discussion
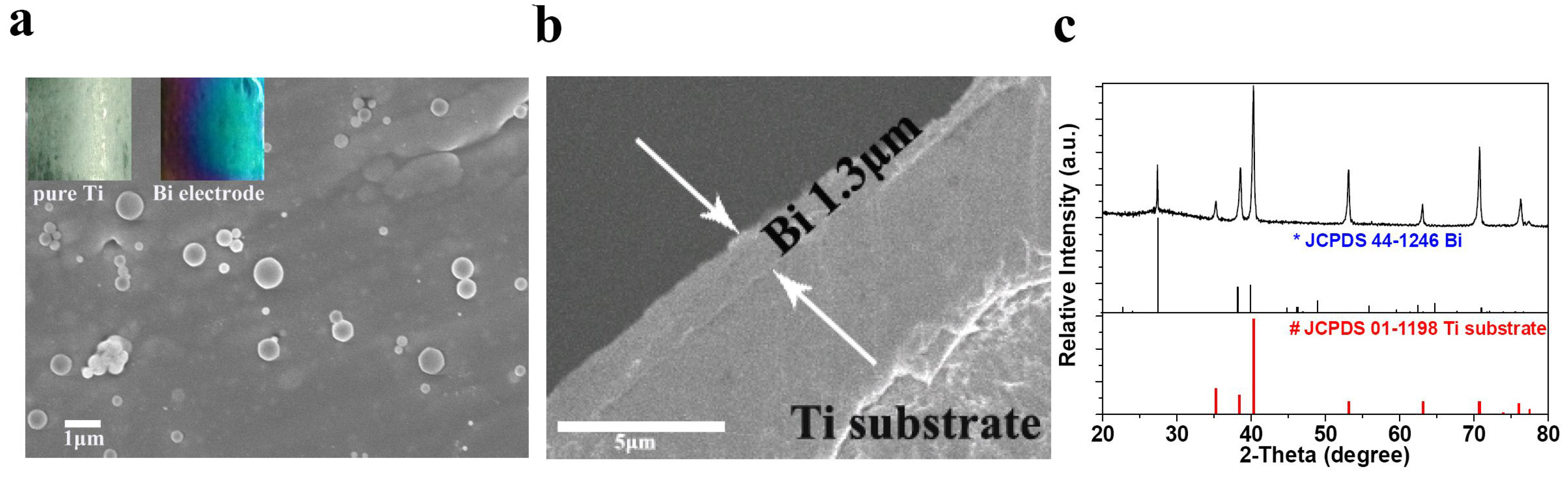
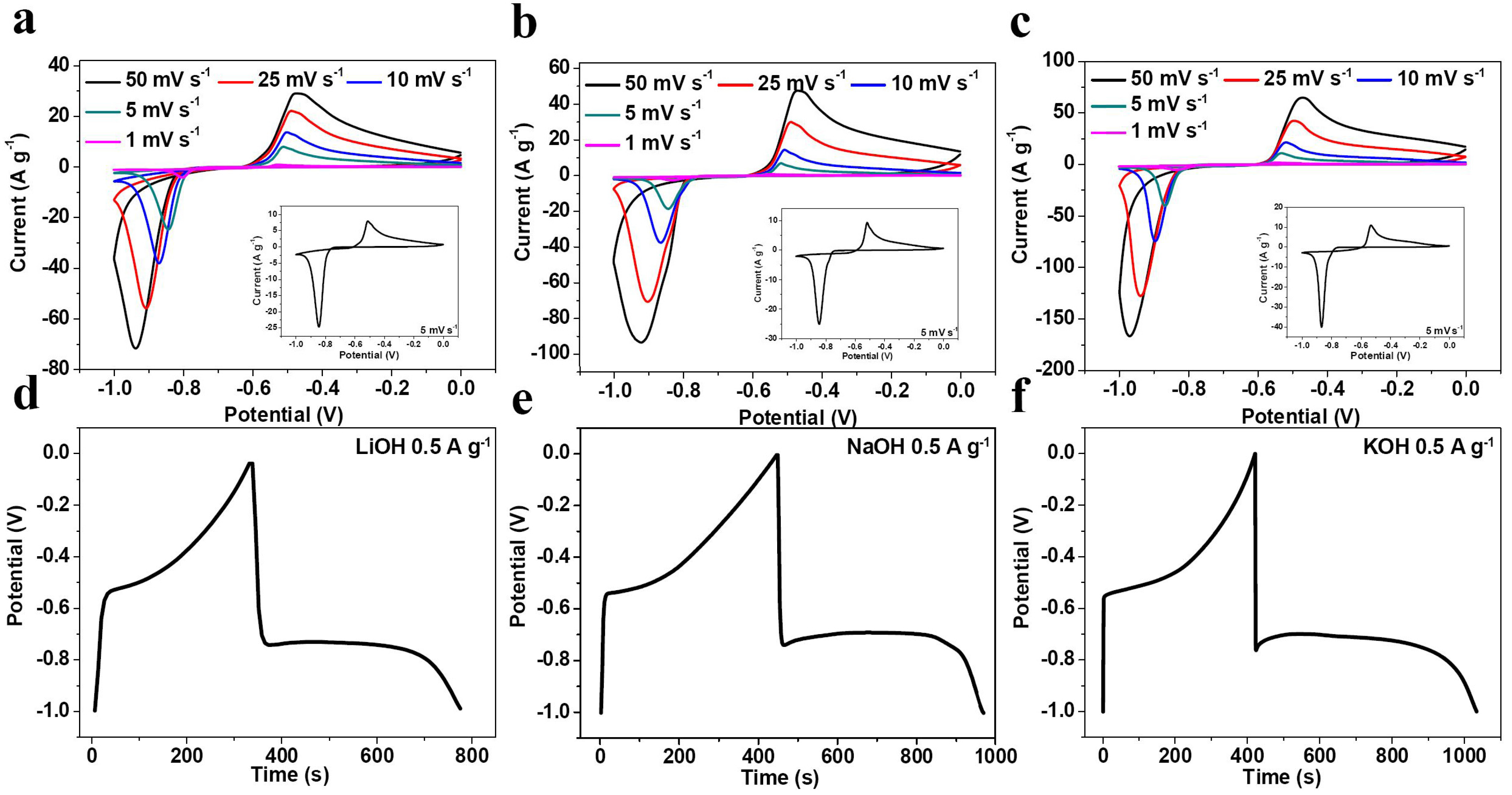
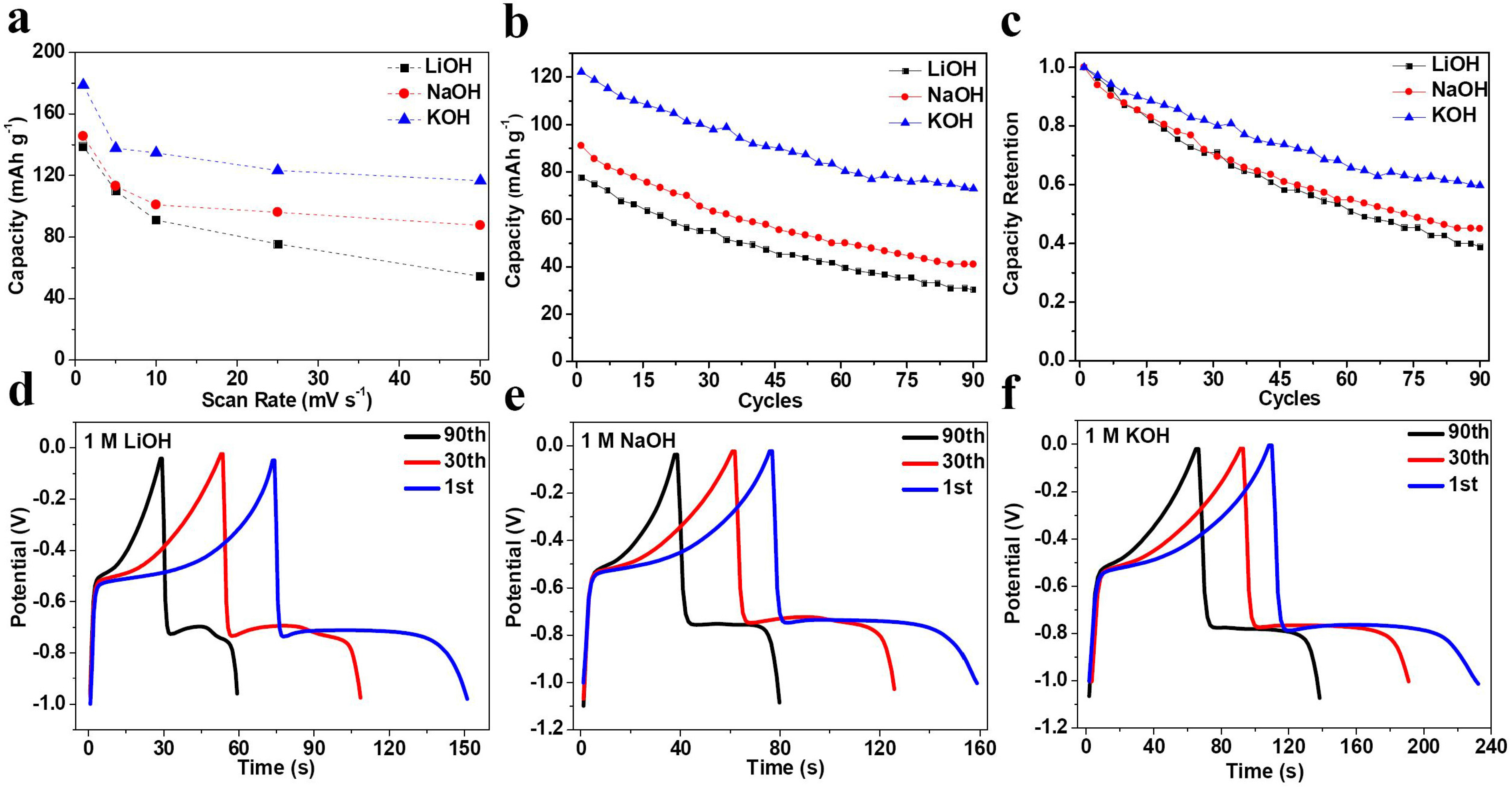
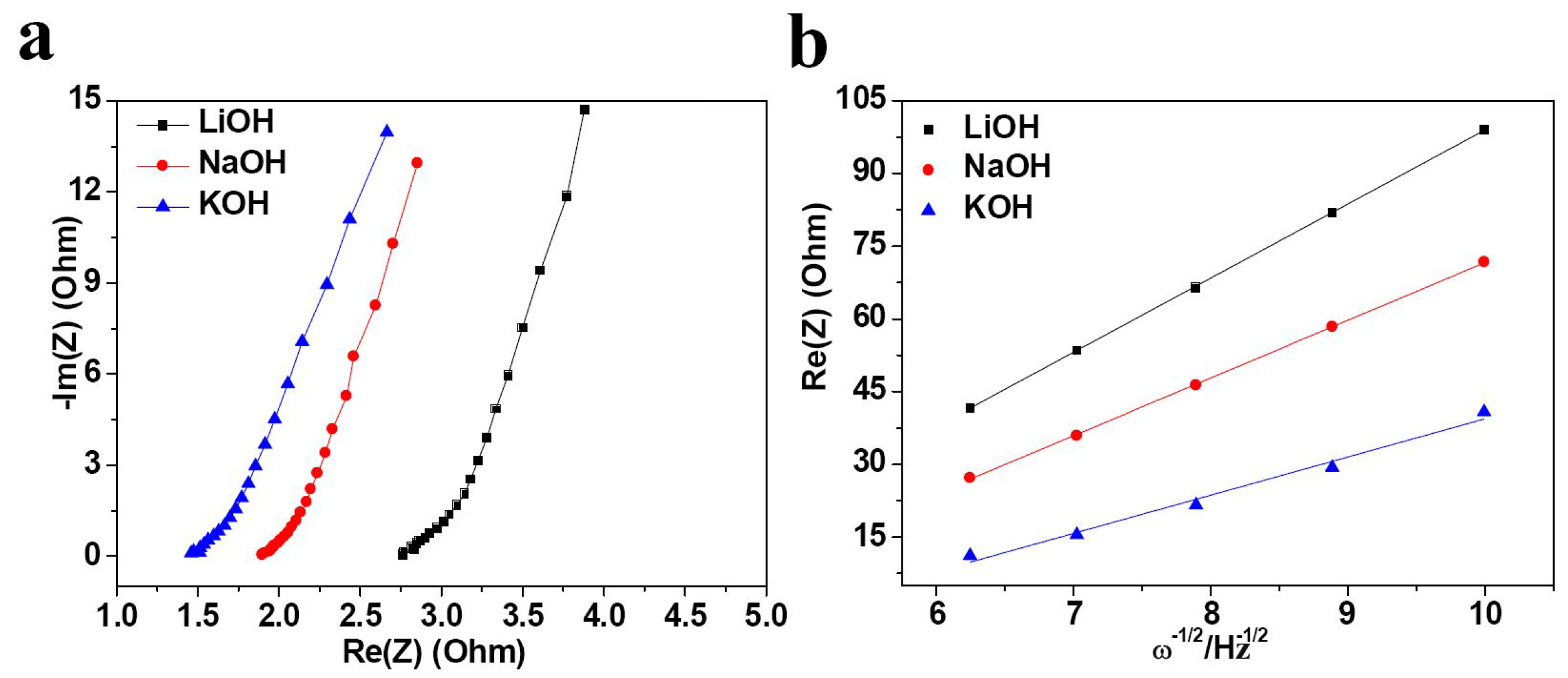
| Electrolyte | Rs (Ω) | σ | D (×10−11 cm2·s−1) |
|---|---|---|---|
| LiOH | 2.76 | 15.28 | 1.67 |
| NaOH | 1.89 | 11.90 | 2.78 |
| KOH | 1.45 | 7.87 | 6.35 |
3. Experimental Section
3.1. Sample Preparation and Characterization
3.2. Electrochemical Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Armand, M.; Tarascon, J. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Minakshi, M.; Singh, P.; Carter, M.; Prince, K. The Zn-MnO2 battery: The influence of aqueous LiOH and KOH electrolytes on the intercalation mechanism. Electrochem. Solid-State Lett. 2008, 11, A145–A149. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, S.; Wu, Y. Hydrothermal-assisted synthesis of the Na7V4(P2O7)4(PO4)/C nanorod and its fast sodium intercalation chemistry in aqueous rechargeable sodium batteries. Nanoscale 2015, 7, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, J.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Park, S.; Gocheva, I.; Okada, S.; Yamaki, J. Electrochemical properties of NaTi2(PO4)3 anode for rechargeable aqueous sodium-ion batteries. J. Electrochem. Soc. 2011, 158, A1067–A1070. [Google Scholar] [CrossRef]
