Effect of Nanosilica on the Undrained Shear Strength of Organic Soil
Abstract
1. Introduction
1.1. Background of the Improvement and Stabilization of Soils
1.2. Organic Soil
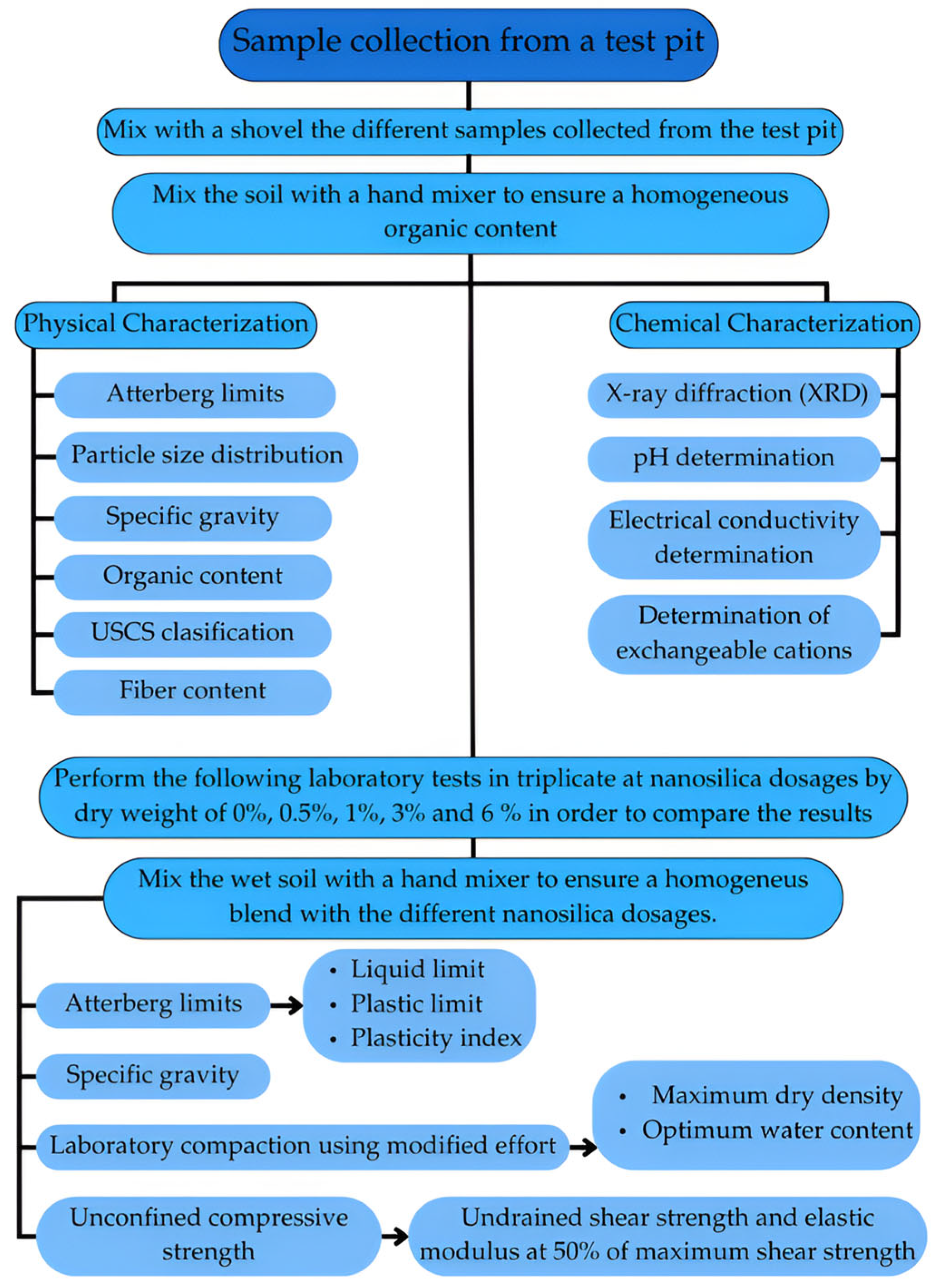
2. Materials and Methods
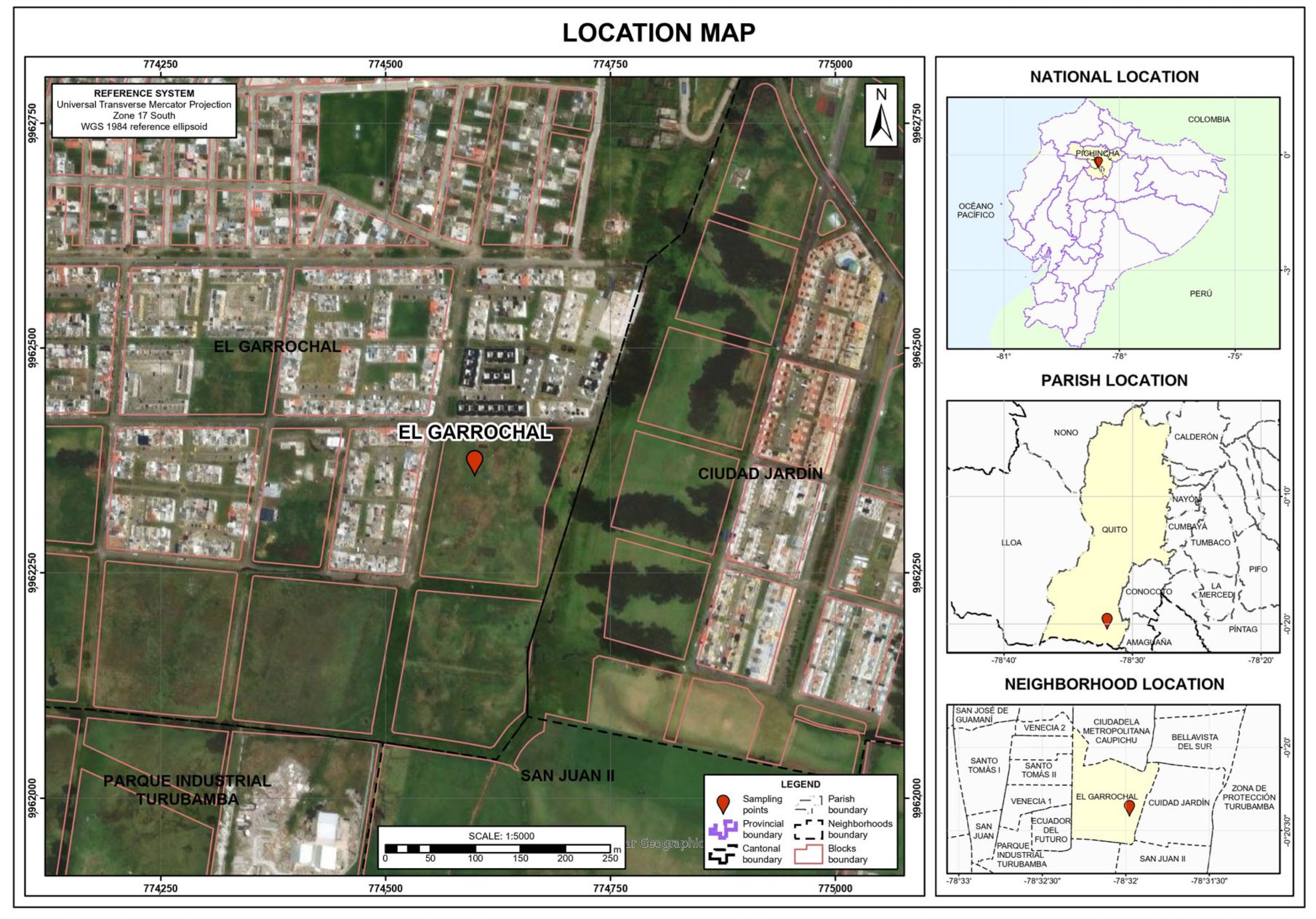
2.1. Sampling Site
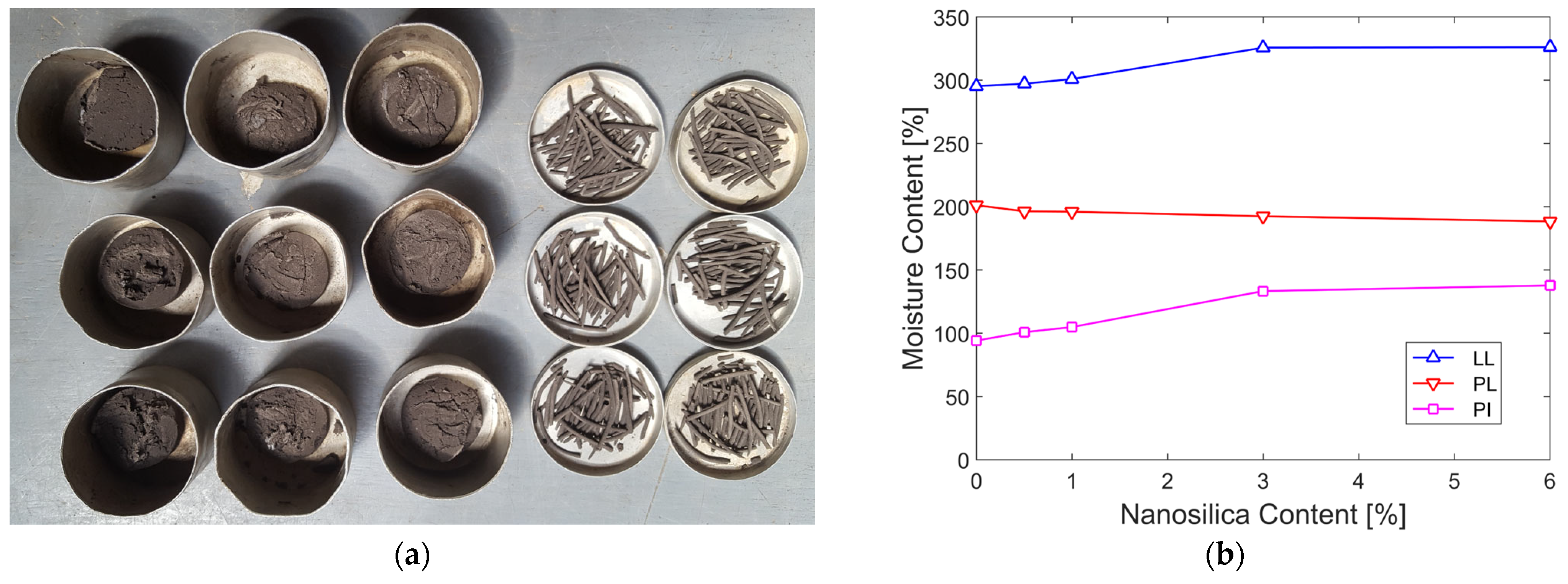
2.2. Sample Extraction and Storage
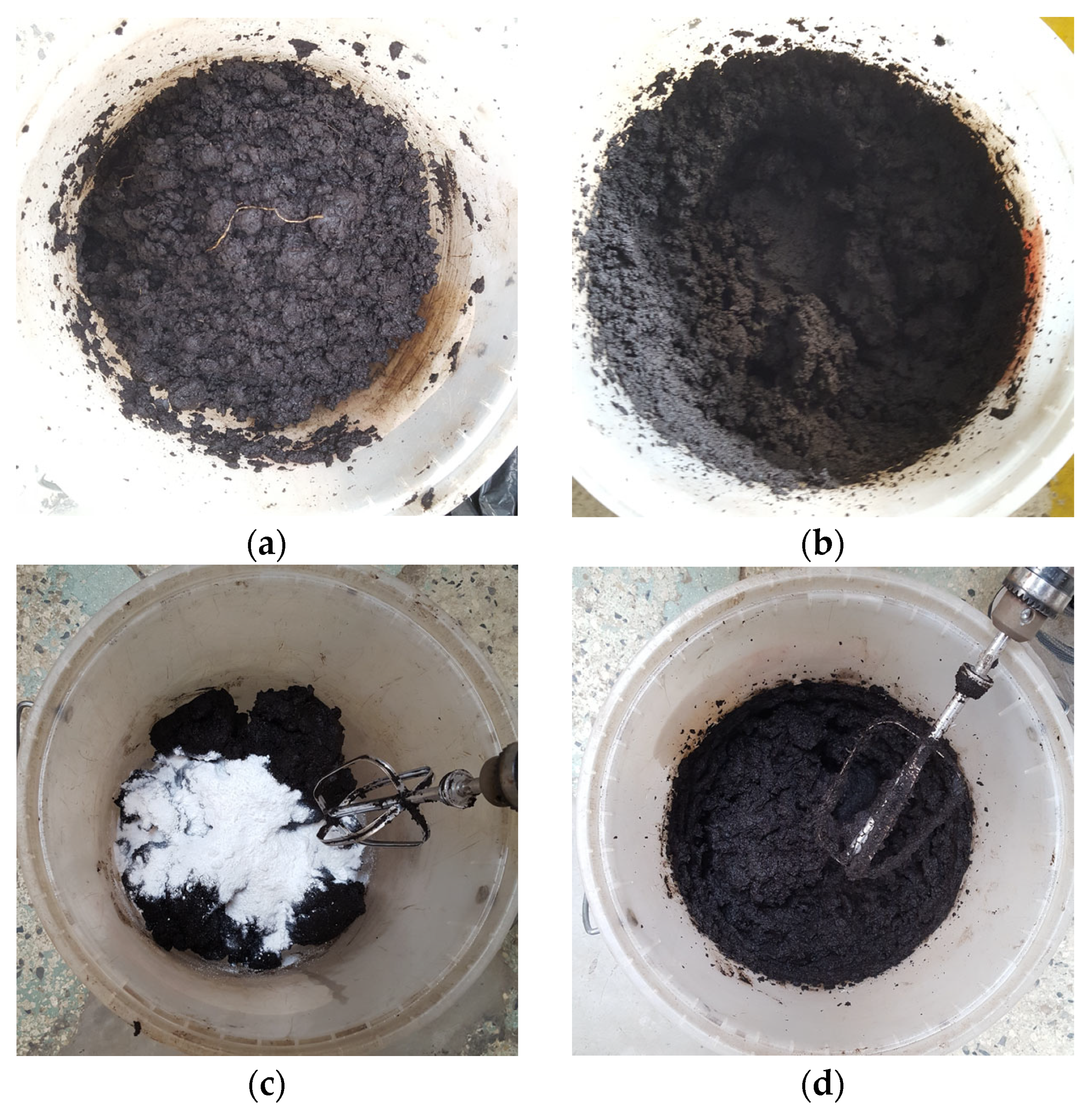
2.3. Testing Process and Proposed Homogenization of Organic Content and Nanosilica
- Determination of the dry weight for accurate dosing;
- Evaluating the degree of dispersion of organic content across the entire sample;
- Assessment of the effectiveness of the mechanical homogenization process using a hand mixer.
2.4. Nanosilica Properties
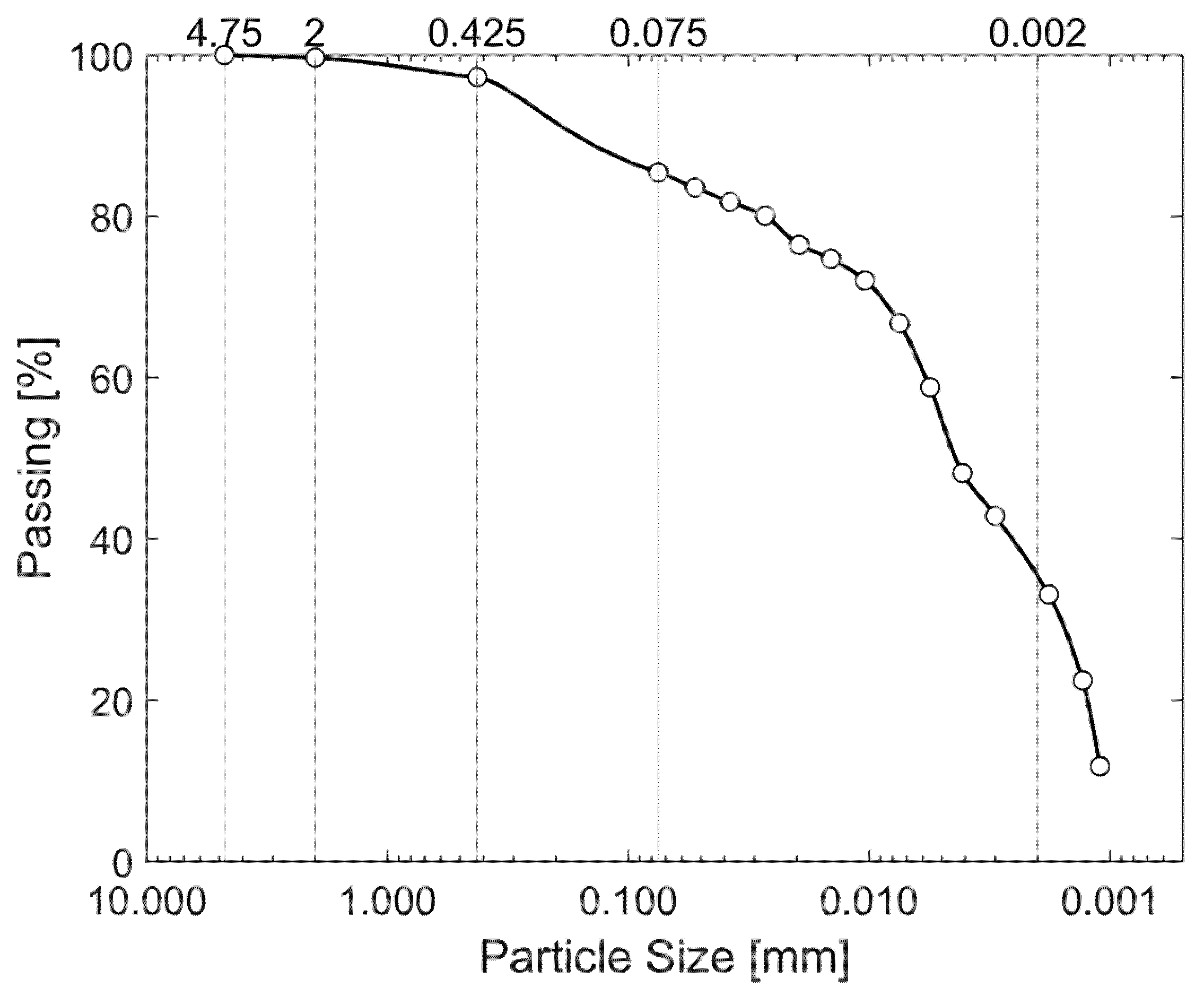
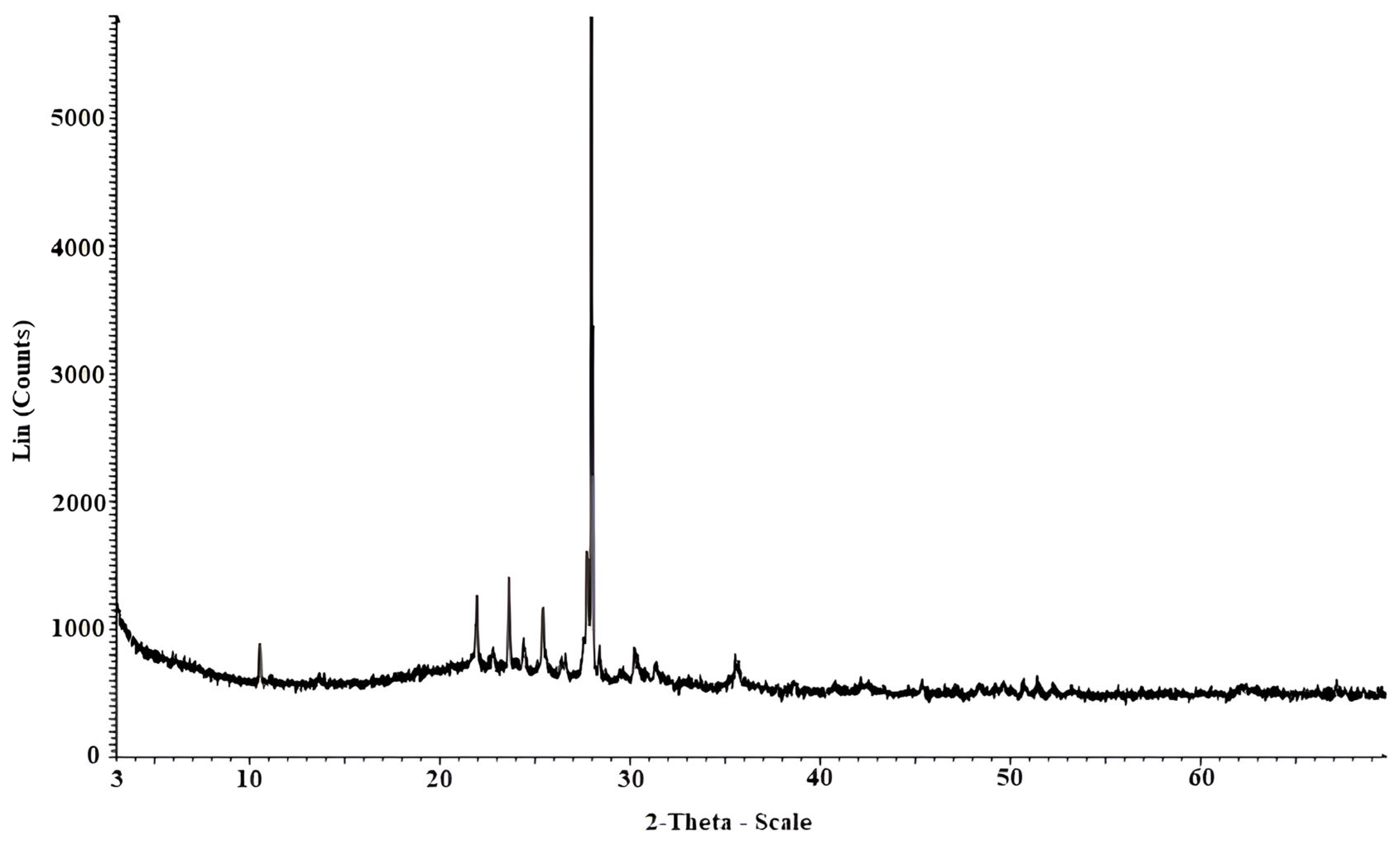