- Trócoli, R.; la Mantia, F. An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion battery chemistry: The rechargeable zinc ion battery. Angew. Chem. 2012, 124, 957–959. [Google Scholar] [CrossRef]
- Manickam, M.; Singh, P.; Issa, T.; Thurgate, S.; de Marco, R. Lithium insertion into manganese dioxide electrode in MnO2/Zn aqueous battery: Part I. A preliminary study. J. Power Sources 2004, 130, 254–259. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.; Yan, N.; Pan, G.; Li, G.; Gao, X. Aluminum storage behavior of anatase TiO2 nanotube arrays in aqueous solution for aluminum ion batteries. Energy Environ. Sci. 2012, 5, 9743–9746. [Google Scholar] [CrossRef]
- Goodenough, J.; Kim, Y. Challenge for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.; Kim, H.; Kim, S.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.; Mohamad, A. Advances of aqueous rechargeable lithium-ion battery: A review. J. Power Sources 2015, 274, 237–251. [Google Scholar] [CrossRef]
- Ruffo, R.; Wessells, C.; Huggins, R.; Cui, Y. Electrochemical behavior of LiCoO2 as aqueous lithium-ion battery electrodes. Electrochem. Commun. 2009, 11, 247–249. [Google Scholar] [CrossRef]
- Tang, W.; Hou, Y.; Wang, F.; Liu, L.; Wu, Y.; Zhu, K. LiMn2O4 nanotube as cathode material of second-level charge capability for aqueous rechargeable batteries. Nano Lett. 2013, 13, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tian, F.; Wang, X.; Yang, Z.; Chen, Q.; Wang, X. LiNi1/3Co1/3Mn1/3O2 as cathode material for aqueous rechargeable lithium batteries. J. Solid State Electrochem. 2012, 16, 491–497. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Appadoo, D.; Martin, D. Synthesis and characterization of olivine LiNiPO4 for aqueous rechargeable battery. Electrochim. Acta 2011, 56, 4356–4360. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Lee, B.; Qiao, R.; Yang, Z.; Xu, S.; Yu, X.; Gu, L.; Hu, Y.; Yang, W.; et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat. Commun. 2015, 6, 6401. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Wang, C.; Li, Y.; Liu, J. Directly grown nanostructured electrodes for high volumetric energy density binder-free hybrid supercapacitors: A case study of CNTs//Li4Ti5O12. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Jiang, W.; Yu, K.; Gao, Y.; Zhu, Z. Hydrothermal synthesis of VO2 (B) nanostructures and application in aqueous Li-ion battery. Electrochim. Acta 2011, 56, 2122–2126. [Google Scholar] [CrossRef]
- Caballero, A.; Morales, J.; Vargas, O. Electrochemical instability of LiV3O8 as an electrode material for aqueous rechargeable lithium batteries. J. Power Sources 2010, 195, 4318–4321. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, G.; Qu, F.; Wang, F.; Song, X. Electrochemical performances of (LiMn0.6Fe0.4PO4/C)//LiV3O8 in different aqueous solution electrolyte. Electrochim. Acta 2015, 151, 50–55. [Google Scholar] [CrossRef]