2.5. Physical and Chemical Soil Characterization
| Laboratory Test | Parameter | Standard | Test Realized Number |
|---|---|---|---|
| Particle-Size Distribution | Gravel, sand, lime, and clay fraction [%] | ASTM D 7928 [49] ASTM D 1140 [50] | 3 for each standard |
| Atterberg Limits | LL, LP, IP [%] | ASTM D 4318 [51] | 3 for each NS Content, for a total of 15 |
| USCS Classification | Soil Classification | ASTM D 2487 [52] | 3 |
| Organic Content | Organic fraction [%] | ASTM D 2974 [53] | 30 |
| Fiber Content | Fiber fraction [%] | ASTM D 1997 [54] | 3 |
| Specific Gravity | Gs | ASTM D 854 [47] | 3 for each NS Content |
| Laboratory Compaction | ɣd max., W opt. | ASTM D 1557 [55] | At least 8 points for each NS Content, for a total of 47 points |
| Unconfined Compressive Strength | qu, E | ASTM D 2166 [56] | 3 for each NS Content, for a total of 15 |
3. Results
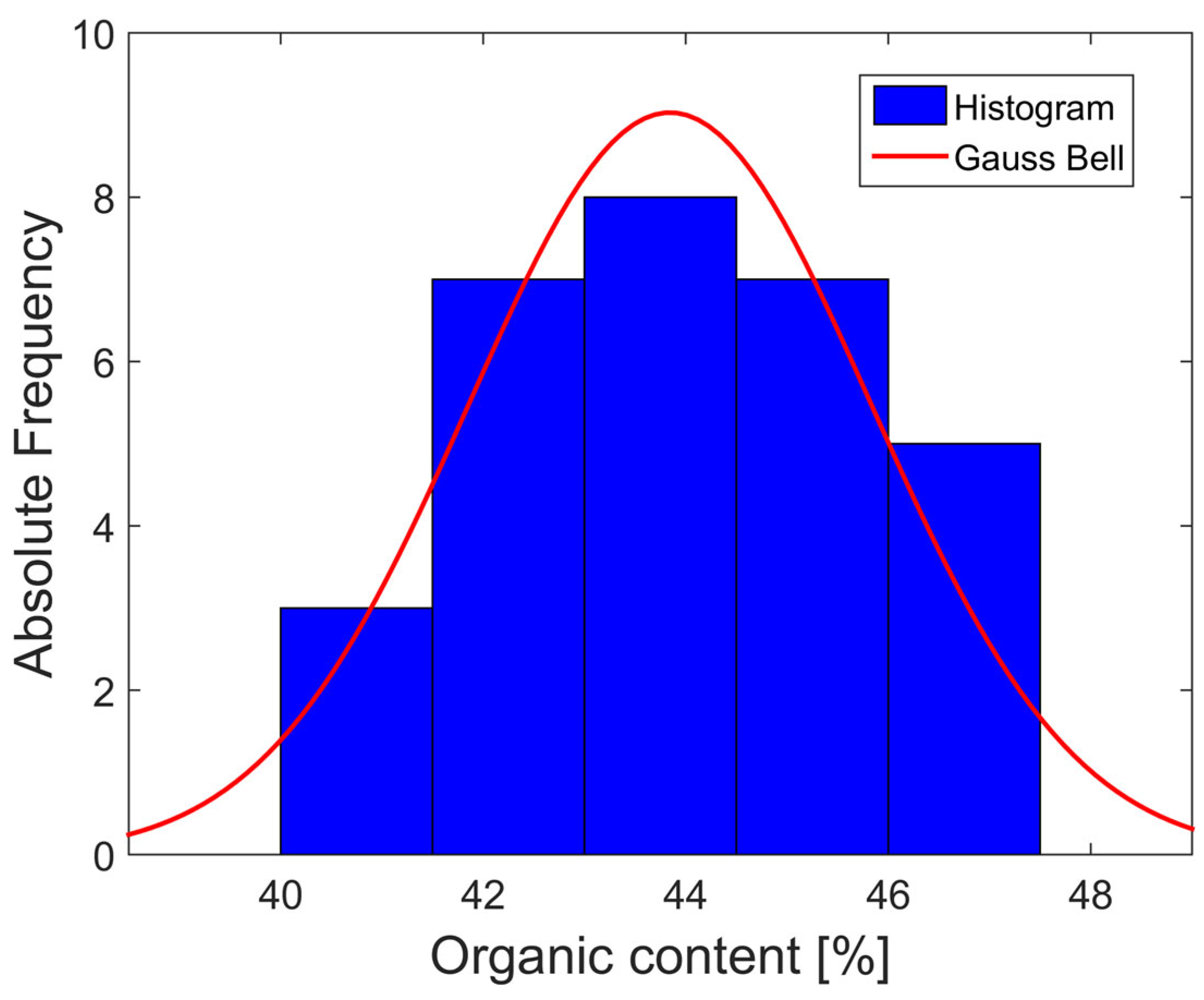
3.1. Organic Content
3.2. Effect of Nanosilica on Plasticity
3.3. Effect of Nanosilica on Specific Gravity
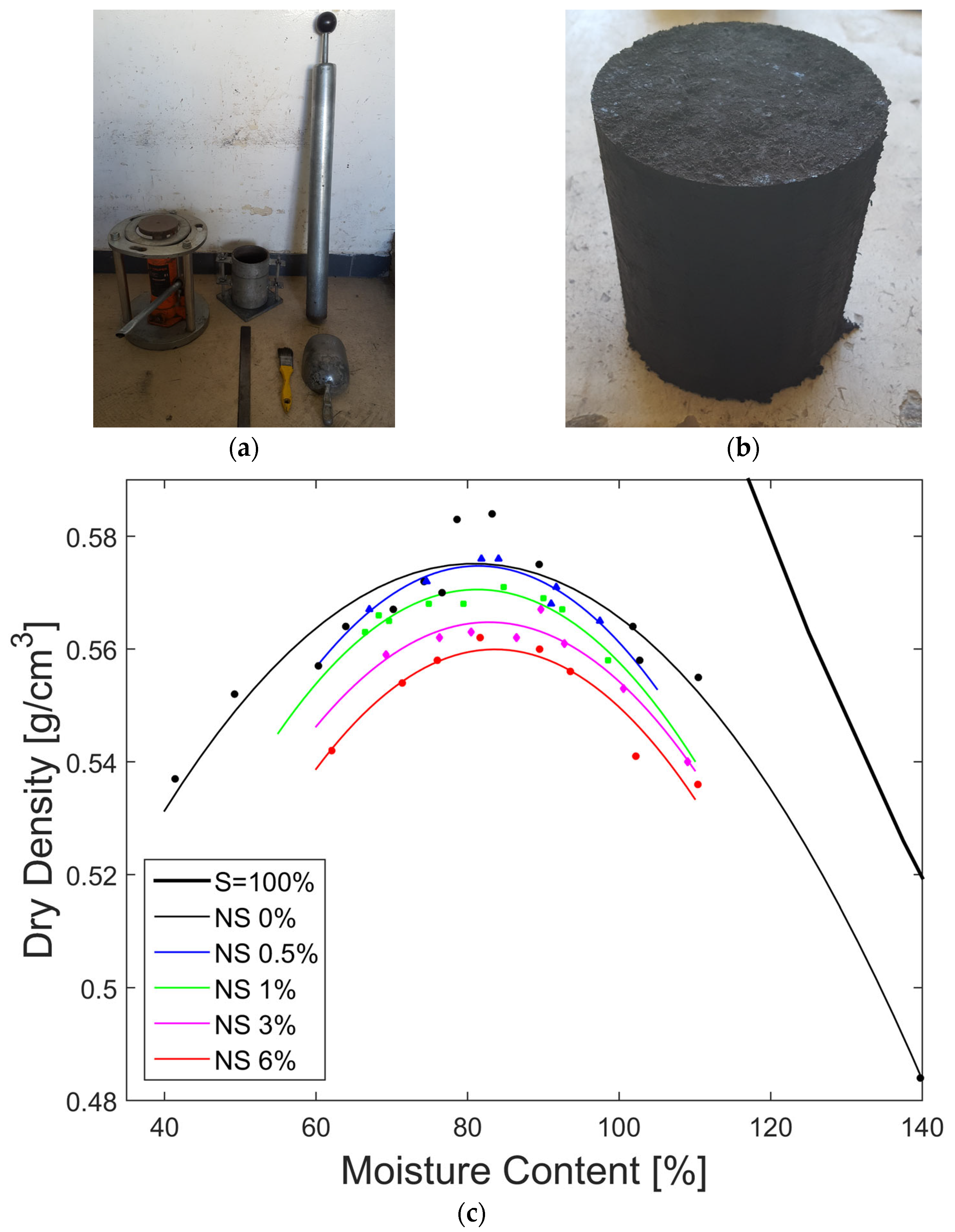
3.4. Effect of Nanosilica on Maximum Dry Density and Optimum Water Content
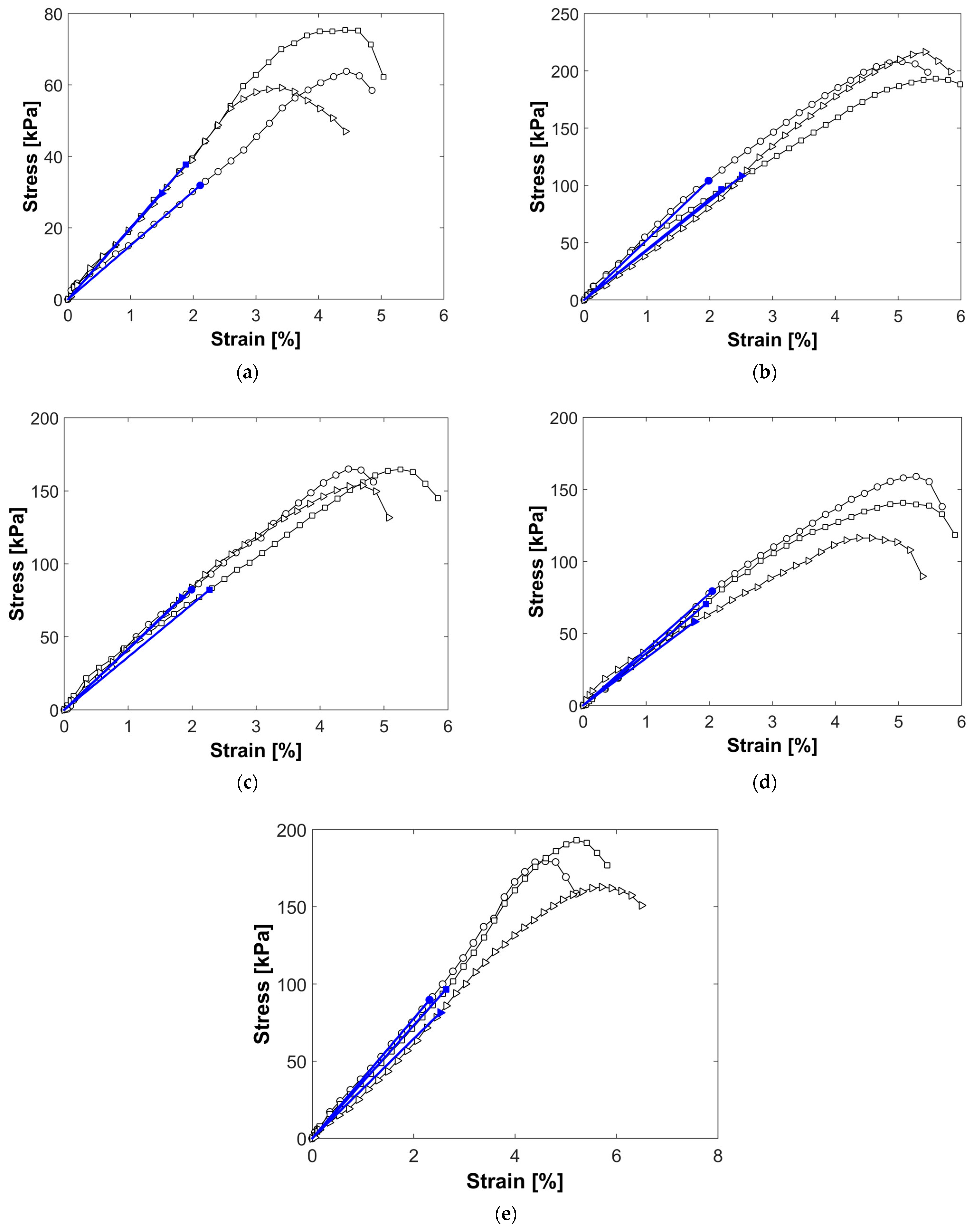
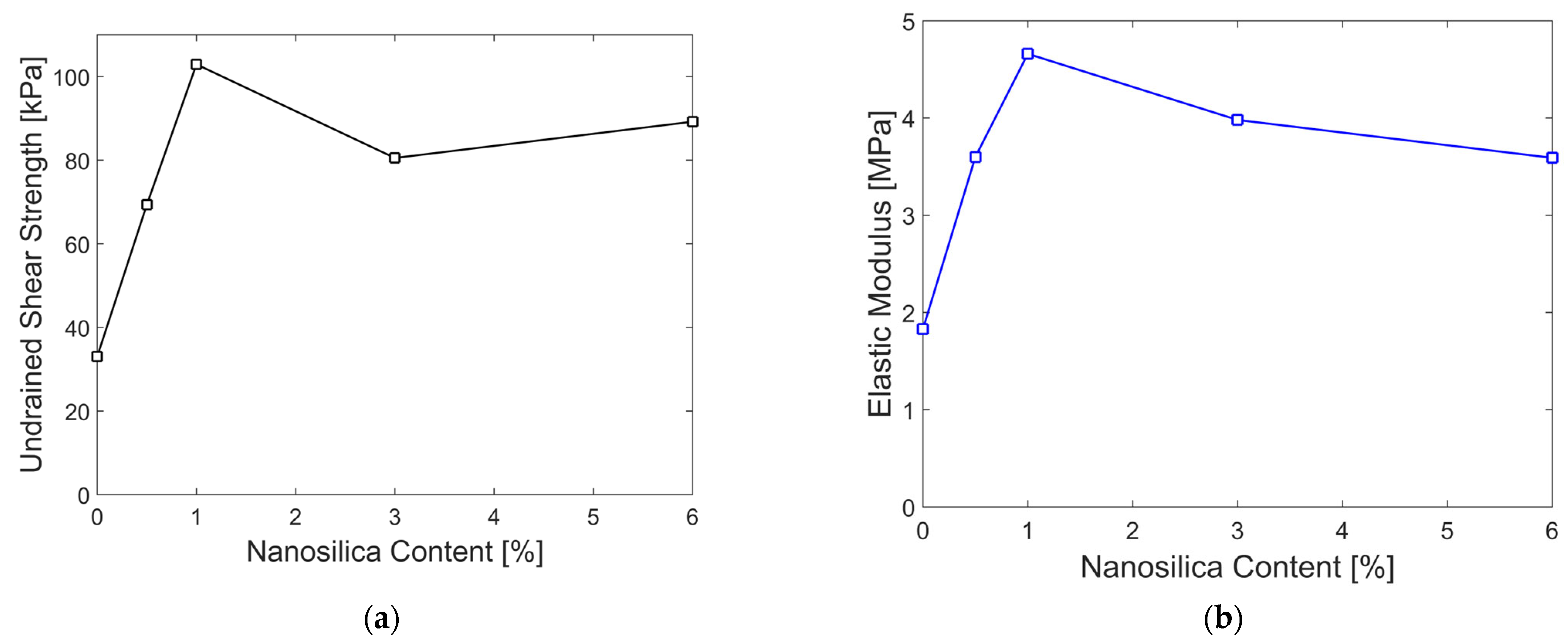
3.5. Effect of Nanosilica on Undrained Shear Strength and Elastic Modulus
4. Discussion
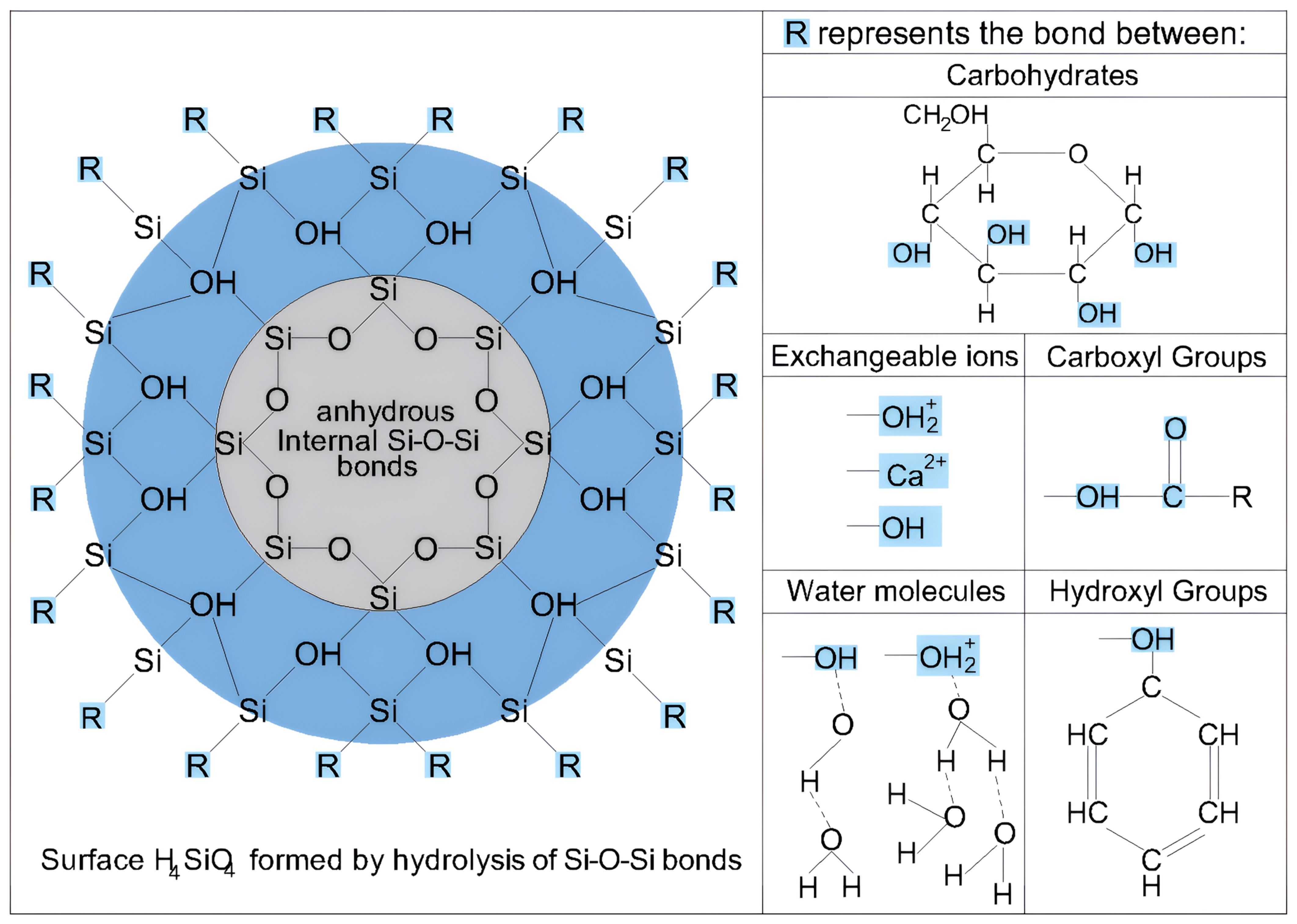
4.1. Role of Soil Mineralogical Components in the Improvement with Nanosilica
4.2. Role of Soil Organic Matter in the Improvement with Nanosilica
5. Conclusions
- Effect on Plasticity: Nanosilica increases the plasticity of organic soil by raising its liquid limit and lowering its plastic