- Sun, D.; Jin, G.; Wang, H.; Liu, P.; Ren, Y.; Jiang, Y.; Tang, Y.; Huang, X. Aqueous rechargeable lithium batteries using NaV6O15 nanoflakes as high performance anodes. J. Mater. Chem. A 2014, 2, 12999–13005. [Google Scholar] [CrossRef]
- Mohamed, A.; Sansone, N.; Kuei, B.; Washburn, N.; Whitacre, J. Using polypyrrole coating to improve cycling stability of NaTi2(PO4)3 as an aqueous Na-ion anode. J. Electrochem. Soc. 2015, 162, A2201–A2207. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zeng, Y.; Yang, S.; Chen, L. Electrochemical properties of TiP2O7 and LiTi2(PO4)3 as anode material for lithium ion battery with aqueous solution electrolyte. Electrochim. Acta 2007, 52, 3280–3285. [Google Scholar] [CrossRef]
- Wu, W.; Shanbhag, S.; Wise, A.; Chang, J.; Rutt, A.; Whitacre, J. High performance TiP2O7 based intercalation negative electrode for aqueous lithium-ion batteries via a facile synthetic route. J. Electrochem. Soc. 2015, 162, A1921–A1926. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Guo, Z.; Wang, Y.; Wang, C.; Che, Y.; Xia, Y. Aqueous lithium-ion batteries using O2 self-elimination polyimides electrodes. J. Electrochem. Soc. 2015, 162, A1972–A1977. [Google Scholar] [CrossRef]
- Qin, H.; Song, Z.; Zhan, H.; Zhou, Y. Aqueous rechargeable alkali-ion batteries with polyimide anode. J. Power Sources 2014, 249, 367–372. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X. Recent advances in metal oxide-based electrode architecture design for electrochemical energy storage. Adv. Mater. 2012, 38, 5166–5180. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, L.; Wang, H.; Zuo, W.; Li, Y.; Liu, J. Fabrication and shell optimization of synergistic TiO2-MoO3 core-shell nanowire array anode for high energy and power density lithium-ion batteries. Adv. Funct. Mater. 2015, 25, 3524–3533. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.; Huang, X.; Liu, J. Carbon-stabilized high-capacity ferroferric oxide nanorod array for flexible solid-state alkaline battery-supercapacitor hybrid device with high environmental suitability. Adv. Funct. Mater. 2015, 25, 5384–8394. [Google Scholar] [CrossRef]
- Crosnier, O.; Brousse, T.; Devaux, X.; Fragnaud, P.; Schleich, D. New anode systems for lithium ion cells. J. Power Sources 2001, 94, 169–174. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, M.; Li, X.; Nie, Z.; Zuo, P.; Li, G.; Liu, T.; Xiao, J.; Cheng, Y.; Wang, C.; Zhang, J.; Liu, J. Highly reversible Mg insertion in nanostructured Bi for Mg ion batteries. Nano Lett. 2014, 14, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Arthur, T.; Singh, N.; Matsui, M. Electrodeposited Bi, Sb and Bi1−xSbx alloys as anodes for Mg-ion batteries. Electrochem. Commun. 2012, 16, 103–106. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Bismuth: A new anode for the Na-ion battery. Nano Energy 2015, 12, 88–95. [Google Scholar] [CrossRef]