limit. This was attributed to the high water adsorption capacity of the nanosilica, which enabled it to retain more water in its plastic state.
- Specific Gravity: Nanosilica had no significant effect on the specific gravity of soil. The slight variation in specific gravity values can be attributed to the variability in organic content, as mentioned previously.
- Maximum Dry Density: The reduction in the maximum dry density with increasing nanosilica content is due to the low density of amorphous and porous nanosilica particles [68]. However, organic soil also exhibits a low dry density owing to the presence of partially decomposed organic matter and the high water adsorption capacity of organic colloids, which results in a large percentage of their weight being water. Therefore, the slight decrease in the maximum dry density is attributed to the similar low densities of the two materials.
- Optimum Moisture Content: The increase in the optimum moisture content is associated with a higher specific surface area of the mixture. This implies that the nanosilica surface, through additional ionic attraction, enhances the adsorption capacity of water molecules, thereby increasing water retention [69].
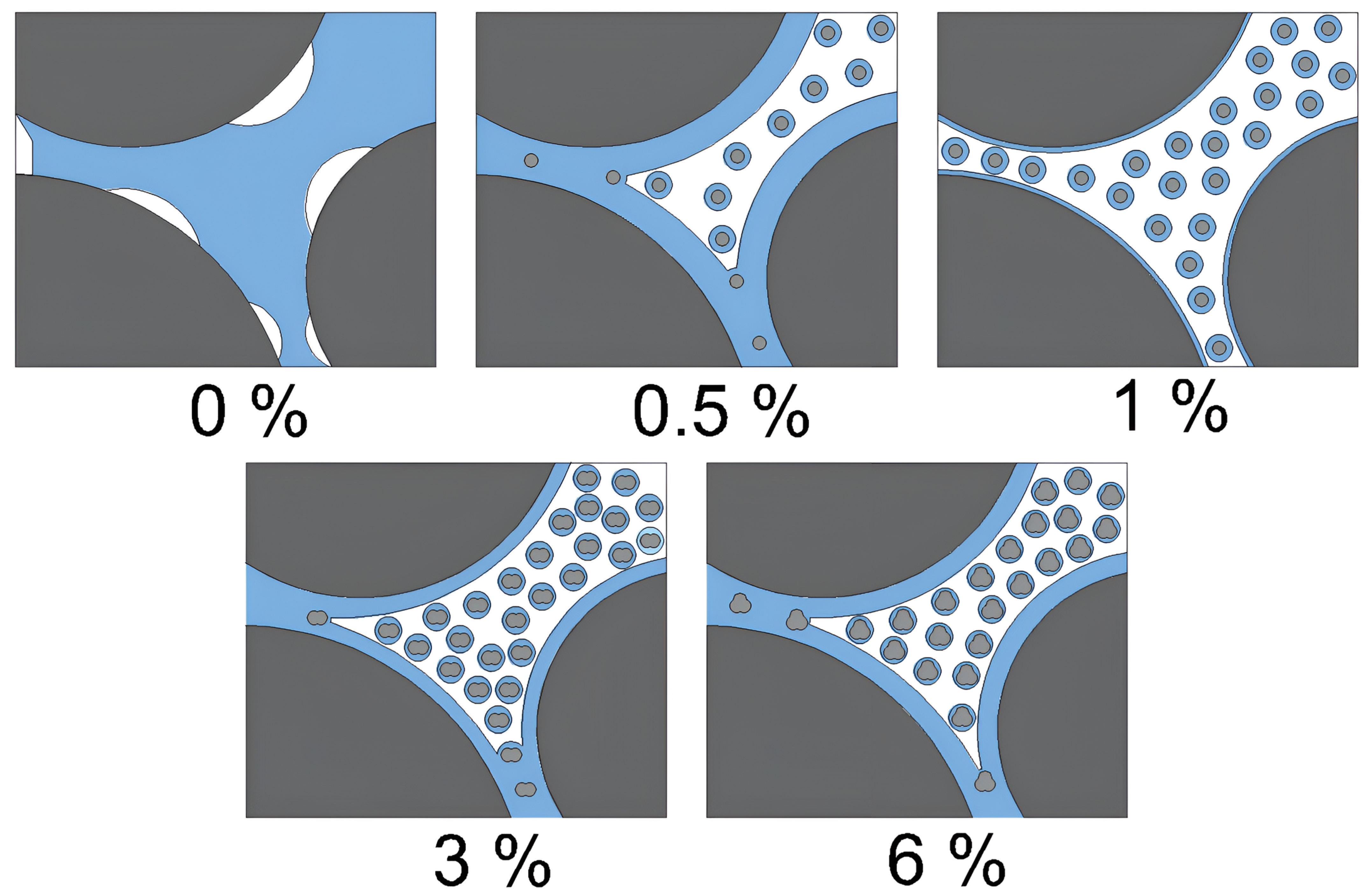
- Undrained Shear Strength and Elastic Modulus: The undrained shear strength and modulus of elasticity of the soil increased significantly with the addition of 1% nanosilica, which was identified as the optimal content. At higher concentrations, nanosilica tends to agglomerate rather than effectively fill soil voids [70]. This increase in strength can be attributed to multiple interaction mechanisms.