- Su, H.; Cao, S.; Xia, N.; Huang, X.; Yan, J.; Liang, Q.; Yuan, D. Controllable growth of Bi2O3 with rod-like structures via the surfactants and its electrochemical properties. J. Appl. Electrochem. 2014, 44, 735–740. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, W.; Xu, P.; Li, Y.; Liu, J. Direct Growth of Bismuth Film as Anode for Aqueous Rechargeable Batteries in LiOH, NaOH and KOH Electrolytes. Nanomaterials 2015, 5, 1756-1765. https://doi.org/10.3390/nano5041756
Zuo W, Xu P, Li Y, Liu J. Direct Growth of Bismuth Film as Anode for Aqueous Rechargeable Batteries in LiOH, NaOH and KOH Electrolytes. Nanomaterials. 2015; 5(4):1756-1765. https://doi.org/10.3390/nano5041756
Chicago/Turabian StyleZuo, Wenhua, Pan Xu, Yuanyuan Li, and Jinping Liu. 2015. "Direct Growth of Bismuth Film as Anode for Aqueous Rechargeable Batteries in LiOH, NaOH and KOH Electrolytes" Nanomaterials 5, no. 4: 1756-1765. https://doi.org/10.3390/nano5041756
APA StyleZuo, W., Xu, P., Li, Y., & Liu, J. (2015). Direct Growth of Bismuth Film as Anode for Aqueous Rechargeable Batteries in LiOH, NaOH and KOH Electrolytes. Nanomaterials, 5(4), 1756-1765. https://doi.org/10.3390/nano5041756