- C-S-H Gel Formation: Although influenced by pH, C-S-H gel can form throughout the 28-day curing process because of the availability of Ca2⁺ ions for cation exchange, and the high reactivity and colloidal properties of nanosilica. Additionally, evaluating the soil at the maximum dry density and optimum moisture content enables a more effective interaction between nanosilica and Ca2⁺ ions, preventing their dispersion into the medium owing to excess free water.
- Interaction with Colloids and Organic Molecules: Nanosilica interacts with colloids and organic molecules, forming multiple chemical bonds that enhance the stiffness of the soil matrix and consequently increase soil strength.
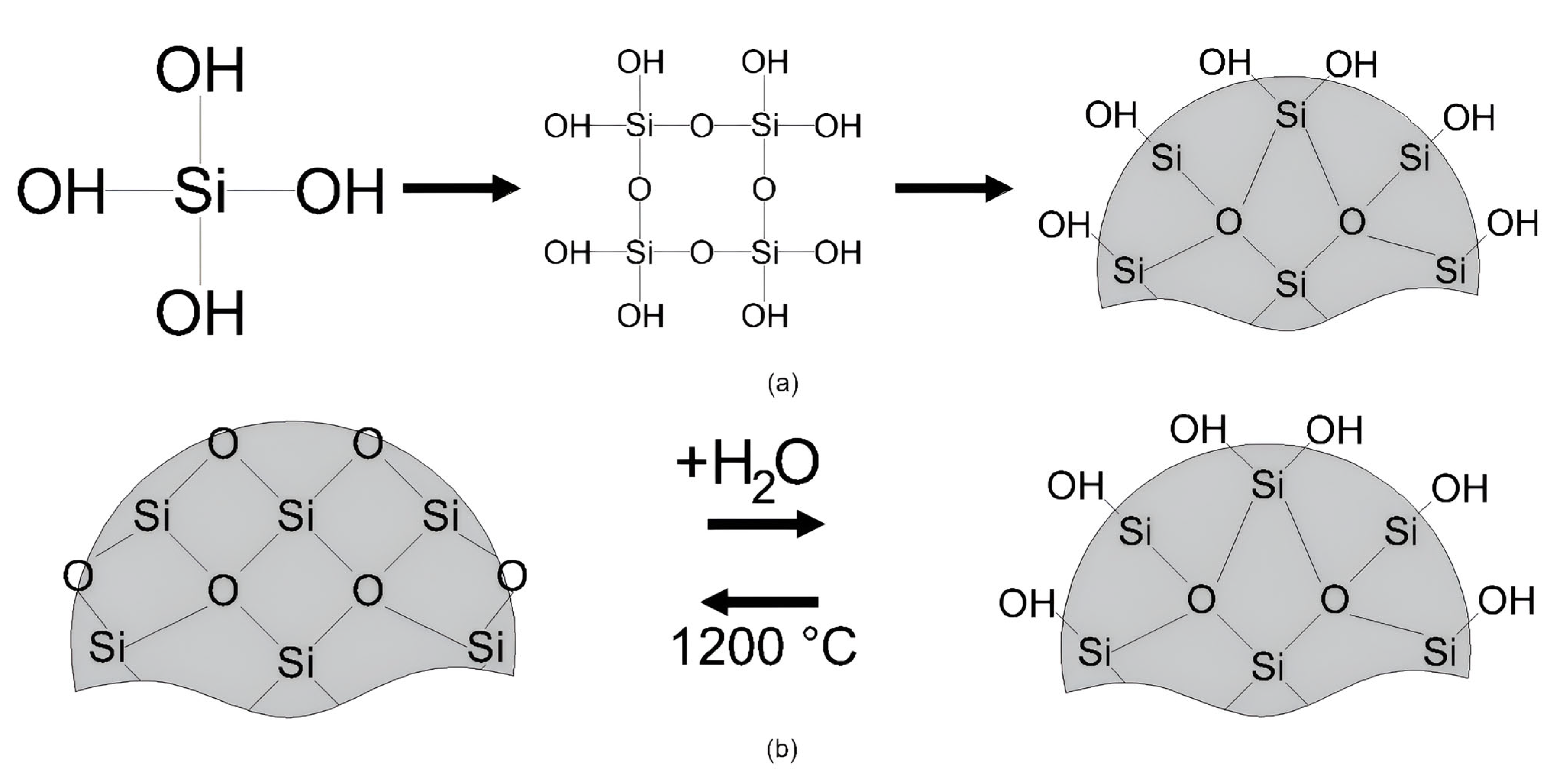
- Enhancement of inter-particle interactions: The compaction process densifies the soil mass by reducing the trapped air in the voids and leaving free water in these spaces. Nanosilica adsorbs free water, promoting greater contact between soil particles, which enhances internal friction and increases resistance. However, excess nanosilica can agglomerate, thereby reducing the adsorption capacity. Figure 16 illustrates this mechanism.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera, J.F.; Aguirre-Guerrero, A.; de Gutiérrez, R.M.; Orobio, A. Estabilización química de suelos—Materiales convencionales y activados alcalinamente (revisión). Inf. Técnico 2020, 84, 43–67. [Google Scholar] [CrossRef]
- Cardona, E.; Jimenez, J.; Ospina, S. Estado del arte, técnicas de mejoramiento y estabilización para rellenos antrópicos. Rev. Espac. 2023, 37, 37–52. [Google Scholar] [CrossRef]
- Ameen, S.K.; Abdulkareem, A.H.; Mahmood, N.S. Shear strength behavior of organic soils treated with fly ash and fly ash-based geopolymer. J. Mech. Behav. Mater. 2023, 32, 20220264. [Google Scholar] [CrossRef]
- Jasim, A.M.; Ahmed, M.D. Assessment of Bearing Capacity and Settlement Characteristics of Organic Soil Reinforced by Dune Sand and Sodium Silicate Columns: A Numerical Study. J. Eng. 2024, 30, 52–67. [Google Scholar] [CrossRef]
- Shah, K.W.; Huseien, G.F.; Xiong, T. Functional nanomaterials and their applications toward smart and green buildings. In New Materials in Civil Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 395–433. [Google Scholar] [CrossRef]
- Rincón-Morantes, J.F.; Reyes-Ortiz, O.J.; Ruge-Cárdenas, J.C. Review of the use of nanomaterials in soils for construction of roads. Respuestas 2020, 25, 213–223. [Google Scholar] [CrossRef]
- Haji, T.R.; Mir, B.A. Effect of nano-gypsum on mechanical properties cement admixed marginal silty soil. Constr. Build. Mater. 2023, 408, 133639. [Google Scholar] [CrossRef]
- Yao, K.; An, D.; Wang, W.; Li, N.; Zhang, C.; Zhou, A. Effect of nano-MgO on Mechanical Performance of Cement Stabilized Silty Clay; Taylor and Francis Ltd.: Abingdon, UK, 2020. [Google Scholar] [CrossRef]
- Kannan, G.; O’Kelly, B.C.; Sujatha, E.R. Geotechnical investigation of low-plasticity organic soil treated with nano-calcium carbonate. J. Rock Mech. Geotech. Eng. 2023, 15, 500–509. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zhao, C.; Deng, L.; Fang, Q. Assessment of strength development of soil stabilized with cement and nano SiO2. Constr. Build. Mater. 2023, 409, 133889. [Google Scholar] [CrossRef]
- AlTawaiha, H.; Alhomaidat, F.; Eljufout, T. A Review of the Effect of Nano-Silica on the Mechanical and Durability Properties of Cementitious Composites. Infrastructures 2023, 8, 132. [Google Scholar] [CrossRef]
- Kani, E.N.; Rafiean, A.H.; Tavakolzadeh, M.; Ghaffar, S.H. Performance enhancement of cementitious soil stabilizers using incorporated nanosilica. Results Eng. 2022, 16, 100713. [Google Scholar] [CrossRef]
- Kannan, G.; Sujatha, E.R. Effect of Nano Additive on Mechanical Properties of Natural Fiber Reinforced Soil. J. Nat. Fibers 2023, 20, 2143980. [Google Scholar] [CrossRef]
- Ghadr, S.; Liu, C.H.; Mrudunayani, P.; Hung, C. Effects of hydrophilic and hydrophobic nanosilica on the hydromechanical behaviors of mudstone soil. Constr. Build. Mater. 2022, 331, 127263. [Google Scholar] [CrossRef]
- Karimiazar, J.; Teshnizi, E.S.; O’Kelly, B.C.; Sadeghi, S.; Karimizad, N.; Yazdi, A.; Arjmandzadeh, R. Effect of nano-silica on engineering properties of lime-treated marl soil. Transp. Geotech. 2023, 43, 101123. [Google Scholar] [CrossRef]
- Gu, J.; Cai, X.; Wang, Y.; Guo, D.; Zeng, W. Evaluating the Effect of Nano-SiO2 on Different Types of Soils: A Multi-Scale Study. Int. J. Environ. Res. Public Health 2022, 19, 16805. [Google Scholar] [CrossRef]
- García, S.; Trejo, P.; Merlos, J.; Castillo, I. Nanosilica to modify the consistency of high compressible lacustrine clays. DYNA 2022, 97, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Nur, A.S.; Alistair, D.G.; Habib, M.M. A Review on Experimental Investigations and Geotechnical Characteristic of Peat Soil Stabilization. Int. J. Adv. Res. Eng. Innov. 2023, 5, 1–19. [Google Scholar] [CrossRef]
- Kaya, Z.; Erol, A. Comparison of bearing capacities of undisturbed organic soils by empirical relations and 2D finite element analysis. Arab. J. Geosci. 2021, 14, 1975. [Google Scholar] [CrossRef]
- Łachacz, A.; Bogacz, A.; Glina, B.; Kalisz, B.; Mendyk, Ł.; Orzechowski, M.; Smólczyński, S.; Sowiński, P. Origin, transformation and classification of organic soils in Poland. Soil Sci. Annu. 2024, 75, 195241. [Google Scholar] [CrossRef]
- Łachacz, A.; Kalisz, B.; Sowiński, P.; Smreczak, B.; Niedźwiecki, J. Transformation of Organic Soils Due to Artificial Drainage and Agricultural Use in Poland. Agriculture 2023, 13, 634. [Google Scholar] [CrossRef]
- Meyer, Z.; Magdalena, O. Analysis of using the empirical model of organic soil consolidation to predict settlement. ACEE 2023, 16, 111–117. [Google Scholar] [CrossRef]
- Chong, W.A.; Chan, C.M.; Sani, S.; Rahman, N.K.A.; Yahya, N.F. Soil stabilisation for laying road foundations: Way forward with organic soil treatment. In IOP Conference Series: Earth and Environmental Science; Institute of Physics: London, UK, 2023. [Google Scholar] [CrossRef]
- Huat, B.; Kazemian, S.; Asadi, A. Experimental Investigation on Geomechanical Properties of Tropical Organic Soils and Peat. J. Eng. Appl. Sci. 2009, 2, 184–188. [Google Scholar]
- Mushtaq, A.; Sachar, E.A. Determination of Shear Strength of Organic Soil in Kashmir. Int. J. Innov. Res. Eng. Manag. 2022, 9, 74–78. [Google Scholar] [CrossRef]
- Navarro, G.; Navarro, S. Química Agrícola, 2nd ed.; Mundi-Prensa: Madrid, Spain, 2003. [Google Scholar]
- Tan, K.H. Soil Gas and Liquid Phases. In Principles of Soil Chemistry, 4th ed.; Taylor and Francis Group, Ed.; CPC Press: Athens, GA, USA, 2011; Chapter 4; pp. 41–42. [Google Scholar]
- Kaneco, S.; Itoh, K.; Katsumata, H.; Suzuki, T.; Masuyama, K.; Funasaka, K.; Hatano, K.; Ohta , K. Removal of natural organic polyelectrolytes by adsorption onto tobermorite. Environ. Sci. Technol. 2003, 37, 1448–1451. [Google Scholar] [CrossRef]
- Tan, K.H. Colloidal Chemistry of Organic Soil Constituents. In Principles of Soil Chemistry, 4th ed.; Taylor and Francis Group, Ed.; CPC Press: Athens, GA, USA, 2011; Chapter 5; pp. 99–105. [Google Scholar]
- Winckell, A.; Marocco, R.; Winter, T.; Huttel, C.; Pourrut, P.; Zebrowski, C.; Sourdat, M. Los Paisajes Naturales Del Ecuador, 1st ed.; Centro Ecuatoriano de Investigación Geográfica: Quito, Ecuador, 1997; Volume 1. [Google Scholar]
- Albuja-Sánchez, J.D. Determination of the indrained shear strength of organic soils using the cone penetration test and Marchetti’s flat dilatometer test. Int. Soc. Soil Mech. Geotech. Eng. 2021, 106, 3–39. [Google Scholar] [CrossRef]
- Villagómez, D. Evolución Geológica Plio-Cuaternaria del Valleinterandino Central en Ecuador (Zona de Quito-Guayllabamba-Sanantonio); Escuela Politécnica Nacional: Quito, Ecuador, 2003. [Google Scholar]
- Mayanquer, J.; Anaguano-Marcillo, M.; Játiva, N.; Albuja-Sánchez, J. New Correlations for the Determination of Undrained Shear, Elastic Modulus, and Bulk Density Based on Dilatometer Tests (DMT) for Organic Soils in the South of Quito, Ecuador. Appl. Sci. 2023, 13, 8570. [Google Scholar] [CrossRef]
- Calderón-Carrasco, A.; Galarza-Poveda, B.; Damián-Chalán, A.; Albuja-Sánchez, J. Effect of Storage Time on the Structural Integrity of Silts and Organic Soils: An Analysis of Moisture Content, Unconfined Compressive Strength, and Elasticity. Appl. Sci. 2024, 14, 8060. [Google Scholar] [CrossRef]
- Kumar, A.; Devi, K. Application of Nanotechnology in Soil Stabilization. J. Build. Mater. Sci. 2023, 5, 25–36. [Google Scholar] [CrossRef]
- Iranpour, B. The influence of nanomaterials on collapsible soil treatment. Eng. Geol. 2016, 205, 40–53. [Google Scholar] [CrossRef]
- Li, W.; O’Kelly, B.C.; Yang, M.; Fang, K.; Li, X.; Li, H. Briefing: Specific gravity of solids relationship with ignition loss for peaty soils. Geotech. Res. 2020, 7, 134–145. [Google Scholar] [CrossRef]
- Kyriacos, D. Polycarbonates. In Brydson’s Plastics Materials, 8th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 457–485. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica nanoparticles by Sol-Gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocompositesa review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A: Physicochem. Eng. Asp. 2000, 173, 1–38. Available online: www.elsevier.nl/locate/colsurfa (accessed on 4 December 2024). [CrossRef]
- Zengin, H.; Erkan, B. Adsorption of acids and bases from aqueous solutions onto silicon dioxide particles. J. Hazard. Mater. 2009, 172, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Rücker, C.; Winkelmann, M.; Kümmerer, K. Are Si–C bonds formed in the environment and/or in technical microbiological systems? Environ. Sci. Pollut. Res. 2023, 30, 91492–91500. [Google Scholar] [CrossRef]
- Tan, K.H. The Colloidal Chemistry of Inorganic Soil Constituents. In Principles of Soil Chemistry, 4th ed.; Taylor and Francis Group, Ed.; CPC Press: Athens, GA, USA, 2011; Chapter 6; pp. 133–134+147–148+172–173. [Google Scholar]
- Carman, P.C. Constitution of colloidal silica. Trans. Faraday Soc. 1940, 36, 967–977. [Google Scholar] [CrossRef]
- ASTM International. Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Biodegradation of biosolids and specific gravity determination. Environ. Geotech. 2016, 6, 47–61. [Google Scholar] [CrossRef]
- ASTM International. Specific Gravity of Soil Solids by the Water Displacement Metod; ASTM International: West Conshohocken, PA, USA, 2023. [Google Scholar] [CrossRef]
- Skempton, A.W.; Petley, D.J. Ignition loss and other properties of peats and clays from avonmouth, king’s lynn and cranberry moss. Géotechnique 1970, 20, 343–356. [Google Scholar] [CrossRef]
- ASTM International. Particle-Size Distribution (Gradation) of Fine-Grained Soils Using the Sedimentation (Hydrometer) Analysis; ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar] [CrossRef]
- ASTM International. Test Methods for Determining the Amount of Material Finer than 75-m (No. 200) Sieve in Soils by Washing; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM International. Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM International. Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM International. Determining the Water (Moisture) Content, Ash Content, and Organic Material of Peat and Other Organic Soils; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- ASTM International. Laboratory Determination of the Fiber Content of Peat Samples by Dry Mass; ASTM International: West Conshohocken, PA, USA, 1997; Available online: http://www.astm.org (accessed on 4 December 2024).
- ASTM International. Laboratory Compaction Characteristics of Soil Using Modified Effort (56,000 ft-lbf/ft3 (2,700 kN-m/m3)); ASTM International: West Conshohocken, PA, USA, 2021. [Google Scholar] [CrossRef]
- ASTM International. Unconfined Compressive Strength of Cohesive Soil; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar] [CrossRef]
- Walpole, R.E.; Myers, R.H.; Myers, S.L.; Ye, K. Probabilidad y Estadística Para Ingeniería y Ciencias, 9th ed.; Pearson: Mexico City, Mexico, 2012. [Google Scholar]
- Birchall, J.D. Silicon-Aluminum Interactions and Biology. In The Coloid Chemistry of Silica; ACS Publications: Washington, DC, USA, 1994; pp. 602–615. [Google Scholar]
- Matysová, P.; Rössler , R.; Götze, J.; Leichmann, J.; Forbes, G.; Taylor, E.L.; Sakala, J.; Grygar , T. Alluvial and volcanic pathways to silicified plant stems (Upper Carboniferous-Triassic) and their taphonomic and palaeoenvironmental meaning. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 292, 127–143. [Google Scholar] [CrossRef]
- Mitchell, R.S.; Tufts, S. Wood Opal-A Tridymite-like Mineral. Am. Mineral. 1973, 58, 717–720. [Google Scholar]
- Sanchez, E.F.; Albuja-Sánchez, J.; Córdova, M. Integrated Geotechnical Analysis of Allophanic Volcanic Ash Soils: SDMT and Laboratory Perspectives. Appl. Sci. 2025, 15, 1386. [Google Scholar] [CrossRef]
- Parida, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Adsorption of organic molecules on silica surface. Adv. Colloid. Interface Sci. 2006, 121, 77–110. [Google Scholar] [CrossRef]
- Nikishina, M.; Perelomov, L.; Atroshchenko, Y.; Ivanova, E.; Mukhtorov, L.; Tolstoy, P. Sorption of Fulvic Acids and Their Compounds with Heavy Metal Ions on Clay Minerals. Soil Syst. 2022, 6, 2. [Google Scholar] [CrossRef]
- Fang, Y.; Lakey, P.S.; Riahi, S.; McDonald, A.T.; Shrestha, M.; Tobias, D.J.; Shiraiwa, M.; Grassian, V.H. A molecular picture of surface interactions of organic compounds on prevalent indoor surfaces: Limonene adsorption on SiO2. Chem. Sci. 2019, 10, 2906–2914. [Google Scholar] [CrossRef]
- Mustoe, G.E. Silicification of Wood: An Overview. Minerals 2023, 13, 206. [Google Scholar] [CrossRef]
- Tian, R.; Seitz, O.; Li, M.; Hu, W.; Chabal, Y.J.; Gao, J. Infrared characterization of interfacial Si-O bond formation on silanized flat SiO2/Si Surfaces. Langmuir 2010, 26, 4563–4566. [Google Scholar] [CrossRef] [PubMed]
- Tiemblo, P.; García, N.; Hoyos, M.; Mejía, A.; de Francisco, R. Organic Modification of Hydroxylated Nanoparticles: Silica, Sepiolite, and Polysaccharides. In Handbook of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–35. [Google Scholar] [CrossRef]
- Kalhor, A.; Ghazavi, M.; Roustaei, M. Impacts of Nano-silica on Physical Properties and Shear Strength of Clayey Soil. Arab. J. Sci. Eng. 2022, 47, 5271–5279. [Google Scholar] [CrossRef]
- Kalkan, E.; Akbulut, S. The positive effects of silica fume on the permeability, swelling pressure and compressive strength of natural clay liners. Eng. Geol. 2004, 73, 145–156. [Google Scholar] [CrossRef]
- Majdi, M.; Uromiei, A.; Nikudel, M. Natural Nanoparticles Effects on Geotechnical Properties of Clayey Soil. J. Eng. Aplied Sci. 2016, 11, 361–368. [Google Scholar]



| Property | Value |
|---|---|
| Chemistry Formula | |
| Morphology and Color | Amorphous white powder |
| Particle Size | 20 nm (0.00002 mm) |
| Specific Surface Area | 145–160 m2/g |
| Purity | 99% |
| Particle Size Distribution by Washing | Particle Size Distribution by Hydrometer | ||
|---|---|---|---|
| Passing 4.75 mm: | 100% | Particles smaller than 0.075 mm: | 83.58% |
| Passing 2.00 mm: | 99.82% | Particles smaller than 0.005 mm: | 55.32% |
| Passing 0.475 mm: | 98.84% | Particles smaller than 0.002 mm: | 36.45% |
| Passing 0.075 mm: | 85.39% | ||
| Property | Value | Property | Value |
|---|---|---|---|
| Sand fraction | 14.55% | Ash content | High ash content |
| Silt fraction | 48.99% | Fiber content | 10.92% |
| Clay fraction | 36.45% | Specific gravity | 1.91 |
| Liquid limit, LL | 295.34% | pH at 0% of NS | 3.79 |
| Plastic limit, PL | 201.18% | pH at 6% of NS | 3.63 |
| Plasticity index, PI | 94.16% | Electric conductivity | 3.71 dS/m |
| Activity Index | 2.58 | Exchangeable cation “Ca” | 10.20 cmol/kg |
| USCS soil classification | Organic Silt (OH) | Exchangeable cation “Mg” | 2.15 cmol/kg |
| Mineral | Chemical Formula | Content (%) |
|---|---|---|
| Plagioclase Group | (Na,Ca)Al(Si,Al)Si2O8 | 85 |
| Anhydrite | CaSO4 | 6 |
| Cordierite | Mg2Al4Si5O18 | 4 |
| Maghemite | Fe2O3 | 3 |
| Sodalite | Na8(AlSiO4)6(ClO4)2 | 2 |
| Test | % Nanosilica | Organic Content | Average (Partial) | Standard Deviation (Partial) | Overall Average | Overall Standard Deviation |
|---|---|---|---|---|---|---|
| USCS | ------- | 43.66 | 43.63 | 0.60 | 43.84 | 1.99 |
| 43.02 | ||||||
| 44.22 | ||||||
| Particle Size Distribution by Hydrometer | ------- | 47.01 | 45.35 | 1.65 | ||
| 45.34 | ||||||
| 43.70 | ||||||
| Specific Gravity | 0 | 41.53 | 44.48 | 2.52 | ||
| 0.5 | 46.66 | |||||
| 1 | 45.61 | |||||
| 3 | 41.99 | |||||
| 6 | 46.60 | |||||
| Consistency Limits | 0 | 43.34 | 42.71 | 0.84 | ||
| 0.5 | 43.64 | |||||
| 1 | 41.69 | |||||
| 3 | 42.01 | |||||
| 6 | 42.88 | |||||
| Maximum Dry Density and Optimum Water Content | 0 | 42.41 | 44.54 | 2.01 | ||
| 40.21 | ||||||
| 0.5 | 43.13 | |||||
| 46.41 | ||||||
| 1 | 44.71 | |||||
| 45.54 | ||||||
| 3 | 46.41 | |||||
| 45.33 | ||||||
| 6 | 45.69 | |||||
| 45.55 | ||||||
| Unconfined Compressive Strength | 0 to 6 | 41.03 | 41.76 | 1.45 | ||
| 42.35 | ||||||
| 40.19 | ||||||
| 43.47 |
| % Nanosilica | Average LP | Average LL | Average IP | LL Increase | LP reduction | IP Increase | LP Standard Deviation | LL Standard Deviation |
|---|---|---|---|---|---|---|---|---|
| 0 | 201.18 | 295.34 | 94.16 | ------- | ------- | ------- | 0.92 | 1.18 |
| 0.5 | 196.38 | 297.19 | 100.82 | 0.63% | 2.39% | 7.07% | 1.41 | 0.51 |
| 1 | 196.05 | 300.94 | 104.89 | 1.90% | 2.55% | 11.40% | 0.69 | 0.63 |
| 3 | 192.46 | 325.76 | 133.31 | 10.30% | 4.33% | 41.58% | 1.28 | 1.69 |
| 6 | 188.35 | 326.12 | 137.77 | 10.42% | 6.38% | 46.31% | 0.85 | 1.44 |
| % Nanosilica | GS | Average | Standard Deviation |
|---|---|---|---|
| 0 | 1.90 | 1.91 | 0.035 |
| 1.94 | |||
| 1.87 | |||
| 0.5 | 1.92 | 1.90 | 0.026 |
| 1.89 | |||
| 1.87 | |||
| 1 | 1.91 | 1.92 | 0.015 |
| 1.91 | |||
| 1.93 | |||
| 3 | 1.92 | 1.91 | 0.017 |
| 1.89 | |||
| 1.91 | |||
| 6 | 1.87 | 1.88 | 0.020 |
| 1.86 | |||
| 1.90 |
| % Nanosilica | Maximum Dry Density (g/cm3) | Optimum Water Content (%) | R2 | Max Dry Density Reduction | Optimum Water Content Increase |
|---|---|---|---|---|---|
| 0 | 0.58 | 80.92 | 0.96 | ------- | ------- |
| 0.5 | 0.57 | 81.40 | 0.86 | 1.72% | 0.59% |
| 1 | 0.57 | 81.84 | 0.86 | 1.72% | 1.14% |
| 3 | 0.56 | 82.81 | 0.95 | 3.45% | 2.34% |
| 6 | 0.56 | 83.61 | 0.93 | 3.45% | 3.32% |
| % Nanosilica | Average Water Content [%] | Average Dry Density [g/cm3] | Average Void Ratio | Average Degree of Saturation [%] |
|---|---|---|---|---|
| 0 | 80.41 | 0.58 | 2.30 | 66.86 |
| 0.5 | 80.71 | 0.57 | 2.32 | 66.08 |
| 1 | 82.38 | 0.57 | 2.36 | 67.03 |
| 3 | 83.22 | 0.56 | 2.38 | 66.74 |
| 6 | 85.33 | 0.56 | 2.38 | 67.50 |
| % Nanosilica | Undrained Shear Strength (Su) [kPa] | Elastic Modulus (E50) [MPa] | (Su) Standard Deviation | (E50) Standard Deviation | Su Increase [%] | E50 Increase [%] |
|---|---|---|---|---|---|---|
| 0 | 33.06 | 1.83 | 4.17 | 0.28 | ------- | ------- |
| 0.5 | 69.36 | 3.60 | 10.68 | 0.30 | 109.80 | 96.72 |
| 1 | 102.91 | 4.66 | 5.91 | 0.51 | 211.28 | 154.64 |
| 3 | 80.52 | 3.98 | 3.20 | 0.32 | 143.56 | 117.49 |
| 6 | 89.20 | 3.59 | 7.61 | 0.33 | 169.81 | 96.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solórzano-Blacio, C.; Albuja-Sánchez, J. Effect of Nanosilica on the Undrained Shear Strength of Organic Soil. Nanomaterials 2025, 15, 702. https://doi.org/10.3390/nano15090702
Solórzano-Blacio C, Albuja-Sánchez J. Effect of Nanosilica on the Undrained Shear Strength of Organic Soil. Nanomaterials. 2025; 15(9):702. https://doi.org/10.3390/nano15090702
Chicago/Turabian StyleSolórzano-Blacio, Carlos, and Jorge Albuja-Sánchez. 2025. "Effect of Nanosilica on the Undrained Shear Strength of Organic Soil" Nanomaterials 15, no. 9: 702. https://doi.org/10.3390/nano15090702
APA StyleSolórzano-Blacio, C., & Albuja-Sánchez, J. (2025). Effect of Nanosilica on the Undrained Shear Strength of Organic Soil. Nanomaterials, 15(9), 702. https://doi.org/10.3390/nano15090702









